Abstract
Proteins destined for translocation across the prokaryotic cytoplasmic membrane are synthesized as precursors carrying transient N-terminal extensions known as signal sequences. They facilitate initial engagement of precursor proteins with the sec-dependent translocase to initiate active threading of the polypeptide across the membrane. The translocated precursor is then processed by a transcytoplasmic signal peptidase anchored to the inner membrane. The temporal nature of cleavage of the signal sequence during pre-protein translocation has remained elusive. Using an engineered mammalian cytochrome b5 precursor we demonstrate that the signal peptide processing in Escherichia coli is an event that can occur after almost complete exocytoplasmic translocation of the preprotein is accomplished. We discuss implications of the findings in light of the known working model of sec-dependent pre-protein translocon.
| Abbreviations | ||
| GRAVY | = | Grand average hydropathy |
| MIS | = | membrane insertion sequence |
| Spase | = | signal peptidase |
| SS | = | signal sequence. |
Introduction
Many proteins destined for export to the periplasmic space or localization to the outer membrane of Escherichia coli are synthesized as precursor forms carrying amino-terminal extensions known as signal peptides or signal sequences (SS) Citation[1], Citation[2]. Although the primary structure of SS is highly variable (18–30 amino acids), it contains a conserved, ordered and a tripartite structure. The N-terminal region (n) ranging in size from 1–5 amino acids contains a net positive charge. The central hydrophobic transmembrane region (h) stretches from 6–15 amino acids and has a preference for a α-helical conformation promoted by interaction of the positively-charged N-terminus with anionic phospholipids at the cytoplasmic side. The polar C-terminal segment (c) composed of 3–7 residues has the cleavage site for SPase which recognizies residues at −1 and −3 position relative to the scission bond. In E. coli, the secretory (sec) pathway directs vast majority of the cytoplasmically-synthesized precursor proteins post-translationally to a protein-conduction channel (pre-protein translocase) in the inner membrane Citation[3]. Fully-synthesized precursor proteins are initially maintained in an unfolded or translocation-competent state with the aid of molecular chaperones such as SecB Citation[4] or SRP Citation[5] prior to its engagement with the translocon. The E. coli Sec translocase is composed of the peripheral SecA and membrane proteins SecYEGDFYajC; the SecA acts as SecYEG-bound receptor for the secreted proteins. Both the N- and H- domains of the SS are essential for precursor recognition by SecA Citation[6] which then binds with high affinity to SecYEA complex to form the aqueous polypeptide-conducting channel in the inner membrane. In this bound complex, Sec A functions as an ATP-dependent motor protein Citation[7], Citation[8] that sequentially drives unfolded polypeptide across the protein-aligned membrane pore through the use of the proton motive force Citation[9]. The precise role of SS in activation and maintenance of the translocation process/machinery has remained obscure except that either during or following complete polypeptide translocation, the transposed SS is cleaved off by an exocytoplasmic Spase Citation[10] to generate the mature region.
Previously, Randall and co-worker Citation[11] employed an elegant limited proteolytic approach to dissect the temporal nature of SS processing of native bacterial precursor proteins. Since almost complete polypeptide transposition was coupled with SS processing, irrespective of co- or post-translational translocation, it was suggested that transfer of entire domain of the preprotein occurred prior to SS processing. Here we readdress the temporal nature of SS processing by using a chimeric mammalian cytochrome b5 secretory protein, N-terminally linked to E. coli alkaline phosphatase secretory sequence. The fusion protein was produced as an intact, unprocessed precursor that was post-translationally integrated via sec-dependent export and eventually anchored in the cytoplasmic membrane with its globular haem-binding domain displaced towards the periplasmic space. Precursor processing of the translocated globular domain was aborted due to intercalation of the transmembrane C-terminal cytochrome anchor and the N-terminal SS which in turn masked accessibility of the cleavage site to Spase. Introduction of nested deletions extending inwards from the C-terminus gradually alleviated cleavage inhibition with concomitant periplasmic localization of the processed mature globular b5 in the periplasmic space. Our data suggests that SS processing of the mammalian cytochrome b5 precursor in E. coli is an event that occurs following complete translocation of the protein. We discuss this model in the light of current workings of the pre-protein translocase.
Materials and methods
Unless stated otherwise, all chemicals were of Analar grade and purchased from Sigma Chemicals. Electrophoresis reagents were purchased from BioRad Labs (Herts, UK) and BDH (Poole, UK). Restriction endonucleases and DNA modifying enzymes were supplied by New England Biolabs (Herts, UK). Synthetic oligonucleotides were supplied by MWG (Ebersberg, Germany). Plasmid DNA was purified using Qiagen plasmid isolation kit (Qiagen, UK).
Plasmids and heterologous expression
The following strains were used in this study:
E. coli TB1: F-, araΔ(lac-proAB) [φ80 ΔlacZ ΔM15] rpsL (StrR) thi hsdR
E. coli MC4100: F- araD139?(argF-lac)U169 rspL150 relA1 flbB5301 fruA25 deoC1 ptsF25
E. coli MM52: secA51(Ts) derivative of MM4100
E. coli IQ85: secY24(Ts) zhd::Tn10 rpsE-derivative of MM4100
Figure 1. Gene constructs. Ampicillin-resistant plasmid segments encoding native and SS-appended cytochrome b5 under the transcriptional control of E. coli alkaline phosphate promoter (PphoA); S/D, Shine/Dalgarno sequence; SS, signal sequence.
