Abstract
Nicotinic acetylcholine receptors (nAChRs) are members of an extensive super-family of neurotransmitter-gated ion channels. In humans, nAChRs are expressed within the nervous system and at the neuromuscular junction and are important targets for pharmaceutical drug discovery. They are also the site of action for neuroactive pesticides in insects and other invertebrates. Nicotinic receptors are complex pentameric transmembrane proteins which are assembled from a large family of subunits; seventeen nAChR subunits (α1-α10, β1-β4, γ, δ and ε) have been identified in vertebrate species. This review will discuss nAChR subunit diversity and factors influencing receptor assembly and trafficking.
Introduction
Nicotinic acetylcholine receptors (nAChRs) are members of a super-family of ligand-gated ion channels which also includes receptors for the neurotransmitters adenosine triphosphate (ATP), γ-aminobutyric acid (GABA), glutamate, glycine and 5-hydroxytryptamine (5-HT) Citation[1], Citation[2]. Nicotinic receptors are also members of a smaller sub-family of ligand-gated ion channels which also includes the ionotropic receptors for GABA, glycine and 5-HT and which is often referred to as the ‘Cys-loop’ family of receptors Citation[1].
Nicotinic receptors are pentameric complexes assembled from an extensive family of subunits. In vertebrates, the 17 nAChR subunits (α1-α10, β1-β4, γ, δ and ε) can assemble into a variety of pharmacologically distinct receptor subtypes. Nicotinic receptors are expressed at the neuromuscular junction and also within the central and peripheral nervous system. More recently, nicotinic receptors have been identified in several non-neuronal cell types Citation[3]. In humans, nAChRs have been implicated in several neuromuscular and neurological disorders Citation[4–6], as a consequence of which they are major targets for pharmaceutical drug discovery Citation[7], Citation[8]. Nicotinic receptors are also expressed abundantly in insects and other invertebrates, where they are an important target for neuroactive pesticides Citation[9], Citation[10].
Nomenclature
The first nAChR subtype to be studied in detail was purified from the electric organ of fish such as the marine ray Torpedo and the freshwater eel Electrophorus (for a detailed review, see Citation[11]). Four subunits were identified in the electric organ nAChR and were assigned the Greek letters α, β, γ and δ on the basis of their increasing apparent molecular weights when resolved on polyacrylamide gels. Since only the Torpedo α could be labelled by quaternary ammonium affinity-labelling reagents after polyacrylamide gel electrophoresis, it was assumed that this was the principal agonist binding site Citation[12]. The subsequent molecular cloning of the Torpedo α subunit Citation[13], Citation[14] identified two adjacent cysteine residues (Cys192 and Cys 193), believed to be important in agonist binding. By convention, only nAChRs which contain two cysteine residues at positions analogous to Cys192 and Cys193 in the Torpedo α subunit have been classified as α-type subunits. It was assumed, for example on the basis of affinity-labelling experiments Citation[12], that α subunits were agonist-binding subunits, whereas non-α subunits were ‘structural’ subunits. However, more recent data indicates that nicotinic agonists bind at subunit interfaces Citation[15] and that both α and non-α subunits are able to contribute to the nicotinic agonist binding site Citation[16]. As discussed in more detail below, seventeen nAChR subunits have been identified in vertebrates (α1-α10, β1-β4, γ, δ and ε). There is considerable subunit diversity amongst nAChR subtypes Citation[17], Citation[18] and, consequently, receptor subtypes are commonly referred to by their subunit composition. For example, α4β2 refers to a nAChR subtype containing only α4 and β2 subunits (even though the precise subunit stoichiometry may not be known). By convention, when the subunit composition of a nAChR subtype is less clearly defined this is indicated by an asterisk Citation[19], Citation[20]. For example, α4β2* indicates a nAChR which is known to contain α4 and β2 subunits but which may also contain additional subunit subtypes. Where both subunit composition and also subunit stoichiometry is known, the number of each subunit present in the assembled pentamer is indicated by subscript numbers, for example α75 or α12β11γ1δ1 (or, more commonly, α12β1γδ).
Muscle-type nAChRs
Nicotinic receptors at the vertebrate neuromuscular junction are similar in subunit composition to nAChRs expressed in the electric organ of fish such as Torpedo and Electrophorus Citation[11]. The electric organ has anatomical similarities to skeletal muscle tissue and is an extremely abundant source of nAChRs. Indeed, much of our knowledge about nAChR structure is a consequence of studies performed with electric organ nAChRs. The electric organ nAChR was the first neurotransmitter receptor to be biochemically purified [reviewed by 11] and was the first to be characterized by molecular cloning Citation[13], Citation[14]. It was also the first neurotransmitter receptor to yield functional receptors when expressed in heterologous expression systems such as the Xenopus oocyte Citation[21] and cultured cells Citation[22].
The high density and distribution of nAChRs within the post-synaptic membrane of Torpedo electric organ has made this preparation particularly suitable for electron diffraction studies; as a consequence, this receptor was one of the first ion channels for which high resolution structural information was available Citation[23] (). Such studies have established that the electric organ nAChR is a pentamer composed of two α subunits co-assembled with one each of the β, γ and δ subunits, the subunits being arranged in a clockwise order of α-γ-α-β-δ Citation[23] (). Studies on the Torpedo nAChR have also helped to determine the membrane topology of an individual nAChR subunit Citation[23–25] (). Each subunit contains a single polypeptide chain containing an N-terminal extracellular domain and four α-helical transmembrane domains (M1-M4), with the second of these transmembrane domains (M2) lining the channel pore Citation[23], Citation[26].
Figure 1. The three-dimensional structure of the nAChR from Torpedo electric organ at 4Å resolution. This high resolution structure was obtained from electron images of helical tubes isolated from electric organ post-synaptic membranes from the marine ray Torpedo marmorata Citation[23]. These images were derived from the Protein Data Bank file 2BG9, coloured using the Swiss-Pdb Viewer, Deep View (www.expasy.org/spdbv) and rendered using MegaPov (www.povray.org). Individual subunits have been coloured for identification (α, red; β, blue; γ, green and δ, yellow). (A) A view from the extracellular side of the receptor. (B) A view from the side.
![Figure 1. The three-dimensional structure of the nAChR from Torpedo electric organ at 4Å resolution. This high resolution structure was obtained from electron images of helical tubes isolated from electric organ post-synaptic membranes from the marine ray Torpedo marmorata Citation[23]. These images were derived from the Protein Data Bank file 2BG9, coloured using the Swiss-Pdb Viewer, Deep View (www.expasy.org/spdbv) and rendered using MegaPov (www.povray.org). Individual subunits have been coloured for identification (α, red; β, blue; γ, green and δ, yellow). (A) A view from the extracellular side of the receptor. (B) A view from the side.](/cms/asset/3aba9190-524b-4997-bc16-398f75b64a96/imbc_a_303735_f0001_b.jpg)
Figure 2. A model illustrating nAChR subunit topology and the pentameric arrangement of subunits in an assembled nAChR. A) A cartoon model of a nAChR subunit illustrating four transmembrane domains and the extracellular N- and C-termini. B) As discussed in the text, there is evidence that all assembled nAChRs (whether homomeric or heteromeric) are pentamers. One of the best characterized nAChRs is the heteromeric receptor expressed in the electric organ of the marine ray Torpedo. This representative cartoon image illustrates the known arrangement of subunits in the Torpedo electric organ nAChR. Individual subunits are illustrated by ovals and have been shaded to indicate the subunit type (α, red; β, blue; γ, green and δ, yellow).
