Abstract
AMPA receptors (AMPAR) mediate the majority of fast excitatory neurotransmission in the central nervous system (CNS). Transmembrane AMPAR regulatory proteins (TARPs) have been identified as a novel family of proteins which act as auxiliary subunits of AMPARs to modulate AMPAR trafficking and function. The trafficking of AMPARs to regulate the number of receptors at the synapse plays a key role in various forms of synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD). Expression of the prototypical TARP, stargazin/TARPγ2, is ablated in the stargazer mutant mouse, an animal model of absence epilepsy and cerebellar ataxia. Studies on the stargazer mutant mouse have revealed that failure to express TARPγ2 has widespread effects on the balance of expression of both excitatory (AMPAR) and inhibitory receptors (GABAA receptors, GABAR). The understanding of TARP function has implications for the future development of AMPAR potentiators, which have been shown to have therapeutic potential in both psychological and neurological disorders such as schizophrenia, depression and Parkinson's disease.
| Abbreviations | ||
| AMPA | = | α-amino-3-hydroxy-5-methylisoxazole-4-propinoic acid |
| CGC | = | Cerebellar granule cell |
| CNQX | = | 6-cyano-7-nitroquinoxaline-2,3-dione |
| GABA | = | γ-aminobutyric acid, NMDA, N-methyl-D-aspartate |
| PSD-95 | = | Postsynaptic density protein of 95 kDa |
| TARP | = | Transmembrane AMPA receptor Regulatory Protein |
Introduction
The majority of fast excitatory neurotransmission in the central nervous system (CNS) is mediated via glutamate activation of AMPA receptors (AMPAR), which are tetrameric assemblies of GluR1-4 subunits Citation[1], Citation[2]. Modulation of AMPAR synaptic transmission underlies various forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD), models of learning and memory Citation[3–5]. This review will focus on the role of transmembrane AMPAR regulatory proteins (TARPs), a novel family of proteins which act as auxiliary subunits of AMPARs, to modulate AMPAR trafficking and function. Furthermore, how the failure to express the prototypical TARP, TARPγ2 affects the balance of expression of both excitatory (AMPAR) and inhibitory receptors (GABAA receptors, GABAR) will be discussed, exemplified by the use of the stargazer mutant mouse, an animal model of absence epilepsy and cerebellar ataxia.
AMPA receptors and interacting proteins
To date numerous postsynaptic scaffolding proteins have been reported to interact with the cytoplasmic C-terminal tail of AMPA receptors at the post synaptic density. These interactions occur either via postsynaptic density-95, discs large, zonula occludens (PDZ) domains, which include glutamate receptor interacting protein/AMPA receptor binding protein (GRIP/ABP), protein interacting with C-kinase (PICK1) and synapse-associated protein of 97 kDa (SAP-97), or via non-PDZ domains, to regulate receptor trafficking Citation[6–11].
The first protein identified to interact with AMPA receptors was the 36–41 kDa protein, stargazin, which is completely ablated in the stargazer mutant mouse, an animal model of human absence epilepsy and cerebellar ataxia Citation[12], Citation[13]. Stargazin protein was determined to share a similar putative secondary structure with the γ subunit of skeletal muscle voltage-dependent calcium channel, γ1, comprising four transmembrane domains and cytosolic amino and carboxy termini. Heterologous expression of stargazin demonstrated that the protein had relatively minor effects on P/Q, L- and T-type calcium channel kinetics and cell surface trafficking Citation[13–16]. Stargazin was therefore proposed to be a neuronal counterpart of the skeletal muscle-specific calcium channel subunit, γ1 and was termed γ2 Citation[13].
Transmembrane AMPA receptor regulatory proteins (TARPs)
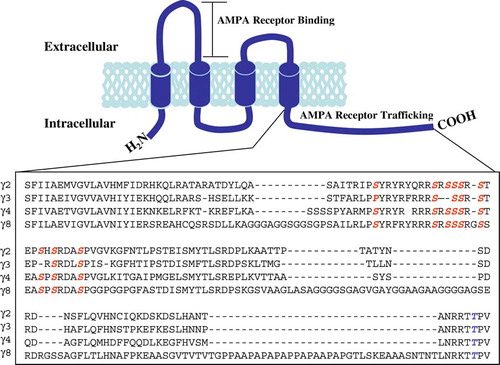
Tomita et al. Citation[17] defined a proposed subgroup of calcium channel subunits, as a family of transmembrane AMPA receptor regulatory proteins (TARPs). The identification of a calcium channel subunit as a member of the TARP family was based on the ability of the subunit to rescue glutamate-evoked responses in stargazer cerebellar granule cells (CGCs), CGCs that fail to express AMPAR at the cell surface as a result of ablation of stargazin expression. Subsequently, stargazin (γ2), γ3, γ4 and γ8 were identified as members of the TARP family Citation[17]. The secondary structure of TARP proteins comprises four transmembrane domains and cytosolic amino and carboxy termini. The carboxy terminus contains a PDZ binding motif (-RR/KTTPV), a protein-protein motif which interacts with PSD-95 at the synapse, which is conserved in all of the TARP isoforms () Furthermore, the C-terminus also contains a number of serine, threonine and tyrosine residues which may act as substrates for phosphorylation.
