Abstract
The effect of dolichol C95 on the structure and thermotropic phase behaviour of dipalmitoylphosphatidylcholine, dipalmitoylphosphatidylethanolamine and stearoyloleoylphosphatidylethanolamine has been examined by synchrotron X-ray diffraction and differential scanning calorimetry. The presence of dolichol C95 had no detectible effects on the temperature of either the gel to ripple or the ripple to liquid-crystal phase transition of dipalmitoylphosphatidylcholine. A proportionate increase of a few degrees in the temperature of the gel to lamellar liquid-crystal phase transition is observed in dispersions of dipalmitoylphosphatidylethanolamine and significantly there is a decrease in the temperature of the lamellar to non-lamellar phase transition of stearoyloleoylphosphatidylethanolamine. There was no significant change in the bilayer repeat spacing of all three mixed dispersions in gel phase in the presence of up to 20 mol% dolichol C95. Electron density calculations showed that there was no change of bilayer thickness of dipalmitoylphosphatidylcholine with incorporation of up to 7.5 mol% dolichol C95. These data suggest that effect of dolichol on the phospholipid model membranes depend on both the head group and the hydrocarbon chains of the phospholipid molecules. The presence of dolichol in phosphatidylcholine bilayers conforms to a model in which the polyisoprene compound is phase separated into a central domain sandwiched between the two monolayers in gel phase. In bilayers of phosphatidylethanolamines dolichol tends to stabilize the bilayers in gel phase at low temperatures and destabilize the bilayers in lamellar disordered structure at high temperatures. Non-lamellar structures coexist with lamellar disordered phase over a wide temperature range suggesting that dolichol is enriched in domains of non-lamellar structure and depleted from lamellar phase. These findings are useful to understand the function of dolichol in cell membranes.
| Abbreviations | ||
| DPPC | = | 1,2-dipalmitoyl-sn-phosphatidylcholine |
| DPPE | = | 1,2-dipalmitoyl-sn-phosphatidylethanolamine |
| SOPE | = | 1-stearoyl-2-oleoyl-sn-phosphatidylethano-lamine |
| SAXS | = | small-angle X-ray scattering (2θ = 0.043–7.9°) |
| WAXS | = | wide-angle X-ray scattering (2θ = 8–60°) |
| Lα | = | lamellar liquid-crystal phase |
| Lβ | = | lamellar gel phase |
| HII | = | inverted hexagonal phase |
Introduction
Dolichols isolated from mammalian tissues are hydrophobic linear polymers, consisting of between 16 and 23 unsaturated isoprene residues, with a hydroxyl group at one end. The molecular conformation of dolichol C95, based on small-angle X-ray scattering and molecular mechanics data is reported to be rod-shaped with a length of 5.3 nm and a width of 3.1 nm Citation[1]. The molecule is believed to consist of three geometrical regions involving a central coiled segment and two flanking domains Citation[2]. Dolichols are present as minor components in membranes of most living organisms from bacteria to mammals, playing a role in the biosynthesis of sugar heteropolymers and glycoproteins Citation[3–6]. Dolichols serve to activate and anchor sugars to membranes and form adducts with proteins during post-translational modification. Thus they assist in assembly of oligosaccharide derivatives from monosaccharide building blocks and the transfer of these oligosaccharides to proteins in the process of N-glycosylation whereby the sugars are attached to amino acids such as asparigine residues.
It has been reported that dolichol enhances vesicle fusion and that dolichol phosphate stimulates the fusion of rat liver microsomes and unilamellar lipid vesicles Citation[7]. There is evidence that dolichol plays a role in membrane traffic within the trans Golgi network, the plasma membrane and the lysosomes Citation[8], and has been assigned as a biomarker in the process of ageing Citation[9]. On the basis of 1H-NMR studies, dolichol has been shown to act as a scavenger of free radicals in cell membranes in which it protects polyunsaturated fatty acids from peroxidation and itself undergoes decomposition Citation[10]. There is other evidence of the role of dolichol as a free radical scavenger in certain diseases such as scrapie and sarcosidosis Citation[11], Citation[12].