Site-specific, nested tail deletions of 15 successive nucleotides, gradually extending inwards from the C-terminus of cytochrome b5, were introduced by inverse PCR involving DNA amplification using HindIII-linearized pM-SCT as the template and the following synthetic primers:
Forward primer: AvrII 5' ATT CCT AGG ATC AAG CTT TAG TTC GTC AAG GCT Reverse primers: AvrII Full-length: ATT CCT AGG TTA ATC TTC TGC CAT GTA CAG ACG GTA Δ129–133: ATT CCT AGG TTA CAG ACG GTA CAT CAG CGC TAC AAC Δ124–133: ATT CCT AGG TTA CGC TAC AAC CAG AGC AGA GAT CGC AGG Δ119–133: ATT CCT AGG TTA AGA GAT CGC AGG GAT AAC CCA GTT AGT Δ114–133: ATT CCT AGG TTA AAC CCA GTT AGT CCA CCA ACT AGA GTT Δ109–133: ATT CCT AGG TTA CCA ACT AGA GTT CGA TTC AAC GGT AGT Δ104–133: ATT CCT AGG TTA TTC AAC GGC AGT GAT CAG AGT TTC GGA Δ99–133: ATT CCT AGG TTA CAG AGT TTC GGA CGG TTT CGC GAT TTT Δ1–133: ATT CCT AGG CCG GGC TTT TGT CAC AGG GGT
PCRs were performed as described previously Citation[14] using Deep VentR@ DNA polymerase. The amplified DNA was digested with AvrII, circularized using T4 DNA ligase and introduced into E. coli TB1. Putative positive clones were identified by PCR using appropriate primer combinations and restriction mapping. Select clone lines were further verified by DNA sequencing using a Long ReadIR 4200 Li-Cor automated fluorescent sequencer (MWG, Germany). The standard procedures involving DNA manipulation including their separation, purification, digestion and bacterial transformation were performed as described Citation[15].
Gene expression and sub-cellular fractionation of cytochrome b5 derivatives
E. coli TB1 harbouring the recombinant plasmids were propagated and cultured in Luria broth containing 1% (w/v), Tryptone, 0.5% (w/v) yeast extract and 1% (w/v) NaCl) and 75 µg ampicillin/ml. A 2% (v/v) Luria broth culture grown to saturation point was used for induction by batch cultivation in the phosphate-limited MOPS medium Citation[15] at 35°C for 8 h with agitation at 125 rev/min. Cells (100 ml) were harvested by centrifugation at 5000 g for 5 min. All subsequent operations were conducted at 4°C. Periplasmic extracts were recovered in 5 ml 0.5 mM MgCl2 by an ‘osmotic shock’ procedure as described previously Citation[16]. The residual cell material was suspended in 10 ml of 50 mM Tris-HCl (pH 8), 5 mM Na2EDTA and lysed by sonication. The pelleted envelope fraction, separated from the cytoplasmic fraction (105,000 g 1 h) was used to prepare the inner and outer membranes by sucrose density ultracentrifugation Citation[17].
Isolated sub-cellular fractions were routinely monitored for the following known marker enzymes. Alkaline phosphatase was assayed using the substrate p-nitrophenylphosphate (1 mM) Citation[18]. Succinate dehydrogenase, malate dehydrogenase, fumarase and isocitrate dehydrogenase activities were assayed as described previously Citation[13]. Cytochrome b5 content was spectrally quantified from the dithionite-reduced Soret absorbance peak at 423 nm using the absorption coefficient of 185 cm−1mM−1. Absolute absorption spectra were recorded by scanning samples contained in the buffer composed of 10 mM Tris-HCl buffer pH (8.0) and 1 mM EDTA, from 350– 450 nm. Apo-protein was converted to holo-haemoprotein on addition of 1 mM haem solution (0.1 M Tris-HCl (pH 8.2), 80% (v/v) ethylene glycol) to both the test and the reference cuvettes at final concentrations ranging from 2.5–10 µM. Protein content was determined using BioRad kit Citation[19] with bovine serum albumin as the standard.
Protein purification and characterization
Recombinant Spase was isolated from 1 lit of low phosphate-induced E. coli/pMC-Spase cell membranes Citation[20] and assayed described previously Citation[12].
SS-fused cytochrome b5 precursor was isolated from E. coli/pM-SCT cells (1lit). Harvested cells (5000 g×5 min) were resuspended in 10 ml of 10 mM Tris-HCl (pH 8), 1 mM EDTA (TE) containing 10 µM bovine haem. After treating with 0.1 mg lysozyme/ml on ice for 30 min, the lysed cells were subjected to 10×15sec sonications (Soniprep 150, MSE) with 5 min cooling on ice between each pulse. The membranes, prepared from a post-cellular fraction (1000 g for 20 min) by centrifugation at 105,000 g for 1 h, were resuspended in 2.5 ml TE buffer and gradually added to 50 ml of 40% (v/v) acetonitrile while stirring in a beaker. A low-speed (8000 g for 30 min) supernatant fraction representing the recombinant haemoprotein extract was diluted with an equal volume of 25 mM Tris-acetate (pH 8) (TA) and applied onto a TA-pre-equilibrated DEAE Sepharose CL-6B column (1 cm ID×10 cm high). After washing the column with TA, the haemoprotein was eluted as a broad peak using a linear NaCl gradient (0–0.5M) in TA containing 1% (w/v) NP-40. Haemoprotein-enriched fractions were pooled, extensively dialyzed against TA, and re-chromatographed on a HR5/5 Mono Q column (Pharmacia-LKB, UK) using an automated Pharmacia-LKB FPLC system, following the procedure essentially as described above. Passage of the final preparation through an Amberlite XAD-2 resin column removed residual NP-40.
Immuno-electrophoresis
Polypeptides were separated by polyacrylamide gel electrophoresis in the presence of 0.1% (w/v) SDS employing the Laemli discontinuous buffer system Citation[21]. The recombinant cytochrome b5 isoforms were immunologically identified by Western blotting Citation[22]. Electrophoresed protein profiles (25 µg lane), transferred from unstained polyacrylamide gels onto nitrocellulose acetate membranes (Schleicher and Schleicher, Germany), were probed with goat anti-rat cytochrome b5 serum and detected by the activity of horse radish peroxidase coupled to the second guinea pig anti-IgG antibody using a chemiluminescent substrate (Amersham Biosciences, UK). The N-terminal sequences of the proteins were determined using Applied Biosystems sequencer (model 473A) after protein bands of interest on SDS-PAGE were electrophoretically transferred onto Bio-RAD PVD membranes.