Figure 1. TARP protein structure. Schematic representation of the secondary structure of TARP proteins, comprising four transmembrane domains and cytosolic carboxy and amino termini. Amino acid sequence of the cytosolic C-terminal tail aligned in TARPγ2, γ3, γ4 and γ8. Red letters indicate phosphorylated serine residues and blue letters, phosphorylated threonine residues. This Figure is reproduced in colour in Molecular Membrane Biology online.
Mapping of TARP isoform mRNA and protein distribution by a combination of in situ hybridization and Western blotting techniques demonstrated that TARPs had overlapping distributions in the brain, such that one cell type could possess a number of TARP isoforms (). However, no two TARP isoforms have been shown to associate within the same AMPAR/TARP complex. The highest levels of expression of TARPγ2 were demonstrated in the cerebellum, TARPγ3 in the cerebral cortex, TARPγ4 in the neonatal forebrain and TARPγ8 in the hippocampus. Immunogold and immunofluorescence studies using pan-TARP antibodies revealed the selective location of TARPs at punctate sites on dendrites corresponding to excitatory synapses. This was evidenced by overlapping staining with the AMPAR GluR2 subunit and postsynaptic density protein of 95 kDa (PSD-95) Citation[22]. Immunoprecipitations of Triton X-100 soluble brain extracts further confirmed the association of TARPs with AMPAR ().
Figure 2. Immunoaffinity purification of TARPγ2 and its associated AMPAR subunits. (A) Immunoblot analysis of TARPγ2 protein throughout the purification assay. Immunoblot probed with an anti-TARPγ2 antibody raised to the extreme C-terminus. Input is cerebellar membranes from control (+/+:+/stg) and stargazer (stg) mice. Insol is the proportion of +/+:+/stg and stg material that remained in the insoluble fraction following Triton X-100 solubilization. FT is the flow-through the column, the soluble material that did not bind to the immunoaffinity column. Output, pH eluted fractions 1–5 are the purified material acid-eluted from the immunoaffinity column. (B) GluR2 solubilization and co-purification with TARPγ2 in control (+/+:+/stg) material. (C) GluR4 solubilization and co-purification with TARPγ2 in control (+/+:+/stg) material.
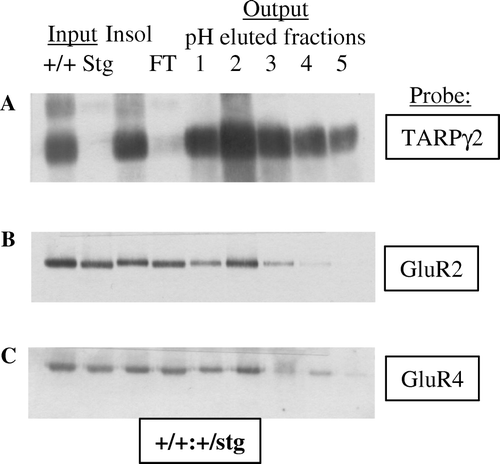
Table I. TARP isoforms.
Stargazin co-immunoprecipitated with AMPAR GluR1, 2 and 4 subunits in cerebellum, γ3 co-associated with AMPAR subunits in cortex, γ4 in neonatal forebrain, and γ8 in hippocampus Citation[17], Citation[23].
More recently Kato and co-workers have identified calcium channel γ7 as a member of the TARP family, which was previously suggested not to act as a TARP. TARPγ7 has been shown to selectively bind AMPAR subunits and PSD-95 in Purkinje neurons in the molecular layer and glomerular synapses in the cerebellar granule cell layer of the cerebellum Citation[24].
TARPs in AMPA receptor trafficking
TARPγ2 contributes to multiple stages in the trafficking of AMPAR, from receptor biogenesis to trafficking from the endoplasmic reticulum (ER) to the Golgi, cell surface and postsynaptic density (PSD) (see and ).
Figure 3. AMPAR trafficking at the synapse. Two monomeric AMPAR subunits form a dimer (1), followed by the association of two dimers of AMPAR subunits to form a tetramer (2) in the endoplasmic reticulum (ER). TARPs associate with the tetrameric AMPAR to act as an auxiliary subunit (3) permitting the efficient export of the AMPAR from the ER to the Golgi (4). nPIST binds to the C-terminal tail of TARPγ2 in the Golgi (5) and acts to chaperone the AMPAR complex to the cell surface in vesicles (6). The TARPγ2-AMPAR complex diffuses into the PSD where PSD-95 binds to the PDZ binding domain of the C-terminal tail of TARPγ2 to anchor the complex at the synapse (7). nPIST is then recycled to the Golgi (8). This Figure is reproduced in colour in Molecular Membrane Biology online.