The matrix of biological membranes consists of a bilayer of phospholipids into which sterols and small amounts of other lipids like fat-soluble vitamins and dolichols are interpolated. Dolichol is distributed widely in the different membranes of living cells and is particularly enriched in lysosomal membranes Citation[13]. The distribution within the lipid bilayer matrix, however, is not random. There is evidence from model membrane studies that dolichol does not promote the creation of membrane rafts isolated from liposome dispersions by detergent extraction methods and nor is dolichol found in the detergent resistant fraction Citation[14]. Based on a number of biophysical studies including ESR spectroscopic probe measurements Citation[15], Citation[16], 2H-NMR Citation[17], Citation[18] and 31P-NMR spectroscopy Citation[19] Troy and co-workers proposed a model of the location of dolichol C95 in phospholipid bilayers. In this model dolichol C95 is said to be sandwiched between the two lamellae of the phospholipid bilayer. A more precise positioning of the molecule has been proposed on the basis of energy minimization and molecular modelling calculations which place dolichol C95 in the hydrophobic domain of fluid bilayers of dimyristoylphosphatidylcholine with the hydroxyl group in proximity of the carbonyl groups of the phospholipid but with the remainder of the molecule shielded from contact with water at the surface of the bilayer Citation[20].
To further characterize the location and interaction of dolichol C95 in phospholipid model membranes we have examined co-dispersions of bilayer-forming and non-bilayer forming phospholipids with varying proportions of dolichol C95. The polymorphism of lipid bilayers has been well characterized by X-ray diffraction methods Citation[21] and differential scanning calorimetry has been used extensively to examine thermotropic phase behaviour Citation[22]. In this study, bilayers of 1,2-dipalmitoyl-sn-phosphatidylcholine (DPPC), 1,2-dipalmitoyl-sn-phosphatidylethanolamine (DPPE) and 1-stearoyl-2-oleoyl-sn-phosphatidylethanolamine (SOPE) containing varying proportions of dolichol C95 have been examined using synchrotron X-ray diffraction and differential scanning calorimetry. The results provide evidence supporting the current model of the disposition of dolichol C95 in phospholipid bilayers and provide additional information on the effect of dolichol on the creation of non-lamellar phases.
Materials and methods
Sample preparation
DPPC, DPPE and SOPE were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Dolichol C95 from porcine liver was purchased from Sigma-Aldrich (St Louis, MO, USA). The lipids were dissolved in chloroform and mixed in appropriate proportions to achieve the desired molar fractions (mol% = 100%×mole dolichol /(mole dolichol + mole phospholipid)). The solvent was evaporated under a stream of oxygen-free dry nitrogen and stored for 24 h in vacuo to remove any remaining traces of solvent. The lipid mixtures were hydrated at 80°C for at least 1 h and dispersed by a rotamixer until homogeneous dispersions were obtained. The lipid dispersions were stored at 0–4°C for 24 h before examination. The method of preparation and storage gave reproducible phase behaviour when samples prepared at different times were examined by X-ray diffraction.
Simultaneous X-ray diffraction/calorimetric methods
Experiments were performed at station 8.2 of the Daresbury Synchrotron Radiation Laboratory, U.K. The combination of a quadrant detector, an INEL detector and a differential scanning calorimetry system was used simultaneously. The beam line generates a flux of 4×1010 photons per second with a focal spot size of 0.3×3 mm2 (V×H) when the synchrotron radiation source is operating at a nominal 200 mA. The samples were mounted in a slot (1×5 mm) cut in a 1 mm thick copper plate sandwiched between a pair of thin mica sheets. The sandwich was clamped to the silver block containing the temperature sensing and modulating elements of a cryomicroscope stage (Linkam Scientific Instruments Ltd., UK). Temperature scans in heating, cooling and reheating modes were performed at 2°/min. X-ray scattering intensities at small angles (2 θ = 0.043–7.9°) and wide-angles (2θ = 8–60°) were recorded using the multiwire quadrant detector and the INEL curved linear-wire detector (INstrumentation Electronique, France), respectively. Data were acquired in 400 consecutive time frames of 5s and separated by a dead time between frames of 50 ms. To obtain simultaneous synchrotron X-ray diffraction and differential scanning calorimetric measurements samples of DPPC and dolichol were transferred to aluminium calorimeter pans and sealed with a cap of aluminium foil 15 µm thick.