Mass spectrometry
Gel-eluted cytochrome b5 derivatives (1–10 nmol) were recovered in a final volume of 100 µl containing 100 mM potassium phosphate buffer (pH 7.4) and 0.1% (w/v) sodium cholate. A 5 µl of the dialyzed protein sample was mixed with an equal volume of matrix solution composed of acetonitrile/water/trifluoroacetic acid (300:700:1), pre-saturated with sinapinic acid. The mixture (2 µl) was spotted onto a sample plate, air-dried and analysed using a Perceptive Biosystems MALDI-TOF mass spectrometer precalibrated with myoglobin.
The spectra were also recorded by electron-spray in the positive ion mode using a VG Quattro II instrument (Fisons Instruments, Manchester, UK). The probe was operated with a capillary voltage of 3.04 kV by pumping (10 µl/min) water as the solvent into which 100 pmol of cytochrome b5 dissolved in 10 µl of 1% (v/v) formic acid was applied. Scans were acquired in a continuous mode with a 1 s scan duration and an interscan delay of 0.11 s.
Results
Expression, targeting and characterization of the SS-appended cytochrome b5 in E. coli
The maps of the engineered gene constructs coding for the SS-less and SS-appended cytochrome b5 in the plasmids pM-CT and pM-SCT are shown in . The chimeric gene in pM-SCT, composed of alkaline phosphatase SS linked to the native full length sequence of rat liver cytochrome b5, was placed downstream of the phoA promoter, inducible by growth of E. coli in a phosphate-limited MOPS medium Citation[13]. Induction of E. coli/pM-SCT directed efficient synthesis of the haemoprotein spectrally identified in the cell lysate as a b-type cytochrome by its characteristic Soret absorption peak at 423 nm and visible peaks at 555 nm and 527 nm. The spectra were indistinguishable in comparison with the native or globular cytochrome b5 Citation[23].
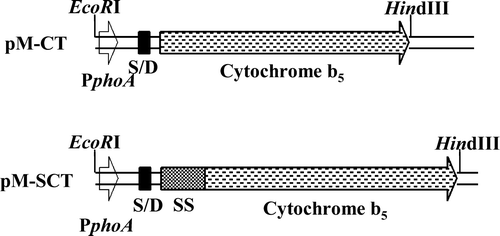
The cellular location of the recombinant cytochrome b5 in E. coli was investigated by subfractionating the induced bacterial cells into periplasmic and cytoplasmic extracts and the envelope membrane fraction. Marker enzymes known to be specific for each of the subcellular fractions were assayed along with cytochrome b5. shows that the periplasm had been efficiently segregated from the cytoplasm and cytochrome b5 was clearly localized in the envelope fraction. The envelope was further fractionated into inner and outer membranes by sucrose density centrifugation. The specific contents of cytochrome b5 in the isolated inner and outer membranes were 10 and 2 nmol cytochrome/mg of protein, respectively.
Table I. Subcellular localization of signal-appended cytochrome expressed in Escherichia coli.
Electrophoretic analysis of the total- and sub-cellular fractions () showed that a 21 kDa induced protein band was both intensely and specifically concentrated in the inner membrane fraction. The fact that this induced 21 kDa protein gave a strong and specific anti-globular cytochrome b5 immuno-reactive signal on Western blots (, lanes 1, 4, 5 and 6) indicated that it was related to the haemoprotein. However, the electrophoretically estimated size of the anti-cytochrome b5 immuno-reactive recombinant protein (21 kDa) was significantly larger than either the processed (15 kDa) or unprocessed form (18 kDa). MALDI-TOF-MS-analysis of the eluted protein yielded a mass of 17,748 which was comparably close to the theoretical or predicted [M+H]+ of 17,751 Da of the unprocessed precursor. Moreover, sequence analysis of the first N-terminal 5 amino acids of the eluted protein (induced band B in ) gave the sequence M K Q S T that was identical to that coded for by the E. coli alkaline phosphatase SS gene Citation[24]. This provided further evidence that the cytochrome b5 was expressed as a membrane-integrated, unprocessed precursor form but exhibited an anomalous electrophoretic mobility.
Figure 2. Sub-cellular distribution of signal-appended cytochrome b5 in induced E. coli/pM-SCT. IM and OM, inner and outer membranes. c, control E. coli harbouring the progenitor plasmid pAF without sct; O, immunoreactive bands against anti-cytochrome b5 polyclonal antibodies.
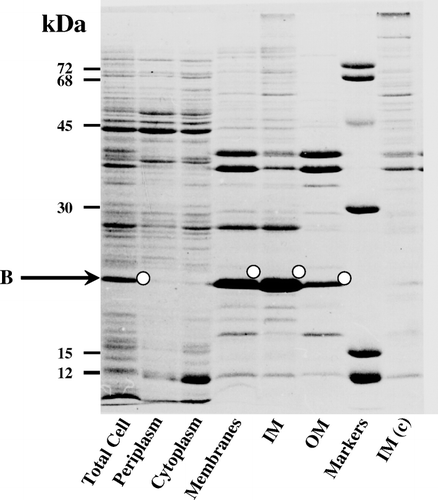
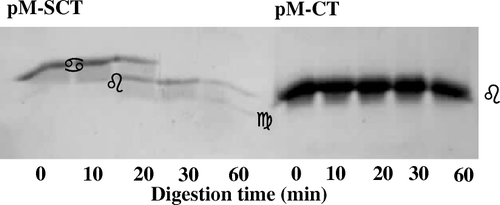
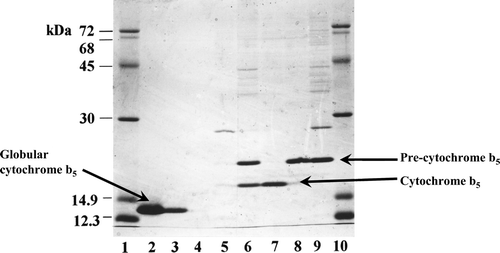
The cytochrome b5 precursor was isolated to homogeneity ( lane 9). Incubation with the purified Spase resulted in a time-dependent processing of the 21 kDa precursor (, lanes 1–8) with concomitant accumulation of a 15 kDa protein. MALDI-TOF-MS analysis of this processed band after electro-elution gave a size of 15,519 compared with the theoretical or predicted [M+H]+ 15,513 of the potential SS-processed cytochrome b5 carrying the N-terminal positive-charged arginine Citation[16]. The sequence of the eluted 15 kDa protein band was R M A E Q S. This matched with the N-terminal sequence of cytochrome b5, providing confirmatory proof that the processed form was indeed derived from the SS-appended precursor form.