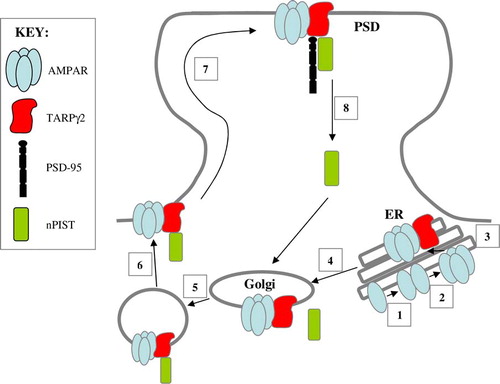
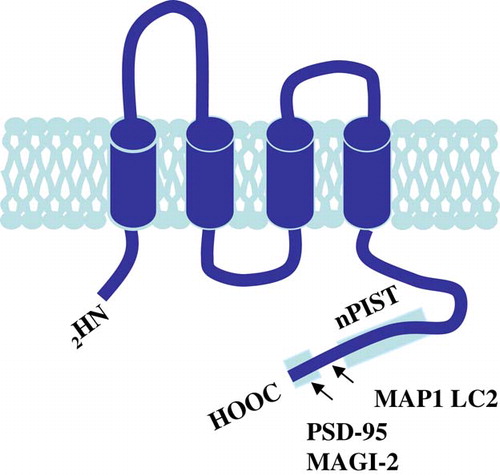
Figure 4. TARPγ2 and its associated proteins. The putative secondary structure of TARPγ2 consists of four transmembrane domains with cytosolic amino and carboxy termini. The C-terminus contains a PDZ binding motif (-RRTTPV) which interacts with MAGUKs (e.g., PSD-95 and MAGI-2) at the synapse. MAP1 LC2 and nPIST interact with TARPγ2 upstream of the PDZ binding domain. This Figure is reproduced in colour in Molecular Membrane Biology online.
Trafficking from the ER to the Golgi
Assembly of AMPAR subunits begins by formation of dimers from monomeric subunits, to formation of a tetrameric structure by the association of two dimers in the ER. The formation of tetramers is required for exit from the ER. TARPγ2 is an auxiliary AMPAR subunit which associates with all four AMPAR subunits, but only within a tetrameric receptor structure Citation[25]. Vandenberghe et al. Citation[25] identified two receptor populations, a functional form which is associated with TARPγ2, and an apo-form which lacks TARPγ2. AMPAR of the stargazer mutant cerebellum show an immature ER-type of glycosylation pattern, consistent with a role for TARPγ2 in the transport of AMPAR from the ER Citation[17], Citation[26]. Furthermore, the unfolded protein response is up-regulated in stargazer CGCs, a homeostatic pathway that up-regulates genes encoding ER chaperones such as Ig binding protein (BiP), to prevent accumulation of unfolded and unassembled proteins in the ER, indicating a role for TARPγ2 in ER processing of AMPAR Citation[27].
Trafficking from the Golgi to the cell surface
TARPγ2 functions to control AMPA receptor number at the synapse by regulating the delivery of AMPAR to the cell surface, a function which does not require the PDZ-domain of the protein Citation[28]. Stargazer cerebellar granule cells express reduced levels of AMPAR subunits GluR2 and 4 Citation[26]; furthermore, functional receptors are not delivered to the cell surface Citation[28], Citation[29]. Transfection of TARPγ2 or TARPγ4 into these cells restores normal AMPAR function Citation[28], Citation[30].
Cuadra and co-workers Citation[31] identified a novel domain within the C-terminus of TARPγ2 (residues 243–283) which was required for TARPγ2 and AMPAR synaptic targeting. Following yeast 2-hybrid screening, they identified a Golgi-enriched protein implicated in the trafficking of transmembrane proteins, neuronal isoform of protein-interacting specifically with TC10 (nPIST). It was suggested that TARPγ2-AMPAR complexes are chaperoned to the PSD by nPIST, where it can interact with other synaptic proteins or directly with PSD-95, allowing the TARPγ2 C-terminus to bind to PSD-95 and stabilize AMPAR at the synapse. nPIST is then recycled to the Golgi to repeat the cycle () Citation[31]. Microtubule-associated protein 1, light chain 2 (MAP1 LC2) has also been implicated in the trafficking of TARPγ2 and the GluR2 subunit of AMPAR Citation[26]. MAP1 LC2 was found to interact with TARPγ2 upstream of the C-terminal PDZ binding domain, forming a tripartite complex of MAP1 LC2, TARPγ2 and GluR2 after exit from the endoplasmic reticulum, prior to incorporation into the synapse ().
Cell surface stabilization and synaptic localization
The second mechanism of AMPAR trafficking for which TARPγ2 is responsible, originally identified by Chen and co-workers in 2000, is the synaptic targeting of AMPAR. Deletion of the final four amino acids of the C-terminal tail of TARPγ2 protein (stargazinΔC) disrupted the association of TARPγ2 with PSD-95, indicating a critical role for the PDZ domain of TARPγ2 in the synaptic clustering of AMPAR Citation[28]. Chimeric analysis, by systematically switching sections of the calcium channel subunit γ5 structure with TARPγ2 domains identified the first intracellular loop and intracellular tail of TARPγ2 (C-terminal residues 212–268) to be critical in AMPAR trafficking Citation[32]. However, more recently calcium channel γ7, a subunit which is highly related to γ5 in structure, has been identified as a member of the TARP family of proteins and is itself involved in AMPAR trafficking Citation[24].