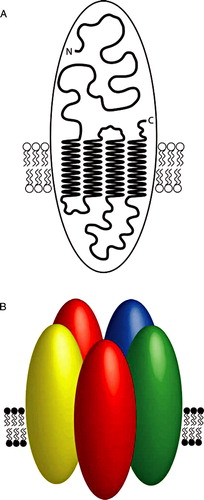
Five nAChRs subunits are expressed in skeletal muscle (α1, β1, γ, δ and ε), two of which (γ and ε) are developmentally regulated (the γ subunit being expressed in embryonic muscle, whereas the ε subunit is expressed in adult muscle). As a consequence of this developmental switch in gene transcription, nAChRs in embryonic muscle have the subunit composition α12β1γδ, whereas those in adult muscle have the composition α12β1δε. The subunit composition and stoichiometry of the embryonic and adult forms of muscle-type nAChRs are highly constrained. As discussed previously Citation[27], it is thought that cells achieve such a tightly controlled assembly process through subunit oligomerization occurring along a fixed and ordered pathway. While there is some debate about the precise order in which subunits interact Citation[27–29], the ordered interaction of subunits is likely to provide a mechanism by which complex heteromeric proteins such as the muscle nAChR can be assembled in a fixed stoichiometry. In one model (often referred to as the ‘heterodimer’ model), the α subunits undergo extensive folding events prior to formation of αγ and αδ heterodimers, which subsequently associate with the β subunit to form an assembled pentamer Citation[30–33]. A second (‘sequential’) model concludes that α, β and γ subunits rapidly assemble into trimers, followed by the addition of a δ subunit and second α subunit. A feature of the sequential model is that important subunit folding events occur only after initial subunit interactions Citation[34–36] and, indeed, there is evidence for conformational changes to one subunit occurring after co-assembly with a partner subunit Citation[37]. Recently, an attempt has been made to develop a model of nAChR subunit assembly based on computer homology modelling Citation[38]. The computer model, which uses theoretical hydrophobic and electrostatic parameters for individual receptor subunits, does not agree exactly with either of the experimentally based assembly models for the muscle-type nAChR and it is likely that a consensus concerning the two opposing empirical assembly models will require further experimental data.
Subunit composition of neuronal nAChRs
In contrast to the homogeneous population of nAChRs found at the neuromuscular junction of embryonic or adult muscle, there is considerable diversity among the sub-family of neuronal nAChRs Citation[17], Citation[18]. In addition to the five subunits found in muscle, twelve vertebrate ‘neuronal’ nAChR subunits (α2–α10 and β2–β4) have been identified which, with some exceptions Citation[3], are expressed predominantly in the central and peripheral nervous system.
Early studies of neuronal nAChRs immunoprecipitated from bovine, chicken, rat and human brain suggested that a major receptor subtype is assembled from only two types of subunit, α4 and β2 Citation[39–41]. Similar studies of native neuronal nAChRs from chick ganglia have identified receptors containing either two or three different types of subunit Citation[42], Citation[43]. These findings provided an early indication that neuronal nAChRs might differ in terms of subunit stoichiometry from the better-characterized skeletal muscle nAChRs. Indeed, there is evidence for neuronal nAChRs containing only one type of subunit (homomeric receptors) and also for those containing two, three and four different subunit sub-types (heteromeric receptors) Citation[17], Citation[18]. Despite such heterogeneity in subunit composition and stoichiometry, several lines of evidence suggest that all nAChRs contain a total of five co-assembled subunits Citation[44–47].
Neuronal nAChR subunits such as α2, α3, α4, β2 and β4 are able, at least in heterologous expression systems, to co-assemble into functional heteromeric nAChRs containing just two different types of subunit (i.e., in ‘pair-wise’ combinations). Heterologous expression studies in Xenopus oocytes demonstrate that functional heteromeric nAChRs are generated when any of the three α subunits α2, α3, α4 are co-expressed with β2 or β4 but not when any of these five subunits is expressed alone Citation[16], Citation[48], Citation[49]. These findings are consistent with data obtained from heterologous expression studies in mammalian cell lines Citation[50–53]. Heterologous expression studies have also demonstrated that, when these subunits are expressed alone, they are retained within the cell Citation[37], Citation[54].
The use of reporter mutations in oocyte expression studies suggests that nAChRs such as α4β2 and α3β4 contain two α subunits coassembled with three β subunits Citation[45], Citation[55]. This conclusion is supported by biochemical studies Citation[44]. There is, however, evidence that some neuronal nAChRs (e.g., α4β2) can assemble into nAChRs of alternative stoichiometries (either α42β23 or α43β22), thereby influencing the functional properties of assembled receptors Citation[56–59].
The α5 and β3 subunits have been described as ‘orphan’ subunits, largely because, unlike other previously-cloned neuronal nAChR subunits, they fail to generate functional recombinant nAChR even when expressed in pair-wise combinations with other subunits Citation[60], Citation[61]. However, both α5 and β3 can co-assemble into ‘triplet’ nAChRs (which contain three different types of subunit). In heterologous expression systems, subunit combinations containing α5 can be distinguished from ‘pair-wise’ subunit combinations on the basis of altered electrophysiological properties and include: α3α5β2 Citation[62], Citation[63], α3α5β4 Citation[62–64] and α4α5β2 Citation[65]. Co-assembly of β3 into functional α3β3β4 nAChRs has been demonstrated by heterologous expression in oocytes using a reporter mutation approach Citation[66], leading to the proposal that the stoichiometry of this subunit combination is α32β31β42 Citation[55]. As discussed below, there is also evidence for the preferential assembly of β3 into α6-containing nAChRs Citation[67–69]. In contrast to such evidence for the functional assembly of β3 into triplet nAChRs such as α3β3β4 and α6β3β4, recent studies have shown instead a dominant-negative effect on nAChR functional expression when β3 co-assembles with several other nAChR subunit combinations Citation[70]. A recently developed approach which may prove useful in determining the composition and stoichiometry of recombinant nAChRs is that of artificial subunit concatemers Citation[58], Citation[71], Citation[72], although there are several potential problems associated with such approaches which may need to be considered Citation[71], Citation[73].
Immunoprecipitation studies conducted on receptors purified from chick ciliary ganglion neurones have identified native nAChRs containing the α5 subunit Citation[74–76]. Most, if not all, of the α5 subunits in this preparation are co-assembled with α3 and β4 and about 20% of α5-containing nAChRs also contain β2 Citation[76], suggesting the formation of α3α5β4 and α3α5β2β4 subunit complexes. Immunoprecipitation studies with nAChRs native to chick optic lobe reveal high levels of α2-containing nAChRs, all of which also contain β2, with more than half also containing α5, suggesting the existence of α2α5β2 nAChRs Citation[77]. Evidence has been obtained for the presence of α4α5β2 nAChRs in chick and rat brain Citation[74], Citation[78], Citation[79], supporting electrophysiological data which demonstrates the co-assembly of these subunits in oocytes Citation[65]. Further support for the formation of α4α5β2 nAChRs has come from immunoprecipitation studies performed in transfected mammalian cells Citation[78]. Subunit deletion experiments, in which embryonic chick sympathetic neurones were treated with antisense oligonucleotides, support the conclusion that the α5 subunit can co-assemble into functional nAChRs, some of which appear also to contain the α7 subunit Citation[80].
Like α5 and β3, the α6 subunit was also considered for several years to be an ‘orphan’ subunit, because of difficulties encountered in its functional expression in recombinant nAChRs. Heterologous expression in Xenopus oocytes has, however, produced functional nAChRs when either the chicken or rat α6 is co-expressed with the human β4 Citation[81]. Despite forming functional nAChRs in combination with the human β4 subunit, the chicken α6 subunit failed to generate detectable whole-cell currents when co-expressed with chicken β4 Citation[81]. Evidence obtained from radioligand binding studies in oocytes indicates that α6 is able to co-assemble with the β2 subunit but that α6β2 complexes do not generate functional nAChRs and are inefficiently expressed on the cell surface Citation[67]. In contrast to these studies in oocytes, expression in transfected mammalian cells has provided evidence for the co-assembly of functional α6β2 nAChRs Citation[82]. Heterologous expression studies have also demonstrated the preferential co-assembly of α6 into triplet subunit combinations. These include α6β3β4 Citation[67], Citation[68] and also α3α6β4 and α3α6β2 Citation[67], Citation[82]. Immunoprecipitation studies on nAChRs from chick retina are largely in agreement with the heterologous expression data. These studies indicate that almost all α6-containing nAChRs in chick retina also contain the β4 subunit Citation[83]. About half also contain the α3 subunit, about half contain β3 and less than 10% contain the β2 subunit Citation[83]. Immunoprecipitation studies with nAChRs expressed in rat and mouse dopamine neurones has identified α6-containing nAChR subtypes such as α6β2* and α4α6β2* Citation[79], Citation[84]. Similar studies with nAChRs expressed in rat retina identified a heterogeneous population of receptor subtypes, of which α4(non-α6)* nAChRs comprised 60% of the total, α6* nAChRs 26% and non-α4/non-α6 nAChRs (such as α2* and α3* nAChRs) 14% Citation[85]. Recently, the assembly and subcellular trafficking of α6 and β3 subunits has been examined by use of Förster resonance energy transfer (FRET) Citation[86]. This is a powerful experimental approach which has led to the conclusion that multiple subunit stoichiometries exist for α4- and α6-containing receptors, whereas only a single β3 subunit is incorporated into assembled nAChRs.