Experimental data were analysed using the OTOKO software (EMBL, Hamburg, Germany) programme Citation[23]. Scattering intensities at low-angles were corrected for fluctuations in beam intensity and detector response recorded from a 55Fe source. Spatial calibrations were obtained using 21-orders of wet rat-tail collagen (d = 67 nm) Citation[24]. The scattering intensity data recorded by the INEL detector was corrected for scattering from an empty cell and spatial calibrations were established from high-density polyethylene (0.4166, 0.3780, 0.3014 nm) Citation[25]. The reciprocal spacing S = 1/d = 2 sin(θ)/λ, where d, λ, θ are the repeat distance, X-ray wavelength and the diffraction angle, respectively. To calculate electron density profiles the Bragg peaks from 5-orders of lamellar reflections were fitted by Voigt functions using PeakFit software (MMIV Systat Software, Inc). A Lorentz correction was applied to the powder diffraction images by multiplying each peak intensity (peak area) by its corresponding wave vector s2. Finally, the square root of the corrected peak intensity determined the form factor F of each respective reflection. The electron density contrast was calculated by the Fourier synthesis.1 where F(h,k) is the amplitude of the peak at the position s(h, k), h,k are the Miller indices and a(h,k) is its corresponding phase. For centrosymmetric structures, as in multilamellar dispersions, the phases for each diffraction order are either ‘ + 1’ or ‘ − 1’. At 22°C, five orders of reflection of the gel phases could be observed. The electron density distribution across the unit cell was calculated for all possible combinations of phase angles and the only plausible fit (-- + --) was used. Differential scanning calorimetric data was dumped simultaneously at 1 sec intervals onto the station computer.
Results
The effect of dolichol on the phase behaviour of saturated phosphatidylcholine
To determine the effect of dolichol on the phase behaviour of saturated phosphatidylcholine, mixtures of dolichol C95 and DPPC were examined by differential scanning calorimetry and synchrotron X-ray diffraction simultaneously. shows the calorimetric scans of different mixtures during the initial heating and subsequent cooling scan; a reheating scan was also recorded (data not shown). Incorporation of up to 10 mol% of dolichol C95 had little effect on the temperature of the pre and main phase transitions. In all mixtures, both of the pre (35°C) and main (41°C) phase transitions appeared during heating scans (a), which is consistent with the corresponding X-ray diffraction data ().
Figure 1. Differential scanning calorimetric heating (a), cooling (b) thermograms of dipalmitoylphosphatidylcholine codispersed with dolichol C95. The proportion of dolichol C95 (mol%) in the phospholipid dispersion is indicated on the respective scan.
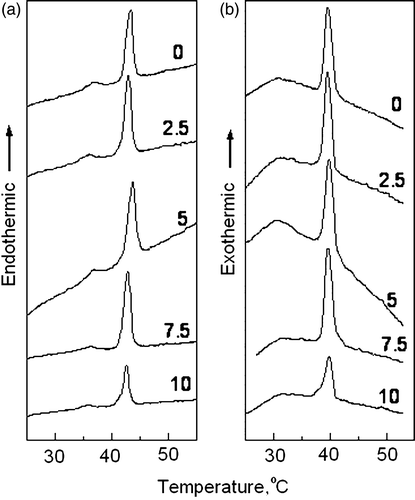
Figure 2. Plots of successive small-angle X-ray scattering intensity profiles versus reciprocal spacing recorded of a fully hydrated dispersion of dipalmitoylphosphatidylcholine (a) and a codispersion of 10 mol% dolichol C95 in dipalmitoylphosphatidylcholine (b) recorded during a heating scan at 2°/min. Each diffraction pattern represents scattering accumulated in 7 seconds.
The pretransition, however, was not observed in successive cooling and reheating scans. The temperature of the main phase transition seen in the first heating scans is identical to that recorded in the second heating scan (data not shown) but has a temperature hysteresis, judged from the peak temperature of the transition, of about 2°C during cooling scans (b).