In support of the role of TARPγ2 in the synaptic targeting of AMPAR to the cell surface, Schnell et al. Citation[33] demonstrated that overexpression of TARPγ2 resulted in a selective increase in extrasynaptic AMPAR excitatory postsynaptic currents (EPSCs). Clustering of these receptors at the synapse required increased expression of the synaptic anchor, PSD-95. Transfection of the stargazinΔC clone, lacking the PDZ domain, into slice cultures resulted in decreased clustering of AMPAR and decreased AMPAR EPSCs Citation[33]. It has recently been shown that there is an exchange of AMPAR by lateral diffusion between extrasynaptic and synaptic sites, which depends on the interaction of TARPγ2 and PSD-95 Citation[34]. Furthermore, a disruption of this interaction increases AMPAR diffusion, preventing accumulation at postsynaptic sites. A further membrane associated guanylate kinase (MAGUK) which has been reported to interact with the cytoplasmic C-termini of TARPs is membrane associated guanylate kinase, WW and PDZ domain containing 2, MAGI-2 Citation[35]. MAGI-2 was shown to recruit TARPγ2 to the cell membrane in the presence of the TARPγ2 C-terminal TTPV motif.
Modulation of TARPγ2 synaptic location
Synaptic plasticity involves protein phosphorylation cascades that alter the number of AMPAR at the synapse. The cytosolic C-terminus of TARPγ2 contains a number of serine, threonine and tyrosine residues that may act as substrates for phosphorylation (). Interestingly, the PDZ domain of the TARPγ2 C-terminal tail overlaps with the consensus sequence for phosphorylation by protein kinase A (PKA). TARPγ2 is basally phosphorylated at a critical threonine residue (T321) by PKA in the brain Citation[36]. As a consequence of phosphorylation, TARPγ2 selectively loses its interaction with PSD-95. Choi et al. Citation[36] demonstrated that mutations mimicking phosphorylation at T321 disrupted interactions with PSD-95 and SAP-97 both in the yeast 2-hybrid system and biochemical associations and clustering of TARPγ2 and PSD-95 in heterologous cells. Furthermore, a TARPγ2 phosphomimic (Thr 321→ Glu) transfected into cultured hippocampal neurons acted to reduce the frequency and amplitude of AMPAR-mediated EPSCs Citation[37].
In contrast, Tomita et al. Citation[38] proposed that only serine residues of the C-terminal tail of TARPγ2 are phosphorylated in cultured cerebrocortical neurons. The group demonstrated that phosphorylation of TARPγ2 was dynamically regulated by activation of calcium-calmodulin kinase II (CaMKII) and protein kinase C (PKC) to induce phosphorylation, whereas activation of protein phosphatases, PP1 and PP2B dephosphorylate serine residues. Phosphorylation of TARPγ2 facilitates synaptic trafficking of AMPAR to the surface through interaction with PDZ proteins. Furthermore, phosphorylation of TARPγ2 was found to be important in hippocampal LTP, while dephosphorylation mediates LTD Citation[38].
TARPs and AMPAR function
In addition to a significant role in AMPAR trafficking, TARPs have been shown to act to alter the electrophysiological properties of AMPAR Citation[39–42]. AMPARs which interact with TARPs show enhanced current responses to glutamate and kainate Citation[39], Citation[43], prolonged channel opening, increased kainate efficacy, increased probability of attaining high conductance opening levels, decreased receptor desensitization Citation[40–42] and enhanced recovery from desensitization which act to accelerate the recovery of AMPAR to the unbound (activating) state.
More recently, it has been proposed that the extent of TARP-mediated enhancement of agonist-induced responses is dependent on the individual AMPAR subunit and/or TARP Citation[30], Citation[43–45]. Cho and co-workers have proposed the possibility of two subgroups of TARPs which have differing effects on AMPAR functional properties. TARPγ2 and TARPγ3 have been reported to have smaller effects on the kinetics of glutamate-evoked currents and larger increases in kainate efficacy compared to TARPγ4 and TARPγ8, which results from differences in the first extracellular domain of the TARP Citation[30], Citation[43]. Furthermore, it has been reported that TARPγ4 modulates the desensitization properties of AMPAR more strongly than TARPγ2 Citation[44], Citation[45]. Soto et al. Citation[46] have also recently reported that TARPγ2 reduces the block of calcium-permeable AMPARs (comprising GluR1,3 or 4) by intracellular polyamines, acting to increase single channel conductance.
The stargazer mutant mouse
The stargazer mutant mouse is an animal model of human absence epilepsy and cerebellar ataxia. The phenotype of the stargazer mutant appears at postnatal day 14 (P14) and manifests in cerebellar ataxia, impaired eye-blink conditioning responses and a characteristic head toss, from which the mutant was named (star-gazing) Citation[47], Citation[48]. At P18, the mutant begins to exhibit spontaneous bilaterally symmetrical 6–7 Hz spike-wave discharges at a frequency of ∼ 150 per hour which originate in the reticulothalamocortical circuit, acting to persistently activate neocortical and hippocampal networks Citation[49].