Figure 3. In vitro processing of cytochrome b5 precursor by signal peptidase I. Processing assay with molar precursor protein: enzyme = 3.5:1 was performed in a buffer system composed of 25 mM Tris-HCl (pH 8), 0.1 M NaCl, and 0.5% (w/v) NP-40. At the times indicated, aliquots were withdrawn and reactions were terminated by boiling with an equal volume of SDS sample buffer prior to analyses by SDS-PAGE.
Sec-dependency of pre-cytochrome b5 export
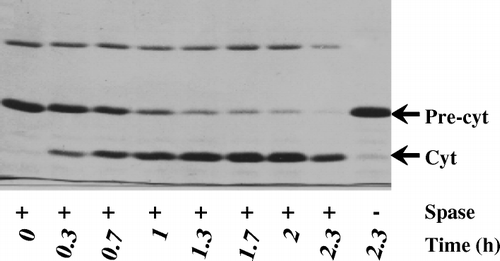
Since export of alkaline phosphatase is sec-dependent Citation[25], it was of interest to investigate whether the artificial-export competent cytochrome b5 fused to the same SS also displayed sec-mediated post-translational translocation. To test this plasmid pM-SCT was introduced into E. coli MC4100 (wild-type) and the temperature-sensitive derivative mutant forms MM52 (secA-) and IQ85 (secY-). These cells were cultured at 33°C for 4 h and then shifted to 40°C for further 4 h of growth (). Steady productivity of pre-cytochrome b5 in the crude isolated membrane fractions was apparent throughout the growth-phase. Whereas, a similar thermal shift caused an almost complete decline of the haemoprotein productivity in the secA and secY deficient backgrounds. This suggested that the chimeric cytochrome precursor employed post-translational, secA-dependent targeting pathway to engage the precursor with the Sec translocon. Interestingly, both the targeting and membrane-localization of the SS-less full-length cytochrome b5 counterpart was independent of SecA and SecY (), implying that the SS in the chimera was an important element for sec-dependent translocation of pre-cytochrome b5.
Figure 4. SecA-and SecY-dependent pre-protein translocation in membrane-localization of pre-cytochrome b5. Seeded cultures (triplicates) were induced (arrow) in MOPS medium at 33°C for 4 h and then the temperature shifted to 40°C for a further 2 h. At each hourly interval cells (100 ml) were harvested and the cytochrome b5 determined spectrophotometrically (A413) in the isolated inner membranes.
The SS-appended cytochrome b5 is integrated in the inner membrane as an unprocessed form with the globular domain facing the periplasm
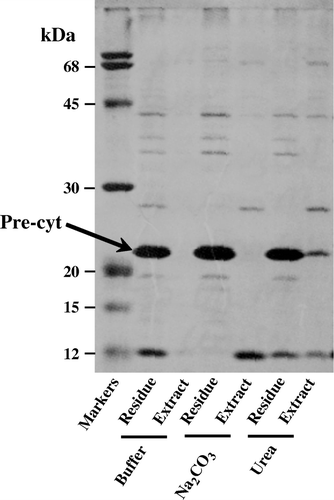
The integral nature of the SS-appended cytochrome b5 was assessed by its extractability from the membranes washed with either a buffered solution or 0.1 M Na2CO3 (pH 10.5) or 4 M urea (). The carbonate treatment converts the closed vesicles into ‘open’ membrane sheets, and peripheral membrane proteins can thus be extracted Citation[26]. Since neither of these extraction procedures displaced the cytochrome b5 precursor from the membranes we concluded that the haemoprotein must be tightly integrated within the lipid bilayer of the inner membrane.
Figure 5. Integral nature of SS-appended cytochrome b5. The isolated inner membranes from E.coli/pM-SCTwere resuspended by gentle homogenization with either buffer 10 mM Tris-HCl (pH 8), 0.1 M Na2CO3 or 4 M Urea. After incubation at 22°C for 15 min and centrifugation at 105,000 g for 1 h at 4°C, the proteins in the residual membranes and the extract were analysed by SDS-PAGE.
To assess the lateral disposition of the catalytic domain of cytochrome b5 in the inner membranes, trypsin-mediated proteolysis was conducted in induced E. coli/pM-SCT cells. Sucrose-plasmolyzed cells were permeabilized by EDTA treatment and then treated with trypsin. The proteolytic changes in the total cellular protein were monitored as a function of time by Western blotting using cytochrome b5 antibodies (). The cleavage profile of cytochrome b5 precursor, monitored by Western blotting, showed initial generation of 15 kDa which is then converted to 12.5 kDa form, both of which displayed comparable mobilities with the isolated forms of the native and globular cytochrome b5, respectively, suggesting that trypsin cleavage of SS is followed by the cleavage of the membrane anchoring tail portion. Thus pre-cytochrome b5 is targeted to the inner membrane-anchored with its globular domain facing the periplasmic space.
Figure 6. Dissection of lateral topology of signal-appended cytochrome b5. Induced E. coli/pM-SCT cells (100 ml culture) were gently suspended in 10 ml of 40% (w/v) sucrose, 33 mM Tris-HCl (pH 8), 1 mM EDTA and incubated at 25°C. After addition of 0.1 mg TCPK-treated trypsin/ml, 1 ml aliquots were withdrawn at the indicated times and treated with trypsin inhibitor and phenylmethylsulphonylfluoride at 0.15 mg/ml and 2.5 mM final concentrations, respectively. The samples were analysed immuno-electrophoretically., Precursor (•), signal-processed (▪) and globular cytochrome b5 (▴).