Most nAChR subunits appear to be unable to generate functional nAChRs unless co-assembled with at least one other type of subunit to generate a heteromeric complex. The first evidence that some nAChR subunits can generate homomeric nAChRs came from studies in which the α7 subunit was expressed in Xenopus oocytes Citation[87]. An α8 nAChR subunit, with close sequence similarity to α7, has been isolated by molecular cloning from a chicken cDNA library Citation[88] but an analogous subunit has not been identified in any mammalian species. However, like α7, the avian α8 subunit is able to generate functional homomeric nAChRs when expressed in Xenopus oocytes Citation[89], Citation[90]. Studies of native nAChRs suggest that homomeric α7 nAChRs are a major subtype in chicken brain, whereas homomeric α8 nAChRs are a major subtype in retina Citation[91]. Native heteromeric nAChRs containing both α7 and α8 subunits have also been identified by immunoprecipitation, but they appear to be a minor population Citation[78], Citation[90–92]. Purification of native chick nAChRs (either with α-bungarotoxin or with antibodies specific for the α7 or α8 subunit) has revealed the existence of multiple (three or more) protein bands in SDS-polyacrylamide gel electrophoresis Citation[93–95], suggesting that these may represent complexes containing other subunits in addition to α7 or α8. The identity of these co-precipitated proteins has not been established but they do not appear to be any of the known nAChR subunits Citation[95]. In contrast, other studies of native nAChRs purified from chick optic lobe have indicated the presence of only a single major polypeptide Citation[96]. The possibility of other (as yet unidentified) subunits co-assembling with α7 is supported by immunoprecipitation studies on receptors isolated from chick ciliary ganglia Citation[97]. Further evidence for the co-assembly of other subunit subtypes with α7 has also been provided by experiments in which chick sympathetic neurones were treated with antisense oligonucleotides Citation[98]. Studies with α7 nAChRs purified from rat brain have not, however, detected evidence for its co-assembly with other known nAChR subunits Citation[99]. Further evidence supporting the conclusion that mammalian α7 nAChRs are homomeric comes from protein cross-linking studies with receptors expressed endogenously by the rat adrenal pheochromocytoma cell line, PC12 Citation[47]. There have, however, been reports of native α7-containing nAChRs found in rat brain which differ in their electrophysiological properties from recombinant homomeric α7 nAChRs Citation[100], Citation[101] and there is evidence that this may result from the co-assembly of α7 with β2 into a heteromeric nAChRs Citation[102].
The two most recently characterized nAChR subunits (α9 and α10) are somewhat atypical in terms of their pharmacological properties and in their anatomical distribution. These subunits are expressed predominantly within the hair cells of the cochlea Citation[103], Citation[104] and have been implicated in auditory processing Citation[105]. As had been demonstrated previously with α7 and α8, the α9 subunit is able to generate functional homomeric nAChRs when expressed in Xenopus oocytes Citation[103]. However, it seems likely that native α9-containing nAChRs are heteromeric complexes in which α9 is co-assembled with the α10 subunit. Indeed, the whole-cell currents generated when α10 is co-expressed with α9 are considerably larger than those seen when α9 is expressed alone Citation[104]. Futhermore, the α10 subunit does not generate functional homomeric nAChRs when expressed alone in Xenopus oocytes Citation[104]. Radioligand binding studies conducted with recombinant nAChRs support the conclusion that native α9-containing receptors are heteromeric complexes of the α9 and α10 subunits Citation[106].
Folding and assembly of nAChRs
As reviewed previously Citation[27], the assembly of ion channels such as nAChRs is a relatively slow and inefficient process which is critically dependent upon appropriate subunit folding and requires trafficking of nAChRs from their site of synthesis in the endoplasmic reticulum (ER) to the cell surface. The importance of subunit folding is very clearly illustrated by the difficulties encountered in expressing recombinant α7 nAChRs in several non-neuronal cultured cell lines Citation[107–109]. As discussed later, recent studies have demonstrated the requirement for a molecular chaperone RIC-3 Citation[110–112], since the presence of RIC-3 enables α7 subunits to acquire their correct conformation instead of being retained within the ER Citation[110–114]. In addition to achieving a correctly folded state, nAChR subunits must also assemble correctly into pentameric complexes before they are exported from the ER. This is most apparent in studies of the recombinant nAChR subunits which generate only heteromeric receptors. Several studies with recombinant nAChR subunits have demonstrated that, when expressed alone, such ‘heteromer-only’ subunits are retained within the cell Citation[37], Citation[54], Citation[115]. Studies with mutated and chimeric subunits have demonstrated the importance of the N-terminal domain of nAChR subunits in mediating subunit-subunit interactions Citation[31], Citation[116], Citation[117]. In addition, a series of studies employing subunit chimeras has illustrated the importance of sequences within the intracellular and transmembrane domains of nAChR subunits in determining efficient subunit folding and in influencing the level of cell-surface expression Citation[54], Citation[109], Citation[118–123]. Other recent studies have identified conserved hydrophobic residues within the M3-M4 loop domain as being important for export of nAChRs from the ER Citation[124].
Post-translational modification
After synthesis in the ER, nAChR subunits interact with a wide variety of proteins responsible for post-translational modifications such as glycosylation, disulphide-bond formation, palmitoylation, proline isomerization and proteolytic cleavage Citation[27], Citation[125]. Appropriate glycosylation has been shown to be required for correct assembly of both muscle-type and neuronal nAChRs Citation[126–129]. Correct folding, assembly and cell-surface expression of nAChRs is also dependent upon appropriate disulphide-bond formation Citation[35], Citation[126], Citation[127], Citation[130], Citation[131]. Studies with the neuronal nAChR α7 subunit suggest that inappropriate disulphide-bond formation may underlie the inefficient folding of this subunit which is observed in some mammalian cell lines Citation[132]. An apparent link has also been demonstrated between the efficient folding of the nAChR α7 subunit in Xenopus oocytes and a requirement for the prolyl isomerase enzyme cyclophilin Citation[133], Citation[134]. This prompted studies to investigate whether the co-expression of cyclophilin proteins might alleviate the inefficient folding of α7 observed in some mammalian host cells but such experiments have not, so far, established this Citation[108], Citation[135].
Molecular chaperones and receptor-associated proteins
Nicotinic receptor subunits have been shown to associate with several molecular chaperones. In common with many transmembrane proteins, interactions have been identified between nAChR subunits and ER-resident chaperone proteins such as BiP Citation[136], Citation[137] and calnexin Citation[138], Citation[139]. Yeast two-hybrid studies with the intracellular loop region of the neuronal nAChR α4 subunit identified interactions with a cytoplasmic chaperone protein 14-3-3η and a peripheral membrane protein VILIP-1, both of which have been reported to influence cell-surface expression levels of α4β2 nAChRs Citation[140], Citation[141]. As mentioned earlier (and reviewed more extensively elsewhere Citation[142]), maturation of nAChR α7 and α8 subunits has been shown to be critically dependent on a recently identified putative chaperone protein RIC-3 Citation[110–114]. RIC-3 is an ER-resident transmembrane protein which was originally identified in genetic studies performed in the nematode C. elegans Citation[143], Citation[144] and which is conserved across vertebrate and invertebrate phyla Citation[113], Citation[114]. In contrast to chaperone proteins such as BiP, calnexin and 14-3-3, RIC-3 appears to be highly selective in interacting with nAChRs Citation[142], although it has also been shown to interact with the 5-HT3 receptor Citation[110], Citation[111], Citation[114], Citation[145]. RIC-3 has a strong facilitatory effect on the maturation of α7 nAChRs Citation[110–114]. It has also been reported to have a modulatory effect on several heteromeric neuronal nAChRs Citation[110], Citation[111], Citation[114], Citation[146]. With some heteromeric nAChRs (such as α4β2), RIC-3 has been reported to cause either an enhancement or a suppression of agonist-induced reponses when examined in different expression systems Citation[110], Citation[111], Citation[114], the reasons for which are unclear but may be due to differences in the host cell type Citation[146]. UNC-50 is a transmembrane protein located within the Golgi apparatus which has been shown to have a selective effect on nAChR trafficking Citation[147]. Like RIC-3, the gene encoding UNC-50 was originally identified in C. elegans through genetic screening for suppressor mutations Citation[148]. Reduced binding of the nicotinic ligand levamisole in unc-50 mutants provided evidence that UNC-50 may be required for nAChR assembly Citation[149]. Co-expression of recombinant nAChRs with a mammalian homologue of UNC-50 suggested that it can enhance levels of cell-surface nAChRs; however, this study concluded that this may be a consequence of an RNA-binding activity Citation[150]. More recent studies with UNC-50 in C. elegans suggest that it is required for subtype-selective trafficking of nAChRs through the Golgi apparatus, thereby acting at a later stage in receptor maturation than RIC-3 Citation[147].