Abnormalities in neurotransmission in the stargazer mutant
Excitatory neurotransmission and inhibitory GABAR expression in the stargazer cerebellum
TARPγ2 is heavily expressed in the cerebellum where it is largely restricted to the cerebellar granule cells (CGCs), neurons that normally exclusively express the TARPγ2 isoform of TARPs. As a result, mossy fibre-CGC synapses in stargazer do not express AMPARs and are subsequently electrically silent Citation[28]. It has also been shown that there is a CGC-specific deficit in brain-derived neurotrophic factor (BDNF) expression and signalling in the stargazer Citation[48]. A number of studies have focused on the ability of inhibitory GABAergic networks to adapt to changes in the strength of their excitatory inputs Citation[50–53] and any accompanying changes in BDNF/TrkB signalling Citation[54–56]. Interestingly, GABAR expression in CGCs has previously been shown to be impaired in waggler mice, which also possess a mutated stargazin gene Citation[57]. The GABAR channel kinetics recorded in adult waggler CGCs were comparable to those expressed in CGCs of juvenile control mice Citation[58], implying that the waggler mutation resulted in the developmental arrest of CGCs, such that GABAR expression was restricted to that expected in juvenile neurons. It has been shown that the α6 subunit-containing GABAR subtypes are selectively affected by the mutation Citation[59], including not only the synaptic α6 subunit-containing GABAR subtypes (α6βxγ2 and α1α6βxγ2) but also the extrasynaptic, tonic inhibition-conferring α6βxδ receptors Citation[50], Citation[60], Citation[61]. Extrasynaptic GABAR are responsible for illiciting >97% of GABAR-mediated inhibition in CGCs and are thus pivotal to information transfer in the cerebellum Citation[62] ().
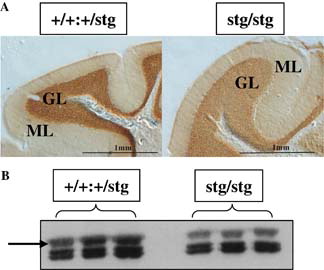
Figure 5. GABAR α6 subunit expression is downregulated in the cerebellum of the stargazer mutant mouse. The GABAR α6 subunit is downregulated in the cerebellar granule cells of the stargazer mutant mouse cerebellum, as a consequence of the stargazer mutation. (A) Immunohistochemical staining of the distribution of the GABAR α6 subunit in the cerebellum of a control (+/+:+/stg, left) and a stargazer (stg/stg, right) mouse. Note the reduction in intensity of staining, in the cerebellar granule cell layer (GL) of the stargazer mutant. ML is the molecular layer. (B) Immunoblot analysis of adult control (+/+:+/stg, left) versus stargazer (stg/stg, right) cerebellum, probed for GABAR α6 subunit expression. Arrow indicates the protein band corresponding to the GABAR α6 subunit.
AMPA receptor activity has selective effects on GABAR subtypes expressed in cerebellar granule cells that may underpin homeostatic GABAergic responses to neuronal excitability. Expression and assembly of α6-containing GABARs is regulated either directly by AMPAR-mediated excitability of CGCs, e.g., depolarization-mediated regulation of α6 expression through a Ca2 + -mediated signal transduction pathway Citation[63], or indirectly as a down-stream consequence of the loss of AMPAR activity e.g. an inability to express BDNF Citation[48]. BDNF has been shown to regulate α6 gene transcription Citation[64]. TARPγ2 and AMPAR subunits, e.g., GluR2, are expressed in maturing CGCs in vitro prior to expression of both GABAR α6 and δ subunits which suggests that expression of these subunits could be regulated by AMPAR activity.
CGCs from control ( (AMPAR-competent, kainate receptor (KAR)-competent) and stargazer (AMPAR-incompetent, KAR-competent) can be cultured in depolarizing media containing either 25 mM KCl or the non-NMDA receptor agonist, kainic acid. Using these cultures, KCl- and kainic acid-mediated depolarization has been shown to downregulate GABAR α1, α6 and β2 but upregulate α4, β3 and δ subunits in control neurons Citation[51], Citation[65]. The KCl-, but not kainic acid-, evoked effects were reciprocated in stargazer neurons, compatible with AMPAR-regulation of GABAR expression. Conversely, GABAR γ2 expression was insensitive to KCl-mediated depolarization but was downregulated by kainic acid-treatment in a CNQX-reversible manner in control and stargazer neurons, compatible with a KAR–mediated response Citation[65]. This implies that failure to express functional AMPARs and subsequent loss of electrical activity of CGCs in stargazer may play a part in the GABAR deficits observed in stargazer CGCs ().