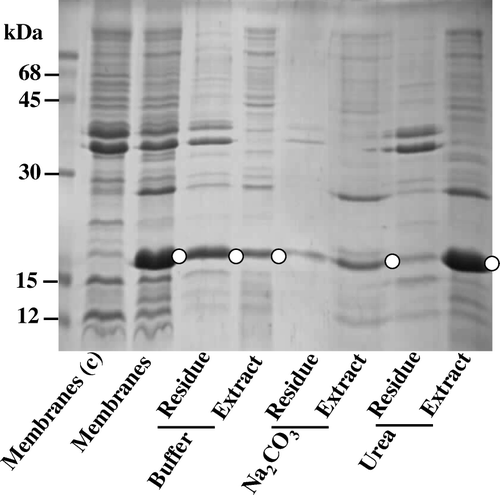
Trypsinization of E. coli/pM-SCT caused eventual degradation of the soluble globular cytochrome b5. However, cytoplasmically-facing precursor could also have been degraded if the spheroplasts were damaged or leaky. In view of this possibility, we constructed a plasmid pM-CT () encoding SS-less cytochrome b5, expressible in E. coli strain under identical conditions to E. coli/pM-SCT. Electrophoretic analysis of the induced E. coli/pM-CT () demonstrated that the haemoprotein of ∼15 kDa was abundantly accumulated in almost equivalent quantities in both the isolated inner and outer membrane fractions. A significant amount of the cytochrome b5 was also found to be isolatable in the cytosolic fraction. The fact that both urea and Na2CO3 treatment readily removes this SS-less cytochrome from the membrane suggested that in contrast to SS-fused counterpart, it was loosely-associated with the membrane (). Trypsinization of the E. coli/pM-CT spheroplasts () did not result in any obvious loss of the haemoprotein implying that the SS-less cytochrome had a cytoplasmic-facing topology. Moreover, these findings confirm that the appendage of SS converts cytochrome b5 as an inner membrane-anchored protein with globular domain disposed towards the periplasmic space.
Figure 7. Polypeptide profiles of E. coli pM-CT and its isolated sub-cellular fractions demonstrating expression of native cytochrome b5 in E.coli. The progenitor plasmid pAF devoid of cytochrome b5 gene is the control (c). immunoreactive bands against anti-cytochrome b5 polyclonal antibodies (O). IM and OM, inner and outer membranes.
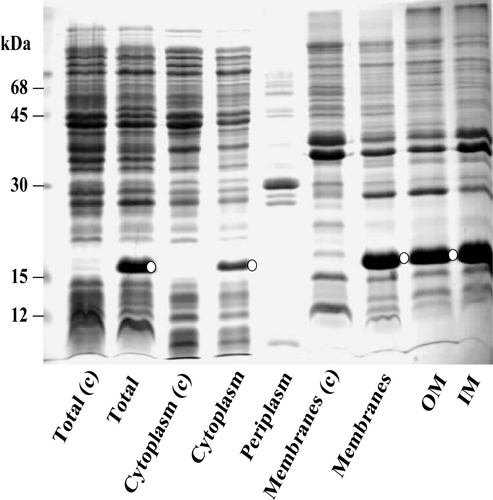
Figure 8. Loose anchorage of cytochrome b5 with E. coli plasma membrane. The progenitor plasmid pAF devoid of cytochrome b5 gene is the control (c); immunoreactive bands against anti-cytochrome b5 polyclonal antibodies (O). The isolated inner membranes from E.coli/pM-CT, gently resuspended in either 10 mM Tris-HCl (pH 8) or 0.1 M Na2CO3 or 4 M Urea. After incubation at 22°C for 15 min and centrifugation at 105,000 g for 1 h at 4°C, the proteins in the residual membranes and the extract were analysed by SDS-PAGE.
Step-wise C-terminal deletion of the tail portion targets SS-processed cytochrome b5 to the periplasm
We have previously reported that bacterial expression of SS-appended globular core cytochrome b5 (devoid of the membrane anchoring portion) resulted in efficient export of the processed, soluble haemoprotein to the periplasm Citation[13], Citation[16], Citation[27]. Moreover, the fact that the isolated cytochrome b5 precursor was efficiently processed, in vitro, by SPase () suggested that, at least during terminal stages of translocation, the membrane anchoring domain of the cytochrome b5 could have annulled processing of SS by the SPase. We have also reported on the potential stop-transfer sequence element within the membrane anchoring region of cytochrome b5 Citation[28]. This proposed stop-transfer sequence (23 residues) in the membrane anchoring domain of rat endoplasmic reticulum cytochrome b5 extends from residues 104–126 () which also acts as a membrane insertion sequence (MIS). Hence we considered the possibility that the appearance of stop-transfer sequence in the preprotein translocase channel may have not only terminated translocation but also interacted with the SS to have sterically inhibited its processing. The potential role of this stop-transfer sequence on the export/processing of the pre-cytochrome b5, in vivo, to abort SS-cleavage was investigated by performing systematic nested deletions of penta-amino acids extending from the C-terminus (). Proteins in the periplasmic and inner membrane fractions prepared from induced E. coli strains were analysed by both the standard Coomassie-blue staining and the more sensitive immuno-electrophoretic approach (). The combined detection reveals abundance of the precursors, intermediates and the secreted counterparts produced thus making it amenable to analyse their potential identities. The detection by the more sensitive immunological means revealed additional protein bands (not readily detectable by staining with Coomassie blue R-250) in the membrane fractions; the identities of some these intermediates remain obscure. Some of these are of the sizes comparable to unprocessed precursors and SS-processed forms. As documented previously Citation[13], Citation[27] that albeit efficient export of the mammalian cytochrome b5 addressed with alkaline phosphatase there is an inevitable build-up of the precursor(s) during transit through the membrane. The precursor forms of most of the C-terminally-excised forms extending up to 98 residue cytochrome b5 were overproduced in the membranes (arrowed in ).
Figure 9. Relationship between MIS hydropathy and export rates of signal-appended cytochrome b5 carrying increasing lengths of penta deletions from the C-terminus. The protein bands in A were densitometrically scanned using a HP Scanjet 5300C scanner and the profiles quantified using Phoretix 1D Advanced software (version 3.01) operating under MS Windows XP™. Product formation was determined from integration of the exported/processed periplasmically-recovered cytochrome b5 bands and expressed as% of the corresponding exported 98 amino acid globular cytochrome b5 form (Δ99–133).