There have been relatively few well-characterized examples of interactions between nAChRs and cytoskeletal proteins. The clearest evidence for such an interaction is between muscle nAChRs and rapsyn (or 43K protein), the discovery of which long predates the advent of recombinant proteomic techniques. The interaction between muscle nAChRs and rapsyn has been shown to be important in the clustering of postsynaptic nAChRs at the neuromuscular junction Citation[151], Citation[152]. Further evidence of a role for rapsyn in neuromuscular synaptogenesis has been provided by studies with transgenic mice lacking rapsyn, in which nAChRs fail to cluster at the neuromuscular junction Citation[153]. The importance of rapsyn in anchoring the muscle-type nAChR within the post-synaptic membrane at the neuromuscular junction has been well documented Citation[154], Citation[155]. As well as being expressed in muscle, rapsyn has been detected in brain Citation[156] and in peripheral neuronal cells such as mouse sympathetic neurones Citation[157] and chick ciliary ganglion neurones Citation[158]. However, it appears that rapsyn is not required for clustering of neuronal nAChRs such as those located at ganglionic synapses Citation[157], Citation[159]. Studies using transfected cells indicate that rapsyn is able to cause clustering of neuronal nAChRs such as α3β2, α3β4, α4β2 and α7 Citation[157], Citation[160], but suggest that, at least for some of these subunit combinations, the clustered neuronal nAChRs are retained within the cell and are not transported to the cell surface Citation[157]. Evidence suggesting that rapsyn is not required for nAChR clustering in sympathetic ganglia has come from studies with rapsyn-deficient transgenic mice, in which the number, size and density of nAChR clusters in superior cervical ganglia are similar to those in control animals Citation[157]. Despite the presence of rapsyn mRNA in chick ciliary ganglia, rapsyn protein is either absent or is present at a much lower ratio (relative to nAChR levels) in ganglia than in muscle Citation[159].
In addition to rapsyn, several other cytoskeletal proteins in have been implicated in the regulation of nAChR distribution and function. For example, there is evidence that actin is important in regulating such events for α7nAChRs located in somatic spines of chick ciliary ganglia and on spinal cord neurones Citation[161–163]. Recent studies have identified interactions between neuronal nAChRs and PDZ-domain cytoskeletal proteins such as PSD-93, PSD-95 and PICK1 Citation[164–166]; interactions which, in some cases, influence events such as receptor clustering Citation[165], Citation[166]. As discussed previously Citation[164], the interaction of nAChRs with PDZ-domain proteins is perhaps unexpected, given that interactions with PDZ-domain proteins have generally been identified with receptors containing intracellular C-terminal domains. In addition, recent studies have identified a role for the tumour-suppressor protein APC in synaptic localization of both muscle and neuronal nAChR subtypes Citation[167–169]. A ‘proteomics’ approach has been used to identify 21 proteins associated with the β2 subunit purified from mouse brain, several of which are implicated in regulation of sub-cellular trafficking Citation[170].
Up-regulation and trafficking of nAChRs
There is extensive evidence to indicate that chronic exposure to nicotine, such as that which occurs during tobacco smoking, causes an up-regulation of brain nAChRs Citation[171–173]. This is brought about by a post-translational mechanism Citation[174] and can be mimicked in cultured cell lines by exposure to low concentrations of nicotine for several hours Citation[175–178]. With nAChR subtypes such as α4β2 there is evidence that chronic nicotine treatment can enhance subunit folding and assembly, and induce conformational changes within subunits Citation[37], Citation[179]. Indeed, it has been proposed that nicotine causes receptor up-regulation by acting as a pharmacological chaperone Citation[180], Citation[181] via a direct action at the agonist binding site Citation[182], thereby promoting receptor maturation Citation[183]. There have, however, been several explanations proposed for the mechanism of nicotine-induced nAChR up-regulation, as reviewed elsewhere Citation[184]. Recent studies have revealed that chronic nicotine has a differential effect on up-regulation of functional α4-containing nAChRs in different brain regions, providing a possible explanation for the differential effect of chronic nicotine (sensitization and tolerance) observed in distinct regions of the brain Citation[185]. Nicotine-induced up-regulation has been reported for nAChR subtypes other than α4β2, although there is evidence from studies both in brain and in cultured cell lines that nAChR subtypes differ in the extent to which they are up-regulated by nicotine Citation[186–191].
Recent studies have demonstrated up-regulation of cell-surface α7 nAChRs in hippocampal neurones by tyrosine dephosphorylation Citation[192] and also by brain-derived neurotrophic factor (BDNF) Citation[193]. In the case of tyrosine dephosphorylation, there is evidence that this may occur via SNARE-dependent trafficking Citation[192]. Evidence has also been obtained to indicate that SNARE-dependent trafficking is important for maintaining functional coupling between α7–responses and downstream signalling in somatic spines Citation[194].
Influence of subunit domains upon receptor trafficking
Assembled nAChRs are targeted to both pre- and post-synaptic sites. Within the brain, pre-synaptic receptors are important in, for example, modulating neurotransmitter release Citation[195], Citation[196]. Post-synaptic nAChRs are important in mediating synaptic transmission at the neuromuscular junction and in autonomic ganglia Citation[197] and, more recently, post-synaptic nAChRs have been identified in the mammalian brain Citation[198]. The differential targeting of nAChRs (for example to pre- or post-synaptic sites) has prompted a search for signals which may regulate receptor targeting.
The influence of the M3-M4 cytoplasmic loop region of neuronal nAChR subunits upon receptor targeting has been examined by the expression of subunit chimeras in chick ciliary ganglion neurones by retrovirus-mediated gene transfer Citation[199]. Whereas the α7 subunit is located peri-synaptically, chimeric subunits in which the M3-M4 cytoplasmic loop of the α7 subunit is replaced by the analogous loop region of the α3 subunit are targeted to post-synaptic sites. In contrast, synaptic targeting is not observed with similar chimeric subunits containing the cytoplasmic loop domain from either the α5 or β4 subunit Citation[199], Citation[200]. These studies implicate sequences within the M3-M4 cytoplasmic loop of the α3 subunit in the targeting of receptors to post-synaptic regions in ciliary ganglion neurones. More recently, motifs responsible for differential targeting to axons and dendrites have been identified within the M3-M4 loops of the α4 and α7 subunits Citation[201]. The influence of the nAChR M3-M4 loop upon folding, cell-surface expression and targeting has also been examined in a recent study in which a series of fourteen subunit chimeras was constructed, each containing a different M3-M4 loop domain (α1-α10 and β1-β4) Citation[202]. This has confirmed that M3-M4 loop domain can influence subunit folding, cell-surface expression and receptor targeting Citation[202]. Mutagenesis studies have demonstrated the importance of hydrophobic amino acids within the M3-M4 loop domain and also of amino acids within the M1 transmembrane domain in regulating export of nAChRs from the endoplasmic reticulum and trafficking to the cell surface Citation[124], Citation[203].
Conclusion
Studies conducted with both native and recombinant receptors have contributed to our current understanding of nAChR assembly and trafficking, but our knowledge of these complex cellular events is still far from complete. Whereas the subunit composition of nAChRs found at the neuromuscular junction is well established, an important task is to identify the extent of subunit diversity amongst neuronal nAChR subtypes. However, as discussed in this review, a variety of experimental approaches are now helping to answer such questions. Recent work has also revealed the influence of subunit domains upon events such as receptor assembly and trafficking and the interaction of nAChRs with several classes of intracellular proteins. In particular, the role of scaffolding proteins in nAChR targeting and in determining the functional state of these receptors is emerging, as is the importance of molecular chaperones such as RIC-3 in the modulation of nAChR maturation.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci 2004; 27: 329–336
- Millar NS. 2006. Ligand-gated ion channels. Encyclopedia of Life Sciences http://www.els.net/ [doi:10.1038/npg.els.0000154].