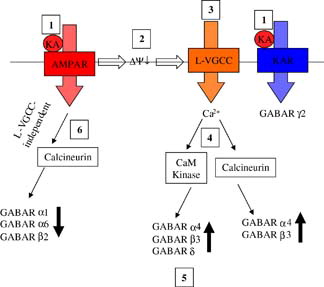
Figure 6. The effects and signalling pathways involved following activation of non-NMDA receptors on GABAR expression in mouse cerebellar granule neurons. Exogenously applied kainic acid activates both AMPAR and KAR (1). AMPAR activation evokes membrane depolarization (2) and subsequent activation of L-type voltage-gated calcium channels (3) leading to Ca2 + influx and activation of Ca2 + /calmodulin dependent protein kinase (CaM Kinase) and calcineurin (4). CaM kinase activation results in upregulation and increased membrane surface expression of GABAR α4, β3 and δ (5). Calcineurin activation by this route leads to upregulation of α4 and β3. AMPAR activation also leads to an L-type VGCC-independent activation of calcineurin resulting in downregulation of GABAR α1, α6 and β2 expression (6). KAR activation leads to downregulation of GABAR γ2 expression via a mechanism that is independent of depolarization, calcineurin, CaM kinase and NMDA receptors. Reproduced with permission from Wiley-Blackwell Publishing, Payne HL, Ives JH, Sieghart W, Thompson CL. AMPA and Kainate receptors mediate mutually exclusive effects on GABAA receptor expression in cultured mouse cerebellar granule neurons. Journal of Neurochemistry. This Figure is reproduced in colour in Molecular Membrane Biology online
Inhibitory neurotransmission in the stargazer dentate gyrus
In contrast to cerebellar granule cells, granule cells of the dentate gyrus (DG) are not electrically silent in the stargazer mutant. The stargazer mutant exhibits spontaneous bilaterally symmetrical 6–7 per second spike-wave discharges in cortical and hippocampal brain areas, which represent ∼20% of total waking electroencephalographic (EEG) activity Citation[49]. The spike-wave pattern of generalized synchronous activity has been shown to generate primarily in the thalamus and neocortex to activate synaptically linked brain areas, such as the hippocampus via the dentate gyrus.
A number of convulsive seizures have been reported to induce rearrangements in the GABAR expression profiles in the hippocampus as an adaptive modification of inhibitory neurotransmission to balance increased excitation Citation[53], Citation[66–68]. In contrast to decreased BDNF mRNA expression and trkB signalling in stargazer cerebellar granule cells (CGCs), the dentate granule cells (DGCs) of the stargazer mutant are subject to intermittent elevated BDNF expression Citation[69], Citation[70]. Activation of trkB signalling in the hippocampus has been shown to influence the maturation of GABAergic factors in cultured hippocampal neurons, including increased expression of GABAR β2/3 subunits Citation[54], increased GABAR cluster number and synaptic localization Citation[56], as well as increased PKC phosphorylation and surface expression of GABAR β3 subunit containing receptors Citation[55].
It has been shown that GABAergic parameters are affected by the stargazer mutation in this brain region. GABAR α4 and β3 subunits were consistently up-regulated, GABAR δ expression was reduced while GABAR α1, β2 and γ2 subunits and the GABAR synaptic anchoring protein, gephyrin were largely unaffected Citation[71]. The α4βγ2 subunit-containing subtype of GABARs, not normally a significant GABAR in DG neurons, was strongly up-regulated in stargazer DG. These GABAR arose at the expense of extrasynaptic α4βδ-containing receptors, indicating a switch from tonic GABA responsive, neurosteroid-sensitive extrasynaptic α4-containing GABARs (α4βδ) to potentially synaptic α4-containing GABARs that are relatively neurosteroid-insensitive and respond phasically to synaptically-released GABA (α4βγ2) Citation[72]. This change was associated with a reduction in neurosteroid-sensitive GABAR-mediated tonic current. A unique, intrinsic adaptation of GABAergic networks in stargazer Citation[71]. TARPγ2 was not detected in stargazer or control DG, rather TARPγ8 was found to be the principal TARP-isoform in the DG and its expression is compromised by the stargazer mutation Citation[71]. These effects on GABAergic parameters and TARPγ8 expression are likely to arise as a consequence of failed expression of TARPγ2 elsewhere in the brain resulting in hyper-excitable inputs to the dentate gyrus.
Conclusion
This review has highlighted the importance of a family of proteins, the transmembrane AMPAR regulatory proteins in the regulation of expression and function of AMPAR at the synapse and subsequently their role in various forms of synaptic plasticity. Moreover, the utility of the stargazer mutant mouse as a model for delineating the roles of TARPs, particularly TARP γ2, has been discussed.
The understanding of TARP function has implications for the future development of AMPAR potentiators (e.g., AMPAkines). These agents have been shown to display therapeutic potential in a wide range of psychological and neurological disorders, such as schizophrenia, depression and Parkinson's disease. Tomita et al. Citation[74] reported that TARPγ2 modulates the pharmacology of AMPAR potentiators, such as cyclothiazide, acting to increase the affinity of AMPAR potentiators for glutamate receptor subunits, and promoting AMPAR signalling by slowing deactivation and reducing receptor desensitization Citation[74]. This may underlie the known effects of such compounds on synaptic plasticity Citation[3–5], Citation[74]. Furthermore, chronic antidepressant treatment has been shown to enhance membrane expression of AMPARs in the hippocampus by increasing the interaction of AMPAR subunits with different neuronal proteins that regulate the insertion and stabilization of AMPARs at the hippocampal membrane. These effects include an increase in the interaction of TARPγ2 and the AMPAR subunit, GluR1, which could contribute to the clinical efficacy of some of the newer antidepressant therapies Citation[73].