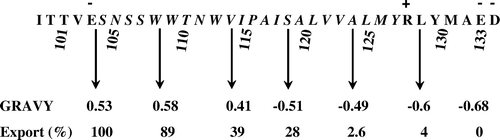
Figure 10. Effect of step-wise serial deletions of penta amino acids from C-terminus on the secretion of cytochrome b5. Periplasmic extracts and membrane fractions were prepared from 6 h-induced E. coli strains harbouring plasmids encoding the indicated lengths of C-terminal deletions. The proteins separated in two separate SDS polyacrylamide gradient (10–20%) gels of which the bands in one were seen by Coomassie blue R-250 (A) and in the other immunologically detected using goat anti-cytochrome b5 antibodies (B). Corresponding protein bands detected by staining and immunologically (o); the white arrows indicate positions of pre-cytochrome b5 forms. The lower three bands in the markers detected by staining are 12, 15 and 20 kDa, respectively.
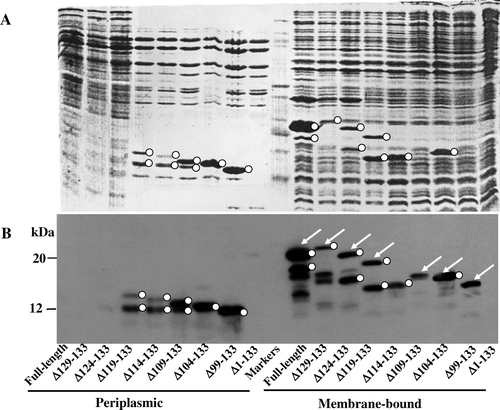
The MS-determined size coupled with partial N-terminal sequences of the gel-eluted proteins (O) demonstrated that deletion of the first five and ten amino acid residues from the carboxy-end yielded similar pattern of targeting in the membranes as the native precursor form. Excision of ten C-terminal residues resulted in miniscule generation of the SS-processed cytochrome b5 in the periplasm. A significantly enhanced secretion (25% of Δ99-133; and ) was apparent in constructs carrying deletions extending to 15 (Δ119–133) and 20 (Δ114–133) amino acids. Deletions extending beyond 25 residues (Δ109–Δ133→Δ99–Δ133) invoked more efficient periplasmic secretion of the SS-processed cytochrome b5 forms. The slower migrating band in the secreted doublet in the constructs Δ119–133→Δ109–133 appear to be C-terminal tethered forms that are correctly SS-processed forms () as deduced from the MS data. They are derived from membrane-bound SS-processed derivatives of: (i) The faster migrating bands in the constructs Δ119–133 and Δ114–133, and (ii) the singular precursor band in Δ109–133. These, however, upon further processing by Spase in the latter together with unspecified intra-membrane-proteolysis, they must be released into the periplasmic space to be further trimmed by a glutamyl-specific-like endopeptidase at the peptide bond 103–104 to generate the eventual globular form constituting the residues 1–103. The constructs carrying the deletions Δ104–133 and Δ99–133 generate abundant precursors (), as previously reported Citation[13], and also efficiently export the globular cytochrome b5 constituting the sequences 1–103 and 1–99 amino acids respectively. The increase in the extent of export/processing of truncated precursors correlated with corresponding decrease in GRAVY indicies () such that deletion of more than half of the membrane-spanning region in the membrane insertion sequence annulled the function of the proposed stop-transfer sequence.
Discussion
Employing a heterologously-expressed SS-appended full-length rat liver cytochrome b5, we demonstrate its Sec-dependent targeting to the inner membrane of E. coli as an integral unprocessed form with a topology disposing the functional globular domain towards the periplasm. We discuss the likely mechanism by which the cytochrome b5 attains this orientation together with the temporal mode of SS processing. Topologically, SS-less loosely-membrane-associated cytochrome b5 faces the cytoplasm (). Thus the role of the MIS as a potential targeting sequence in the translocation of the precursor cytochrome b5 is discounted.
Transmembrane disposition of the SS would be a pre-requisite for both Sec-dependent preprotein translocase-facilitated discharge via linear threading and subsequent SS processing and the polypeptide discharge into the periplasm. Occurrence of SS processing either during or immediately after the start of the translocation step would have eventually yielded a processed, translocated exo-cytoplasmic cytochrome b5 core anchored by the membrane-insertion sequence. Thus, the transposed SS must have remained unprocessed at least throughout the duration of threading involving globular core polypeptide region. The appearance of the terminal MIS in the preprotein translocase appeared to abort processing of SS. Since deletion of the MIS character restores efficient export and processing of the globular core, SS-mediated processing must occur after the polypeptide is completely threaded across the membrane. These findings are in agreement with previous work of Randall Citation[11], who employed an elegant limited proteolytic approach to dissect the temporal nature of SS processing of native bacterial precursor proteins. Since almost complete polypeptide transposition was coupled with SS processing, irrespective of co- or post-translational translocation, it was suggested that transfer of entire domain of the preprotein occurred, a model that is not concilable with the current mode of Sec-dependent preprotein discharge. These observations raise the question as to why the SS should be cleaved post-translocationally? Secondly, how does the appendage of SS to cytochrome b5 inhibit processing of the precursor?
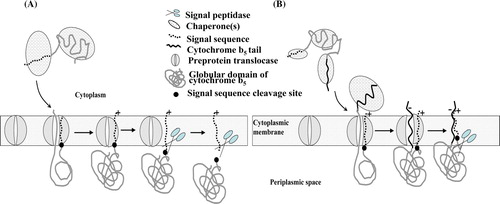
During translocation, the early portion of the precursor is trapped in a so-called clamp composed of SecYEG proteins. A protein-conduction pore in the centre of the complex, formed between two halves of the SecY subunit, is usually blocked by a short plug domain Citation[29]. Precisely how this conduit is activated and maintained in an open state during translocation is unknown. However, during translocation, the inverted SS has been shown to be localized between two transmembrane segments of SecY located adjacent to the channel Citation[30] while the threading polypeptide and SecY were located along the pore Citation[31]. Hence, we suggest that this interaction may be a primary event in maintaining an open channel throughout the phase when the polypeptide is actively driven through the complex by SecA-ATPase motor activity. The interaction of the SS with Sec may be crucial throughout the duration of polypeptide threading across the membrane. However, following completion of polypeptide translocation, pore closure probably disperses the SS away from the TM (transmembrane) domain of secY into the lipid phase. At this temporal event in protein translocation, the dissociated SS would be amenable to cleavage by the Spase (A). Indeed, the bacterial SPase I is a membrane-bound enzyme with its active site facing the periplasm. It is known that only the globular domain is sufficient for recognition of the C-region of SS cleavage to be accomplished Citation[32].