- Sharma G, Vijayaraghavan S. Nicotinic receptor signalling in nonexcitable cells. J Neurobiol 2002; 53: 524–534
- Lindstrom J. Acetylcholine receptors and myasthenia. Muscle Nerve 2000; 23: 453–477
- Weiland S, Bertrand D, Leonard S. Neuronal nicotinic acetylcholine receptors: from the gene to the disease. Behavioural Brain Res 2000; 113: 43–56
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 2006; 27: 482–491
- Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identification, and therapeutic inspirations. J Med Chem 2005; 48: 4705–4745
- Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol 2007; 74: 1092–1101
- Raymond Delpech V, Matsuda K, Sattelle BM, Rauh JJ, Sattelle DB. Ion channels: molecular targets of neuroactive insecticides. Invert Neurosci 2005; 5: 119–133
- Millar NS, Denholm I. Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invert Neurosci 2007; 7: 53–66
- Popot J-L, Changeux J-P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev 1984; 64: 1162–1239
- Weill CL, McNamee MG, Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Comm 1974; 61: 997–1003
- Noda M, Takahashi H, Tanabe T, Toyosato M, Furutani Y, Hirose T, Asai M, Inayama S, Miyata T, Numa S. Primary structure of α-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature 1982; 299: 793–797
- Sumikawa K, Houghton M, Smith JC, Bell L, Richards BM, Barnard EA. The molecular cloning and characterisation of cDNA coding for the α subunit of the acetylcholine receptor. Nucl Acids Res 1982; 10: 5809–5822
- Sine SM. The nicotinic receptor ligand binding domain. J Neurobiol 2002; 53: 431–446
- Luetje CW, Patrick J. Both α- and β-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci 1991; 11: 837–845
- Le Novère N, Corringer P-J, Changeux J-P. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 2002; 53: 447–456
- Millar NS. Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem Soc Trans 2003; 31: 869–874
- Lukas RJ, Changeux J-P, Le Novère N, Albuquerque EX, Balfour DJK, Berg DK, Bertrand D, Chiappinelli AA, Clarke PBS, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International union of pharmacology. XX. current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev 1999; 51: 397–401
- Luetje CW. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol Pharmacol 2004; 65: 1333–1335
- Mishina M, Kurosaki T, Tobimatsu T, Morimoto Y, Noda M, Yamamoto T, Terao M, Lindstrom J, Takahashi T, Kuno M, Numa S. Expression of functional acetylcholine receptor from cloned cDNAs. Nature 1984; 307: 604–608
- Claudio T, Green WN, Hartman DS, Hayden D, Paulson HL, Sigworth FJ, Sine SM, Swedlund A. Genetic reconstitution of functional acetylcholine receptor channels in mouse fibroblasts. Science 1987; 238: 1688–1694
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol 2005; 346: 967–989
- Kubalek E, Ralston S, Lindstrom J, Unwin N. Location of subunits within the acetylcholine receptor by electron image analysis of tubular crystals from Torpedo marmorata. J Cell Biol 1987; 105: 9–18
- Beroukhim R, Unwin N. Three-dimensional location of the main immunogenic region of the acetylcholine receptor. Neuron 1995; 15: 323–331
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature 1995; 373: 37–43
- Green WN, Millar NS. Ion-channel assembly. Trends Neurosci 1995; 18: 280–287
- Green WN. Ion channel assembly: creating structures that function. J Gen Physiol 1999; 113: 163–169
- Keller ST, Taylor P. Determinants responsible for assembly of the nicotinic acetylcholine receptor. J Gen Physiol 1999; 113: 171–176
- Blount P, Smith MM, Merlie JP. Assembly intermediates of the mouse muscle nicotinic acetylcholine receptor in stably transfected fibroblasts. J Cell Biol 1990; 111: 2601–2611
- Gu Y, Forsayeth JR, Verrall S, Yu XM, Hall ZW. Assembly of the mammalian muscle acetylcholine receptor in transfected COS cells. J Cell Biol 1991; 114: 799–807
- Saedi MS, Conroy WG, Lindstrom J. Assembly of Torpedo acetylcholine receptors in Xenopus oocytes. J Cell Biol 1991; 112: 1007–1015
- Kreienkamp H-J, Maeda RK, Sine S, Taylor P. Intersubunit contacts governing assembly of the mammalian nicotinic acetylcholine receptor. Neuron 1995; 14: 635–644
- Green WN, Claudio T. Acetylcholine receptor assembly: subunit folding and oligomerization occur sequentially. Cell 1993; 74: 57–69
- Green WN, Wanamaker CP. The role of the cystine loop in acetylcholine receptor assembly. J Biol Chem 1997; 272: 20945–20953
- Green WN, Wanamaker CP. Formation of the nicotinic acetylcholine receptor binding sites. J Neurosci 1998; 18: 5555–5564
- Harkness PC, Millar NS. Changes in conformation and subcellular distribution of α4β2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras. J Neurosci 2002; 22: 10172–10181
- Ortells MO, Barrantes GE. A model for the assembly of nicotinic receptors based on subunit-subunit interactions. Proteins 2008; 70: 473–488
- Whiting PJ, Lindstrom JM. Purification and characterization of a nicotinic acetylcholine receptor from chick brain. Biochemistry 1986; 25: 2082–2093
- Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci USA 1987; 84: 595–599
- Whiting PJ, Lindstrom JM. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci 1988; 8: 3395–3404
- Halvorsen SW, Berg DK. Affinity labeling of neuronal acetylcholine receptor subunits with an α-neurotoxin that blocks receptor function. J Neurosci 1987; 7: 2547–2555
- Halvorsen SW, Berg DK. Subunit composition of nicotinic acetylcholine receptors from chick ciliary ganglia. J Neurosci 1990; 10: 1711–1718
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem 1991; 266: 11192–11198
- Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature 1991; 350: 235–238
- Palma E, Bertrand S, Binzoni T, Bertrand D. Neuronal nicotinic α7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J Physiol 1996; 491: 151–161
- Drisdel RC, Green WN. Neuronal α-bungarotoxin receptors are α7 subunit homomers. J Neurosci 2000; 20: 133–139
- Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci USA 1987; 84: 7763–7767
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: β4. Neuron 1989; 3: 487–496
- Rogers SW, Gahring LC, Papke RL, Heinemann S. Identification of cultured cells expressing ligand-gated cationic channels. Protein Expr Purif 1991; 2: 108–116
- Whiting P, Schoepfer R, Lindstrom J, Priestley T. Structural and pharmacological characterization of the major brain nicotinic acetylcholine receptor subtype stably expressed in mouse fibroblasts. Mol Pharmacol 1991; 40: 463–472
- Wong ET, Holstad SG, Mennerick SJ, Hong SE, Zorumski CF, Isenberg KE. Pharmacological and physiological properties of a putative ganglionic nicotinic receptor α3β4, expressed in transfected eucaryotic cells. Mol Brain Res 1995; 28: 101–109
- Ragozzino D, Fucile S, Giovannelli A, Grassi F, Mileo AM, Ballivet M, Alemà S, Eusebi F. Functional properties of neuronal nicotinic acetylcholine receptor channels expressed in transfected human cells. Eur J Neurosci 1997; 9: 480–488
- Cooper ST, Harkness PC, Baker ER, Millar NS. Upregulation of cell-surface α4β2 neuronal nicotinic receptors by lower temperature and expression of chimeric subunits. J Biol Chem 1999; 274: 27145–27152
- Boorman JPB, Groot-Kormelink PJ, Sivilotti LG. Stoichiometry of human recombinant neuronal nicotinic receptors containing the β3 subunit expressed in Xenopus oocytes. J Physiol 2000; 529: 565–577
- Zwart R, Vijverberg HPM. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol 1998; 54: 1124–1131
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol 2003; 63: 332–341