Acknowledgements
I would like to thank Drs Paul L Chazot and Victoria Hann for critical reading of this manuscript.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Hollmann M, Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci 1994; 17: 31–108
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharm Rev 1999; 51: 7–61
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress?. Science. 1999; 285: 1870–1874
- Malinow R, Malenka RC. AMPA Receptor Trafficking and Synaptic Plasticity. Ann Rev Neurosci 2002; 25: 103–126
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 2002; 25: 578–588
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997; 386: 279–284
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman GJ, States BA, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, Weinberg RJ, Ziff EB. Novel anchorage of GluR2/3 to postsynaptic density by an AMPA receptor binding protein ABP. Neuron 1998; 21: 581–591
- Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C( binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharm 1999; 38: 635–644
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 1999; 22: 179–187
- Henley JM. Proteins interactions implicated in AMPA receptor trafficking: A clear destination and an improving route map. Neurosci Res 2003; 45: 243–254
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron 2003; 40: 361–379
- Noebels JL, Qiao X, Bronson RT, Spencer C, Davisson MT. Stargazer: A new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res 1990; 7: 129–135
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett II FS, Mori Y, Campbell KP, Frankel WN. The mouse Stargazer gene encodes a neuronal Ca2 + -channel ( subunit. Nat Gen 1998; 19: 340–347
- Klugbauer N, Dai S, Sprecht V, Lacinová L, Marais E, Bohn G, Hofmann F. A family of γ-like calcium channel subunits. Fed Euro Biochem Soc Lett 2000; 470: 189–197
- Rousset M, Cens T, Restituito S, Barrere C, Black III JL, McEnery MW, Charnet P. Functional roles of γ2, γ3 and γ4, three new Ca2+ channel subunits, in P/Q-type Ca2+ channel expressed in Xenopus oocytes. J Physiol 2001; 532.3: 583–593
- Green PJ, Warre R, Hayes PD, McNaughton NCL, Medhurst AD, Pangalos M, Duckworth DM, Randall AD. Kinetic modification of the α1I subunit-mediated T-type Ca2+ channel by a human neuronal Ca2 + channel γ subunit. J Physiol 2001; 533.2: 467–478
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol 2003; 161: 805–816
- Sharp AH, Black III JL, Dubel SJ, Sundarraj S, Shen JP, Yunker AMR, Copeland TD, Mcenery MW. Biochemical and anatomical evidence for specialized voltage-dependent calcium channel γ isoform expression in the epileptic and ataxic mouse, Stargazer. Neuroscience 2001; 105: 599–617
- Chu PJ, Robertson HM, Best PM. Calcium channel γ subunits provide insights into the evolution of this gene family. Gene 2001; 280: 37–48
- Moss FJ, Viard P, Davies A, Bertaso F, Page KM, Graham A, Canti C, Plumpton M, Plumpton C, Clare JJ, Dolphin AC. The novel product of a five-exon stargazin-related gene abolishes Cav2.2 calcium channel expression. Eur Mol Biol Org 2002; 21: 1514–1523
- Inamura M, Itakura M, Okamoto H, Hoka S, Mizoguchi A, Fukazawa Y, Shigemoto R, Yamamori S, Takahashi M. Differential localization and regulation of stargazin-like protein, (-8 and stargazin in the plasma membrane of hippocampal and cortical neurons. Neurosci Res 2006; 55: 45–53
- Fukaya M, Tsujita M, Yamazaki M, Kushiya E, Abe M, Akashi K, Natsume R, Kano M, Kamiya H, Watanabe M, Sakimura K. Abundant distribution of TARP γ-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci 2006; 24: 2177–2190
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP γ-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci 2005; 8: 1525–1533
- Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, (7, differentially regulates AMPA receptors. J Neurosci 2007; 27: 4969–4977
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. PNAS 2005; 102: 485–490
- Ives JH, Fung S, Tiwari P, Payne HL, Thompson CL. Microtubule-associated Protein Light Chain 2 is a Stargazin-AMPA Receptor Complex-interacting Protein in vivo. J Biol Chem 2004; 279: 31002–31009
- Vandenberghe W, Nicoll RA, Bredt DS. Interaction with the Unfolded Protein Response reveals a role for stargazin in biosynthetic AMPA receptor transport. J Neurosci 2005; 25: 1095–1102
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000; 408: 936–943
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse Stargazer. J Neurosci 1999; 19: 6027–6036
- Cho C, St-Gelais F, Zhang W, Tomita S, Howe JR. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron 2007; 55: 890–904
- Cuadra AE, Kuo S, Kawasaki Y, Bredt DS, Chetkovich DM. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J Neurosci 2004; 24: 7491–7502
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science 2004; 303: 1508–1511
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazing control synaptic AMPA receptor number. PNAS 2002; 99: 13902–13907
- Bats C, Groc L, Choquet D. The interaction between stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 2007; 53: 719–734
- Deng F, Price MG, Davis CF, Mori M, Burgess DL. Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain. J Neurosci 2006; 26: 7875–7884
- Choi J, Ko J, Park E, Lee J, Yoon J, Lim S, Kim E. Phosphorylation of stargazin by protein kinase A regulates its interaction with postsynaptic density-95 (PSD-95). J Biol Chem 2002; 277: 12359–12363
- Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. Phosphorylation of the postsynaptic density-95 (PSD-95)/Discs Large/Zona Occludens-1 binding site of Stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci 2002; 22: 5791–5796
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 2005; 45: 269–277
- Yamasaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res 2004; 50: 369–374
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci 2005; 25: 2682–2686
- Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci 2005; 25: 7438–7448
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 2005; 435: 1052–1058
- Kott S, Werner M, Korber C, Hollmann M. Electrophysiological properties of AMPA receptors are differentially modulated depending on the associated member of the TARP family. J Neurosci 2007; 27: 3780–3789
- Korber C, Werner M, Kott S, Ma Z, Hollmann M. The transmembrane AMPA receptor regulatory protein γ4 is a more effective modulator of AMPA receptor function than stargazin (γ2). J Neurosci 2007; 27: 8442–8447
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron 2007; 55: 905–918
- Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat Neurosci 2007; 10: 1260–1267
- Qiao X, Hefti F, Knusel B, Noebels JL. Selective failure of brain-derived neurotrophic factor mRNA expression in the cerebellum of stargazer, a mutant mouse with ataxia. J Neurosci 1996; 16: 640–648
- Qiao X, Chen L, Gao H, Bao S, Hefti F, Thompson RF, Knusel B. Cerebellar brain derived neurotrophic factor-TrkB defect associated with impairment of eyeblink conditioning in Stargazer mutant mice. J Neurosci 1998; 18: 6990–6999
- Qiao X, Noebels JL. Developmental analysis of hippocampal mossy fiber outgrowth in a mutant mouse with inherited spike-wave seizures. J Neurosci 1993; 13: 4622–4635
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 1998; 18: 1693–1703
- Ives JH, Drewery DL, Thompson CL. Differential cell surface expression of GABAA receptor α1, α6, β2 and β3 subunits in cultured mouse cerebellar granule cells influence of cAMP-activated signalling. J Neurochem 2002; 80: 317–327
- Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABAA receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol 2004; 557: 473–487
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci 2004; 24: 8629–8639
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci 2002; 22: 7580–7585
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity and cell-surface stability. J Neurosci 2004; 24: 522–530
- Elmiariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signalling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J Neurosci 2004; 24: 2380–2393
- Chen L, Bao S, Qiao X, Thompson RF. Impaired cerebellar synapse maturation in waggler, a mutant mouse with a disrupted neuronal calcium channel γ subunit. PNAS 1999; 96: 12132–12137
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci 1996; 16: 3630–3640
- Payne HL, Connelly WMK, Ives JH, Lehner R, Furtmuller B, Sieghart W, Tiwari P, Lucocq JM, Lees G, Thompson CL. GABAA α6-containing receptors are selectively compromised in cerebellar granule cells of the ataxic mouse, stargazer. J Biol Chem 2007; 282: 29130–29143
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol 1996; 497: 753–759
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci 1999; 19: 2960–2973
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 2002; 33: 625–633
- Suzuki K, Sato M, Morishima Y, Nakanishi S. Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J Neurosci 2005; 25: 9535–9543
- Bulleit RF, Hsieh T. MEK inhibitors block BDNF-dependent and -independent expression of GABAA receptor subunit mRNAs in cultured mouse cerebellar granule neurons. Dev Brain Res 2000; 119: 1–10
- Payne HL, Ives JH, Sieghart W, Thompson CL. 2007. AMPA and Kainate receptors mediate mutually exclusive effects on GABAA receptor expression in cultured mouse cerebellar granule neurons. J Neurochem ( in press).
- Brooks-kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med 1998; 4: 1166–1172
- Shumate MD, Lin DD, Gibbs JW, III, Holloway KL, Coulter DA. GABAA receptor function in epileptic human dentate granule cells: Comparison to epileptic and control rat. Epilepsy Res 1998; 32: 114–128
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABAA receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci 2003; 17: 1607–1616
- Chaftez RS, Nahm WK, Noebels JL. Aberrant expression of neuropeptide Y in hippocampal mossy fibers in the absence of local cell injury following onset of spike-wave synchronisation. Mol Brain Res 1995; 31: 111–121
- Nahm WK, Noebels JL. Nonobligate role of early or sustained expression of immediate-early gene proteins c-Fos, c-Jun, and Zif/268 in hippocampal mossy fiber sprouting. J Neurosci 1998; 18: 9245–9255
- Payne HL, Donoghue PS, Connelly WMK, Hinterreiter S, Tiwari P, Ives JH, Hann V, Sieghart W, Lees G, Thompson CL. Aberrant GABAA receptor expression in the dentate gyrus of the epileptic mutant mouse Stargazer. J Neurosci 2006; 26: 8600–8608
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 2002; 87: 2624–2628
- Martinez-Turrillas R, Del Rio J, Frechilla D. Neuronal proteins involved in synaptic targeting of AMPA receptors in rat hippocampus by antidepressant drugs. Biochem Biophys Res Com 2007; 353: 750–755
- Tomita S, Sekiguchi M, Wada K, Nicoll RA, Bredt DS. Stargazin controls the pharmacology of AMPA receptor potentiators. PNAS 2006; 103: 10064–10067