Figure 11. Models depicting post-translational translocation of precursor forms of cytochrome b5. (A) The fully-synthesized globular cytochrome b5 precursor in a chaperone-mediated unfolded state is targeted to the pre-protein translocase. During translocation SS interacts with the TM domain of SecY to maintain the channel open. Following translocation the SS dispersal into the lipid phase makes cleavage site accessible to Spase. (B), Targeting and translocation of full-length cytochrome b5 precursor as in (A) but following translocation of the globular domain, interaction of the SS with the MIS introduces steric hindrance to processing by Spase. This Figure is reproduced in colour in Molecular Membrane Biology online.
So how does the MIS of cytochrome b5 abort processing of SS? Consideration of the potential structure and biochemical properties of the precursor may shed light on the mode of inhibition. The isolated recombinant precursor protein displays amphipathic properties similar to the native cytochrome b5 but not the soluble globular form of b5, in that it is completely recovered with the micellar Triton X-114 after temperature-induced phase separation Citation[33] of the detergent-solubilized membranes (supplementary , online version only). Hydropathy plot analysis suggests that the cytochrome b5 precursor is a three domain protein in which the central globular domain is bounded by terminal hydrophobic cores of SS and the MIS. This is validated by trypsin-mediated serial removal from the inner membrane of the SS, MIS and finally the globular domain of precytochrome b5 (). The spectral and biochemical properties of the cytochrome b5 precursor was indistinguishable from the native form. This indicates retention of the structural integrity of the globular b5 domain, which must have been correctly folded and preserved against likely perturbations by the N- and C-terminal hydrophobic segments. Molecular modelling of the cytochrome b5 precursor, based on data derived from prediction methods and the crystal structure of the globular form, validates this amphipathic structure of the chimera. In this model, a protruding hydrophobic cylinder anchors the core to the membrane (B), the cylinder, formed by apolar interactions between the MIS and the inverted SS, and stabilized by terminal ionic bonds on the cytoplasmic side must have masked the SPase cleavage site. Further evidence for this comes from the finding that a comparable derivative devoid of the membrane-insertion sequence was both efficiently processed and exported to the periplasmic space in E. coli Citation[30], Citation[34]. This implied that the appendage of MIS converted SS-appended globular form into a non-exportable, membrane bound form. Consistent with this model, nested C-terminal deletions of the membrane-insertion sequence gradually restored full-processing capability of the truncated precursors which were then localized in the periplasm as soluble forms ( and ). Models depicting the outcome of targeting of the full length and globular precursors are shown in . In this model the SS, although dissociated from TM domain of SecY, becomes capable of interacting with the MIS of cytochrome b5 thus sterically impeding its cleavage by Spase. Further support for this model is the fact that detergent solubilization of the membranes initiates immediate processing of the precursor ( and Supplementary ). Moreover, deletion of the MIS renders its GRAVY to that of a soluble counterpart thus re-instating both efficient translocation and processing of the globular domain (). The significant build-up of cytochrome b5 precursor is a feature of the engineered cytochrome b5 precursor arising from proton-motive retardation imposed by the positive Arg residue preceeding the SS cleavage site Citation[27]. The presence of breakdown of precursor detectable by immuno-electrophoretic approach may be related to slow but significant level of intramembrane proteolysis of SS or MIS Citation[34], Citation[35].
We are aware that the engineered cytochrome b5 precursor may not have a counterpart in prokaryotes, nevertheless, it continues to provide a useful probe to dissect the protein translocation machinery.
Supplementary Figure 1. Triton X-114 phase separation of globular (1–98 residues) and SS-fused native cytochrome b5 (1–133 amino acid residues). Preparations of purified globular cytochrome b5 (25 µg protein, lane 1) or isolated E. coli pM-SCT membranes (120 µg protein, lane 9) in a final volume of 200 µl containing 150 mM NaCl, 10 mM Tris-HCl (pH 7.6) and 2% (w/v) Triton X-114 were incubated on ice for 10 min and then overlaid on a cushion of 0.25 M sucrose containing 150 mM NaCl and 10 mM Tris-HCl (pH 7.6) and 0.06% (w/v) Triton X-114 in an Eppendorf tube. After incubation at 30°C for 3 min and centrifugation in a swing-out rotor at 1700 g for 3 min at room temperature the upper aqueous phase was carefully harvested and made up to 0.5% (w/v) with Triton X-114 and subjected to a second round of the phase separation, as described above, over the previous sucrose cushion. Half of the final volumes were analysed by electrophoresis. Molecular weight marker proteins (lanes 1 and 10); aqueous (lane 3) and detergent (lane 4) phases of globular cytochrome b5 extractrions. Aqueous (lane 5) and detergent (lane 6) phases of E. coli pM-SCT membranes. Note that detergent-treatment of pM-SCT membranes initiate processing of SS-fused cytochrome (lane 6). Lanes 7 and 8 shows purified isolates of native and its corresponding SS-appended cytochrome b5 forms (1–133 residues), respectively.