- Zhou Y, Nelson ME, Kuryatov A, Choi CH, Cooper J, Lindstrom J. Human α4β2 acetylcholine receptors formed from linked subunits. J Neurosci 2003; 23: 9004–9015
- Tapia L, Kuryatov A, Lindstrom J. Ca2 + permeability of the (α4)3(β2)2 stoichiometry greatly exceeds that of (α4)2(β2)3 human acetylcholine receptors. Mol Pharmacol 2007; 71: 769–776
- Deneris ES, Boulter J, Swanson LW, Patrick J, Heinemann S. β3: a new member of nicotinic acetylcholine receptor gene family is expressed in brain. J Biol Chem 1989; 264: 6268–6272
- Couturier S, Erkman L, Valera S, Rungger D, Bertrand S, Boulter J, Ballivet M, Bertrand D. α5, α3, and non-α3. Three clustered avian genes encoding neuronal nicotinic acetylcholine receptor-related subunits. J Biol Chem 1990; 265: 17560–17567
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor α5 subunit with α3, β2, and β4 subunits. J Biol Chem 1996; 271: 17656–17665
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 subunit alters desensitization, pharmacology, Ca+ + permeability and Ca+ + modulation of human neuronal α3 nicotinic receptors. J Pharm Exper Ther 1998; 286: 311–320
- Fucile S, Barabino B, Palma E, Grassi F, Limatola C, Mileo AM, Alemà S, Ballivet M, Eusebi F. α5 subunit forms functional α3β4α5 nAChRs in transfected human cells. Neuroreport 1997; 8: 2433–2436
- Ramirez-Latorre J, Yu CR, Qu F, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature 1996; 380: 347–351
- Groot-Kormelink PJ, Luyten WHML, Colquhoun D, Sivilotti LG. A reporter mutation approach shows incorporation of the “orphan” subunit β3 into a functional nicotinic receptor. J Biol Chem 1998; 273: 15317–15320
- Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human α6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacol 2000; 39: 2570–2590
- Tumkosit P, Kuryatov A, Luo J, Lindstrom J. β3 subunits promote expression and nicotine-induced up-regulation of human nicotinic α6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol Pharmacol 2006; 70: 1358–1368
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Mol Pharmacol 2005; 67: 2007–2015
- Broadbent S, Groot-Kormelink PJ, Krashia PA, Harkness PC, Millar NS, Beato M, Sivilotti LG. Incorporation of the β3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol Pharmacol 2006; 70: 1350–1356
- Groot-Kormelink PJ, Broadbent S, Boorman JP, Sivilotti LG. Incomplete incorporation of tandem subunits in recombinant neuronal nicotinic receptors. J Gen Physiol 2004; 123: 697–708
- Groot-Kormelink PJ, Broadbent S, Beato M, Sivilotti LG. Constraining the expression of nicotinic acetylcholine receptors by using pentameric constructs. Mol Pharmacol 2006; 69: 558–563
- Ericksen SS, Boileau AJ. Tandem couture: Cys-loop receptor concatamer insights and caveats. Mol Neurobiol 2007; 35: 113–128
- Conroy WG, Vernallis AB, Berg DK. The α5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron 1992; 9: 679–691
- Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron 1993; 10: 451–464
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem 1995; 270: 4424–4431
- Balestra B, Vailati S, Moretti M, Hanke W, Clementi F, Gotti C. Chick optic lobe contains a developmentally regulated α2α5β2 nicotinic receptor subtype. Mol Pharmacol 2000; 58: 300–311
- Conroy WG, Berg DK. Nicotinic receptor subtypes in the developing chick brain: appearance of a species containing the α4, β2 and α5 gene products. Mol Pharmacol 1998; 53: 392–401
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci 2002; 22: 8785–8789
- Yu CR, Role LW. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. J Physiol 1998; 509: 667–681
- Gerzanich V, Kuryatov A, Anand R, Lindstrom J. "Orphan” α6 nicotinic AChR subunit can form a functional heteromeric acetylcholine receptor. Mol Pharmacol 1997; 51: 320–327
- Fucile S, Matter J-M, Erkman L, Ragozzino D, Barabino B, Grassi F, Alemà S, Ballivet M, Eusebi F. The neuronal α6 subunit forms functional heteromeric acetylcholine receptors in human transfected cells. Eur J Neurosci 1998; 10: 172–178
- Vailati S, Hanke W, Bejan A, Barabino B, Longhi R, Balestra B, Moretti M, Clementi F, Gotti C. Functional α6-containing nicotinic receptors are present in chick retina. Mol Pharmacol 1999; 56: 11–19
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 2003; 23: 7820–7829
- Moretti M, Vailati S, Zoli M, Lippi G, Riganti L, Longhi R, Viegi A, Clementi F, Gotti C. Nicotinic acetylcholine receptor subtypes expressed during rat retina development and their regulation by visual experience. Mol Pharmacol 2004; 66: 85–96
- Drenan RM, Nashmi R, Imoukhuede P, Just H, McKinney S, Lester HA. Subcellular trafficking, pentameric assembly and subunit stoichiometry of neuronal nicotinic acetylcholine receptors containing fluorescently labeled α6 and β3 subunits. Mol Pharmacol 2008; 73: 27–41
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 1990; 5: 847–856
- Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain α-bungarotoxin binding protein cDNAs and mAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron 1990; 5: 35–48
- Gerzanich V, Anand R, Lindstrom J. Homomers of α8 and α7 subunits of nicotinic receptors exhibit similar channels but contrasting binding site properties. Mol Pharmacol 1994; 45: 212–220
- Gotti C, Hanke W, Maury K, Moretti M, Ballivet M, Clementi F, Bertrand D. Pharmacology and biophysical properties of α7 and α7-α8 α-bungarotoxin receptor subtypes immunopurified from the chick optic lobe. Eur J Neurosci 1994; 6: 1281–1291
- Keyser KT, Britto LR, Schoepfer R, Whiting P, Cooper J, Conroy W, Brozozowska-Prechtl A, Karten HJ, Lindstrom J. Three subtypes of α-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. J Neurosci 1993; 13: 442–454
- Anand R, Peng X, Ballesta JJ, Lindstrom J. Pharmacological characterization of α-bungarotoxin-sensitive acetylcholine receptors immunoisolated from chick retina: contrasting properties of α7 and α8 subunit-containing subtypes. Mol Pharmacol 1993; 44: 1046–1050
- Gotti C, Ogando AE, Hanke W, Schlue R, Moretti M, Clementi F. Purification and characterization of an α-bungarotoxin receptor that forms a functional nicotinic channel. Proc Natl Acad Sci USA 1991; 88: 3258–3262
- Gotti C, Hanke W, Schlue W-R, Briscini L, Moretti M, Clementi F. A functional α-bungarotoxin receptor is present in chick cerebellum: purification and characterization. Neurosci 1992; 50: 117–127
- Gotti C, Moretti M, Maggi R, Longhi R, Hanke W, Klinke N, Clementi F. α7 and α8 nicotinic receptor subtypes immunoprecipitated from chick retina have different immunological, pharmacological and functional properties. Eur J Neurosci 1997; 9: 1201–1211
- Norman RI, Mehraban F, Barnard EA, Dolly JO. Nicotinic acetylcholine receptor from chick optic lobe. Proc Natl Acad Sci USA 1982; 79: 1321–1325
- Pugh PC, Corriveau RA, Conroy WG, Berg DK. Novel subpopulation of neuronal acetylcholine receptors among those binding α-bungarotoxin. Mol Pharmacol 1995; 47: 717–725
- Yu CR, Role LW. Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J Physiol 1998; 509: 651–665
- Chen D, Patrick JW. The α-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the α7 subunit. J Biol Chem 1997; 272: 24024–24029
- Shao Z, Yakel JL. Single channel properties of neuronal nicotinic ACh receptors in stratum radiatum interneurons of rat hippocampal slices. J Physiol 2000; 527: 507–513
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol 2000; 527: 515–528
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic receptor α7 and β2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol 2002; 540: 425–434
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 1994; 18: 705–715
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heineman SF, Boulter J. α10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 2001; 98: 3501–3506
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of α9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 1999; 23: 93–103
- Baker ER, Zwart R, Sher E, Millar NS. Pharmacological properties of α9α10 nicotinic acetylcholine receptors revealed by heterologous expression of subunit chimeras. Mol Pharmacol 2004; 65: 453–460
- Kassner PD, Berg DK. Differences in the fate of neuronal acetylcholine receptor protein expressed in neurons and stably transfected cells. J Neurobiol 1997; 33: 968–982
- Cooper ST, Millar NS. Host cell-specific folding and assembly of the neuronal nicotinic acetylcholine receptor α7 subunit. J Neurochem 1997; 68: 2140–2151
- Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, Fox AP, Salman SB, Green WN. Neuronal α-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J Neurosci 1997; 17: 8201–8212
- Castillo M, Mulet J, Gutiérrez LM, Ortiz JA, Castelán F, Gerber S, Sala S, Sala F, Criado M. Dual role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J Biol Chem 2005; 280: 27062–27068
- Lansdell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, Gibb AJ, Millar NS. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharmacol 2005; 68: 1431–1438
- Williams ME, Burton B, Urrutia A, Shcherbatko A, Chavez-Noriega LE, Cohen CJ, Aiyar J. Ric-3 promotes functional expression of the nicotinic acetylcholine receptor α7 subunit in mammalian cells. J Biol Chem 2005; 280: 1257–1263
- Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, Treinin M. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J 2002; 21: 1012–1020
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M. Conservation within the RIC-3 gene family: effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 2003; 278: 34411–34417
- Harkness PC, Millar NS. Inefficient cell-surface expression of hybrid complexes formed by the co-assembly of neuronal nicotinic acetylcholine receptor and serotonin receptor subunits. Neuropharmacol 2001; 41: 79–87
- Yu XM, Hall ZW. Extracellular domains mediating ε subunit interactions of muscle acetylcholine receptor. Nature 1991; 352: 64–67
- Sumikawa K. Sequences on the N-terminus of ACh receptor subunits regulate their assembly. Brain Res Mol Brain Res 1992; 13: 349–353
- Eiselé J-L, Bertrand S, Galzi J-L, Devillers-Thiéry A, Changeux J-P, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 1993; 366: 479–483
- García-Guzmán M, Sala F, Sala S, Campos-Caro A, Criado M. Role of two acetylcholine receptor subunit domains in homomer formation and intersubunit recognition, as revealed by α3 and α7 subunit chimeras. Biochem 1994; 33: 15198–15203
- Vicente-Agulló F, Rovira JC, Campos-Caro A, Rodríguez-Ferrer C, Ballesta JJ, Sala S, Sala F, Criado M. Acetylcholine receptor subunit homomer formation requires compatibility between amino acid residues of the M1 and M2 transmembrane segments. FEBS Lett 1996; 399: 83–86
- Cooper ST, Millar NS. Host cell-specific folding of the neuronal nicotinic receptor α8 subunit. J Neurochem 1998; 70: 2585–2593
- Dineley KT, Patrick JW. Amino acid determinants of α7 nicotinic acetylcholine receptor surface expression. J Biol Chem 2000; 275: 13974–13985
- Gee VJ, Kracun S, Cooper ST, Gibb AJ, Millar NS. Identification of domains influencing assembly and ion channel properties in α7 nicotinic receptor and 5-HT3 receptor subunit chimaeras. Br J Pharmacol 2007; 152: 501–512
- Ren X-Q, Cheng S-B, Treuil MW, Mukherjee J, Rao J, Braunewell KH, Lindstrom J, Anand R. Structural determinants of α4β2 nicotinic acetylcholine receptor trafficking. J Neurosci 2005; 25: 6676–6686
- Drisdel RC, Manzana E, Green WN. The role of palmitoylation in the functional expression of nicotinic α7 receptors. J Neurosci 2004; 24: 10502–10510
- Mishina M, Tobimatsu T, Tanaka K, Fujita Y, Fukuda K, Kurasake M, Takahashi H, Morimoto Y, Hirose T, Inayama S, Takahasi T, Kuno M, Numa S. Location of functional regions of acetylcholine receptor α-subunit by site-directed mutagenesis. Nature 1985; 313: 364–369
- Blount P, Merlie JP. Mutational analysis of muscle nicotinic acetylcholine receptor subunit assembly. J Cell Biol 1990; 111: 2613–2622
- Chen D, Dang H, Patrick JW. Contributions of N-linked glycosylation to the expression of a functional α7-nicotinic receptor in Xenopus oocytes. J Neurochem 1998; 70: 349–357
- Wanamaker CP, Green WN. N-linked glycosylation is required for nicotinic receptor assembly but not for subunit associations with calnexin. J Biol Chem 2005; 280: 33800–33810
- Sumikawa K, Gehle VM. Assembly of mutant subunits of the nicotinic acetylcholine receptor lacking the conserved disulfide loop structure. J Biol Chem 1992; 267: 6286–6290
- Gelman MS, Prives JM. Arrest of subunit folding and assembly of nicotinic acetylcholine receptors in cultured muscle cells by dithiothreitol. J Biol Chem 1996; 271: 10709–10714
- Rakhilin S, Drisdel RC, Sagher D, McGehee DS, Vallejo Y, Green W, N. bungarotoxin receptors contain α7 subunits in two different disulfide-bonded conformations. J Cell Biol 1999; 146: 203–217
- Helekar SA, Char D, Neff S, Patrick J. Prolyl isomerase requirement for the expression of functional homo-oligomeric ligand-gated ion channels. Neuron 1994; 12: 179–189
- Helekar SA, Patrick J. Peptidyl prolyl cis-trans isomerase activity of cyclophilin A in functional homo-oligomeric receptor expression. Proc Natl Acad Sci USA 1997; 94: 5432–5437
- Blumenthal EM, Conroy WG, Romano SJ, Kassner PD, Berg DK. Detection of functional nicotinic receptors blocked by α-bungarotoxin on PC12 cells and dependence of their expression on post-translational events. J Neurosci 1997; 17: 6094–6104
- Paulson HL, Ross AF, Green WN, Claudio T. Analysis of early events in acetylcholine receptor assembly. J Cell Biol 1991; 113: 1371–1384
- Blount P, Merlie JP. BIP associates with newly synthesized subunits of the mouse muscle nicotinic receptor. J Cell Biol 1991; 113: 1125–1132
- Gelman MS, Chang W, Thomas DY, Bergeron JJM, Prives JM. Role of the endoplasmic reticulum chaperone calnexin in subunit folding and assembly of nicotinic acetylcholine receptors. J Biol Chem 1995; 270: 15085–15092
- Keller SH, Lindstrom J, Yaylor P. Involvement of the chaperone protein calnexin and the acetylcholine receptor β-subunit in the assembly and cell surface expression of the receptor. J Biol Chem 1996; 271: 22871–22877
- Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. The chaperone protein 14-3-3η interacts with the nicotinic acetylcholine receptor α4 subunit. J Biol Chem 2001; 276: 28281–28290
- Lin L, Jeanclos EM, Treuil MW, Braunewell K-H, Gundelfinger ED, Anand R. The calcium sensor protein visinin-like protein-1 modulates the surface expression and agonist-sensitivity of the α4β2 nicotinic acetylcholine receptor. J Biol Chem 2002; 277: 41872–41878
- Millar NS. 2008. RIC-3: a nicotinic acetylcholine receptor chaperone. Br J Pharmacol 153:5177–5183.
- Nguyen M, Alfonso A, Johnson CD, Rand JB. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 1995; 140: 527–535
- Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci USA 1996; 93: 12593–12598
- Cheng A, McDonald NA, Connolly CN. Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J Biol Chem 2005; 280: 22502–22507
- Lansdell SJ, Collins T, Yabe A, Gee VJ, Gibb AJ, Millar NS.2008. Host-cell specific effects of the nicotinic acetylcholine receptor chaperone RIC-3 revealed by a comparison of human and Drosophila RIC-3 homologues. J Neurochem [published on-line: doi: 10.1111/j.1471-4159.2008.05235.x].