Acknowledgements
We thank Jim Heald for his excellent technical assistance with MS. We are most grateful to Dr Khawar Abbas (Leeds University) for the sheep anti-recombinant rat cytochrome b5 antibodies. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Izard JW, Kendall DA. Signal peptides: exquisitely designed transport promoters. Mol Microbiol 1994; 13: 765–773
- von Heijne G. The signal peptide. J Membr Biol 1990; 115: 195–201
- de Keyzer J, van der Does C, Driessen AJ. The bacterial translocase: a dynamic protein channel complex. Cell Mol Life Sci 2003; 60: 2034–2052
- Ullers RS, Luirink J, Harms N, Schwager F, Georgopoulos C, Genevaux P. SecB is a bona fide generalized chaperone in Escherichia coli. Proc Natl Acad Sci USA 2004; 101: 7583–7588
- Luirink J, Sinning I. SRP-mediated protein targeting: structure and function revisited. Biochim Biophys Acta 2004; 1694: 17–35
- Akita M, Sasaki S, Matsuyama S, Mizushima S. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J Biol Chem 1990; 265: 8164–8169
- Chen L, Tai PC. Effects of nucleotides on ATP-dependent protein translocation into Escherichia coli membrane vesicles. J Bacteriol 1986; 168: 828–832
- Economou A, Pogliano JA, Beckwith J, Oliver DB, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell 1995; 83: 1171–1181
- Driessen AJ. Precursor protein translocation by the Escherichia coli translocase is directed by the protonmotive force. EMBO J 1992; 11: 847–853
- Tuteja R. Type I signal peptidase: an overview. Arch Biochem Biophys 2005; 441: 107–111
- Randall LL. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell 1983; 33: 231–240
- Kaderbhai N, Kaderbhai MA. Expression, isolation, and characterization of a signal sequence-appended chimeric precursor protein. Protein Express Purificat 1996; 7: 237–246
- Karim A, Kaderbhai N, Evans A, Harding V, Kaderbhai MA. Efficient bacterial export of a eukaryotic cytoplasmic cytochrome. Biotechnology (NY) 1993; 11: 612–617
- Akhtar MK, Kaderbhai NN, Hopper DJ, Kelly SL, Kaderbhai MA. Export of a heterologous cytochrome P450 (CYP105D1) in Escherichia coli is associated with periplasmic accumulation of uroporphyrin. J Biol Chem 2003; 278: 45555–45562
- Ausubel FM, Brent R, Kingston RE, Moore DE, Seidman JG, Smith JA, Struhl KA. 2001. vol. 1 New York: John Wiley & Sons.
- Harding V, Karim A, Kaderbhai N, Jones A, Evans A, Kaderbhai MA. Processing of chimeric mammalian cytochrome b5 precursors in Escherichia coli: reaction specificity of signal peptidase and identification of an aminopeptidase in post-translocational processing. Biochem J 1993; 293: 751–756
- Liu YY, Akhtar MK, Ourmozdi EP, Kaderbhai N, Kaderbhai MA. A chloroplast envelope-transfer sequence functions as an export signal in Escherichia coli. FEBS Lett 2000; 469: 61–66
- Kaderbhai N, Karim A, Hankey W, Jenkins G, Venning J, Kaderbhai MA. Glycine-induced extracellular secretion of a recombinant cytochrome expressed in Escherichia coli. Biotechnol Appl Biochem 1997; 25: 53–61
- Bradford M. A rapid and sensitive method for the quantitation of protein utilising the principle of protein-dye binding. Analyt Biochem 1976; 72: 248–254
- Zwizinski C, Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem 1980; 255: 7973–7977
- Gallagher SR. 2001. One-dimensional SDS gel electrophoresis of proteins. In: Ausubel FM, Brent R, Kingston RE, Moore DE, Seidman JG, Smith JA, Struhl KA Current Protocols in Molecular Biology. vol. 2. New YorkUSA: John Wiley & Sons. pp 10.2A.1–10.2A.29.
- Kaderbhai MA, He MY, Beechey RB, Kaderbhai N. Coexpression of a precursor and the mature protein of wheat ribulose- 1,5-bisphosphate carboxylase small subunit from a single gene in Escherichia coli. DNA Cell Biol 1990; 9: 11–25
- Gallagher J, Kaderbhai N, Kaderbhai MA. Gene-dose-dependent expression of soluble mammalian cytochrome b5 in Escherichia coli. Appl Microbiol Biotechnol 1992; 38: 77–83
- Blattner FR, Plunkett G.3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science 1997; 277: 1453–1474
- Angelini S, Moreno R, Gouffi K, Santini C, Yamagishi A, Berenguer J, Wu L. Export of Thermus thermophilus alkaline phosphatase via the twin-arginine translocation pathway in Escherichia coli. FEBS Lett 2001; 506: 103–107
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment – application to endoplasmic reticulum. J Cell Biol 1982; 93: 97–102
- Kaderbhai MA, Davey HM, Kaderbhai NN. A directed evolution strategy for optimized export of recombinant proteins reveals critical determinants for preprotein discharge. Protein Sci 2004; 13: 2458–2469
- Kaderbhai MA, Morgan R, Kaderbhai NN. The membrane-interactive tail of cytochrome b(5) can function as a stop-transfer sequence in concert with a signal sequence to give inversion of protein topology in the endoplasmic reticulum. Arch Biochem Biophys 2003; 412: 259–266
- Breyton C, Haase W, Rapoport TA, Kuhlbrandt W, Collinson I. Three-dimensional structure of the bacterial protein-translocation complex SecYEG. Nature 2002; 418: 662–665
- Cannon KS, Clemons WM, Jr, Or E, Shibata Y, Rapoport TA. Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. J Cell Biol 2005; 169: 219–225
- Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 1998; 94: 795–807
- Carlos JL, Paetzel M, Brubaker G, Karla A, Ashwell CM, Lively MO, Cao G, Bullinger P, Dalbey RE. The role of the membrane-spanning domain of type I signal peptidases in substrate cleavage site selection. J Biol Chem 2000; 275: 38813–38822
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem 1981; 256: 1604–1607
- Lemberg MK, Bland FA, Weihofen A, Braud VM, Martoglio B. Intramembrane proteolysis of signal peptides: an essential step in the generation of HLA-E epitopes. J Immunol 2001; 167: 6441–6446
- Kim AC, Oliver DC, Paetzel M. Crystal structure of a bacterial signal Peptide peptidase. J Mol Biol 2008; 376: 352–366