- Eimer S, Gottschalk A, Hengartner M, Horvitz HR, Ricmond J, Schafer WR, Bessereau J-L. Regulation of nicotinic receptor trafficking by the transmembrane Golgi protein UNC-50. EMBO J 2007; 26: 4313–4323
- Lewis JA, Wu C-H, Berg H, Levine J. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 1980; 95: 905–928
- Lewis JA, Elmer JS, Skimming J, McLafferty S, Fleming JT, McGee T. Cholinergic receptor mutants of the nematode Caenorhabditis elegans. J Neurosci 1987; 7: 3059–3071
- Fitzgerald J, Kennedy D, Viseshakul N, Cohen BN, Mattick J, Bateman JF, Forsayeth JR. UCNL, the mammalian homologue of UNC-50, is an inner nuclear membrane RNA-binding protein. Brain Res 2000; 877: 110–123
- Froehner SC, Luetje CW, Scotland PB, Patrick J. The postsynaptic 43K protein clusters muscle nicotinic acetylcholine receptors in Xenopus oocytes. Neuron 1990; 5: 403–410
- Phillips WD, Kopta C, Blount P, Gardner PD, Steinbach JH, Merlie JP. ACh receptor-rich membrane domains organized in fibroblasts by recombinant 43-kilodalton protein. Science 1991; 251: 568–570
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 1995; 377: 232–236
- Colledge M, Froehner SC. To muster a cluster: anchoring neurotransmitter receptors at synapses. Proc Natl Acad Sci USA 1998; 95: 3341–3343
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 1999; 22: 389–442
- Yang S-H, Armson PF, Cha J, Phillips WD. Clustering of GABAA receptors by rapsyn/43kD protein in vitro. Mol Cell Neurosci 1997; 8: 430–438
- Feng G, Steinbach JH, Sanes JR. Rapsyn clusters neuronal acetylcholine receptors but is inessential for formation of an interneuronal cholinergic synapse. J Neurosci 1998; 18: 4166–4176
- Burns AL, Benson D, Howard MJ, Margiotta JF. Chick ciliary ganglion neurons contain transcripts coding for acetylcholine receptor-associated protein at synapses (rapsyn). J Neurosci 1997; 17: 5016–5026
- Conroy WG, Berg DK. Rapsyn variants in ciliary ganglia and their possible effects on clustering of nicotinic receptors. J Neurochem 1999; 73: 1399–1408
- Kassner PD, Conroy WG, Berg DK. Organizing effects of rapsyn on neuronal nicotinic acetylcholine receptors. Mol Cell Neurosci 1998; 10: 258–270
- Liu Q-S, Berg DK. Actin filaments and the opposing actions of CaM kinase II and calcineurin in regulating α7-containing nicotinic receptors on chick ciliary ganglion neurons. J Neurosci 1999; 19: 10280–10288
- Shoop RD, Yamada N, Berg DK. Cytoskeletal links of neuronal acetylcholine receptors containing α7 subunits. J Neurosci 2000; 20: 4021–4029
- Roth AL, Berg DK. Large clusters of α7-containing nicotinic acetylcholine receptors on chick spinal cord neurons. J Comp Neurol 2003; 465: 195–204
- Conroy WG, Liu Z, Nai Q, Coggan JS, Berg DK. PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron 2003; 38: 759–771
- Parker MJ, Zhao S, Bredt DS, Sanes JR, Feng G. PSD93 regulates synaptic stability at neuronal cholinergic synapses. J Neurosci 2004; 24: 378–388
- Baer K, Bürli T, Huh K-H, Wiesner A, Erb-Vögtli S, Göckeritz-Dujmovic D, Moransard M, Nishimune A, Rees MI, Henley JM, Fritschy J-M, Fuhrer C. PICK1 interacts with α7 neuronal nicotinic acetylcholine receptors and controls their clustering. Mol Cell Neurosci 2007; 35: 339–355
- Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y, Wang Z-Z. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nature Neurosci 2003; 6: 1017–1018
- Temburni MK, Rosenberg MM, Pathak N, McConnell R, Jacob MH. Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein. J Neurosci 2004; 24: 6776–6784
- Farías GG, Vallés AS, Colombres M, Godoy JA, Toledo EM, Lukas RJ, Barrantes FJ, Inestrosa NC. Wnt-7a induces presynaptic colocalization of α7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci 2007; 27: 5313–5325
- Kabbani N, Woll M, Levenson R, Lindstrom JM, Changeux J-P. Intracellular complexes of the β2 subunit of the nicotinic acetylcholine receptor in brain identified by proteomics. Proc Natl Acad Sci USA 2007; 104: 20570–20575
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain function. J Pharmacol Exp Ther 1985; 235: 619–628
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem 1985; 45: 427–433
- Benwell MEM, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem 1988; 50: 1243–1247
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotine receptor subunit RNA after chronic nicotine treatment. J Neurosci 1992; 12: 2765–2784
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol 1994; 46: 523–530
- Zhang X, Gong Z-H, Hellstrom-Lindahl E, Nordberg A. Regulation of α4β2 nicotinic acetylcholine receptors in M10 cells following treatment with nicotinic agents. Neuroreport 1994; 6: 313–317
- Bencherif M, Fowler K, Lukas R, Lippiello PM. Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. J Pharmacol Exp Ther 1995; 275: 987–994
- Gopalakrishnan M, Monteggia LM, Anderson DJ, Molinari EJ, Piattoni-Kaplan M, Donnelly-Roberts D, Arneric SP, Sullivan JP. Stable expression, pharmacologic properties and regulation of the human neuronal nicotinic acetylcholine α4β2 receptor. J Pharm Exp Ther 1996; 276: 289–297
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, Lester HA. Assembly of α4β2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci 2003; 23: 11554–11567
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Mol Pharmacol 2005; 68: 1839–1851
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux J-P, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron 2005; 46: 595–607
- Kishi M, Steinbach JH. Role of agonist binding site in up-regulation of neuronal nicotinic α4β2 receptors. Mol Pharmacol 2006; 70: 2037–2044
- Corringer P-J, Sallette J, Changeux J-P. Nicotine enhances intracellular nicotinic receptor maturation: a novel mechanism of neuronal plasticity?. J Physiol (Paris) 2006; 99: 162–171
- Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C. Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev 2007; 55: 134–143
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhance long-term potentiation in perforant path. J Neurosci 2007; 27: 8202–8218
- Wang F, Nelson ME, Kuryatov A, Olale F, Cooper J, Keyser K, Lindstrom J. Chronic nicotine treatment up-regulates human α3β2 but not α3β4 acetylcholine receptors stably transfected in human embryonic kidney cells. J Biol Chem 1998; 273: 28721–28732
- Ridley DL, Rogers A, Wonnacott S. Differential effects of chronic drug treatment on α3* and α7 nicotinic receptor binding sites, in hippocampal neurones and SH-SY5Y cells. Br J Pharmacol 2001; 133: 1286–1295
- Sallette J, Bohler S, Benoit P, Soudant M, Pons S, Le Novère N, Changeux J-P, Corringer PJ. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J Biol Chem 2004; 279: 18767–18775
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates α6- and β3-containing nicotinic cholinergic receptors in rat brain. J Pharm Exp Ther 2007; 322: 306–315
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem 2007; 104: 446–456
- Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. 2008. Upregulation of nicotinic receptors by nicotine varies with receptor subtype. J Biol Chem 283:6022–6032.
- Cho C-H, Song W, Leitzell K, Teo E, Meleth AD, Quick MW, Lester RAJ. Rapid upregulation of α7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J Neurosci 2005; 25: 3712–2723
- Massey KA, Zago WM, Berg DK. BDNF up-regulates α7 nicotinic acetylcholine receptor levels on subpopulations of hippocampal interneurons. Mol Cell Neurosci 2006; 33: 381–388
- Liu Z, Tearle AW, Nai Q, Berg DK. Rapid activity-driven SNARE-dependent trafficking of nicotinic receptors on somatic spines. J Neurosci 2005; 25: 1159–1168
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci 1997; 20: 92–98
- MacDermott AB, Role LW, Sigelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci 1999; 22: 443–485
- Berg DK, Shoop RD, Chang KT, Cuevas J, Nicotinic acetylcholine receptors in ganglionic transmission, in Handbook of Experimental Pharmacology, Vol. 144, Neuronal Nicotinic Receptors, Clementi F, Fornasari D, Gotti C,. Springer: Berlin, 2000; pp 247–267.
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci 1999; 22: 555–561
- Williams BM, Temburni MK, Levey MS, Bertrand S, Bertrand D, Jacob MH. The long internal loop of the α3 subunit targets nAChRs to subdomains within individual synapses on neurones in vivo. Nature Neurosci 1998; 1: 557–562
- Temburni MK, Blitzblau RC, Jacob MH. Receptor targeting and heterogeneity at interneuronal nicotinic cholinerigic synapses in vivo. J Physiol 2000; 525: 21–29
- Xu J, Zhu Y, Heinemann SF. Identification of sequence motifs that target neuronal nicotinic receptors to dendrites and axons. J Neurosci 2006; 26: 9780–9793
- Kracun S, Harkness PC, Gibb AJ, Millar NS. 2008. Influence of M3-M4 intracellular domain upon nicotinic acetylcholine receptor assembly targeting and function. Br J Pharmacol [published on-line: doi: 10.1038/sj.bjp.0707676].
- Wang J-M, Zhang L, Yao Y, Viroonchatapan N, Rothe E, Wang Z-Z. A transmembrane motif governs the surface trafficking of nicotinic acetylcholine receptors. Nature Neurosci 2002; 5: 963–970