The structural parameters of binary mixtures of dolichol C95 and DPPC obtained from synchrotron X-ray diffraction measurements were consistent with the thermal results from differential scanning calorimetry. shows small-angle X-ray scattering intensity profiles of pure phospholipid and phospholipid co dispersed with 10 mol% dolichol C95 recorded during an initial heating scan from 20-55°C at 2°/min. A reheating scan performed immediately after the cooling scan (data not shown) showed that the phase transitions were essentially a reversal of the cooling scans. The gel phase in reheating scans was transformed into liquid-crystalline phase as judged by a transition of a sharp WAXS peak centred at about 0.42 nm into a broad scattering band at about 0.46 nm at precisely the same temperature as that observed in the initial heating scans.
The most conspicuous difference between the SAXS diffraction profiles of the pure phospholipid and the co dispersion of 10 mole% dolichol C95 with DPPC is that the Bragg peaks are considerably broadened by the presence of dolichol. This is the case in both gel and fluid phases. The most probable explanation for peak broadening is that there is less order in the bilayer repeats when dolichol is present. Phospholipids with hydrocarbon chain lengths of equal carbon number are known to form planar bilayers compared with phospholipids with asymmetric hydrocarbon chain lengths where the surface is rough and consequently the stacking would be expected to be disordered.
Comparison of the temperature-dependent lamellar repeat spacings of fully hydrated dispersions of DPPC containing up to 10 mol% dolichol is shown in A. It can be seen from these data that lamellar repeat spacings of the multilamellar liposomes containing up to 10mole% dolichol C95 show almost identical patterns as a function of temperature during heating scans. This suggests that the presence of relatively high proportions of dolichol C95 in bilayers of DPPC neither causes a significant perturbation of the temperature of the gel to ripple and ripple to liquid-crystal phase transitions, nor changes the lamellar repeat spacings of the bilayers.
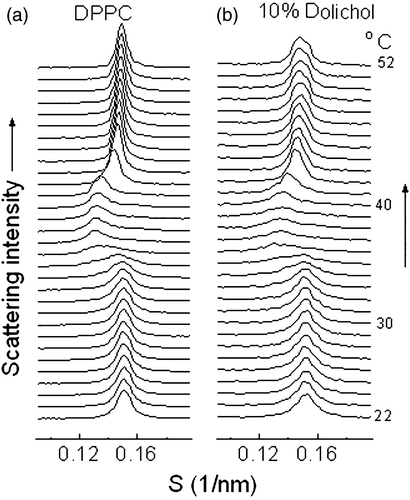
Figure 3. (A) Bilayer repeat spacing versus temperature during heating scans of mixed aqueous dispersions of dipalmitoylphosphatidylcholine containing different proportions of dolichol C95. The proportion of dolichol C95 (mol%) in the phospholipid is indicated on the respective curves. (B) Relative electron density distribution across the lamellar repeat of dispersions of dipalmitoylphosphatidylcholine without and with 7.5 mol% dolichol C95 at 22°C. (C) Wide-angle X-ray scattering intensity profiles recorded at 22°C from aqueous dispersions of DPPC and DPPC containing indicated proportions of dolichol C95.
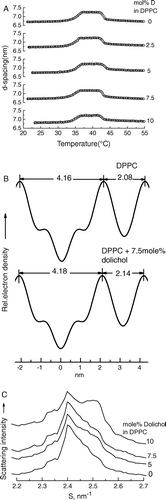
To investigate the effect of dolichol C95 on the structure of bilayers relative electron density calculations through the lamellar repeat were performed using five orders of reflection of the phospholipid in gel phase at 22°C (B). The profiles were calculated using the phase combination (−, −, +, −, −), which was obtained by examination of all possible combinations of phase angles. The set was the only combination found to provide a plausible solution of a centro symmetric structure with two electron dense peaks flanking a region of relatively low electron density. This phase combination has been reported consistently for the DPPC in the gel phase using lattice swelling methods Citation[26–29] to obtain phase angles. The calculated relative electron density profiles showed that there was no significant difference in bilayer thickness (dpp=4.16 nm for DPPC and 4.18 nm for DPPC containing 7.5 mole% dolichol C95). The thickness of the water layer of pure DPPC (dw=2.08 nm) increases by 0.06 nm with the inclusion of 7.5 mol% dolichol C95 in the phospholipid dispersion. Moreover, assuming the relative electron densities at the centre of the water layer and the phosphate groups of the bilayers of DPPC and bilayers of DPPC containing 7.5 mole% dolichol are the same no difference in electron density in the centre of the respective bilayers can be distinguished
Although five orders of reflection were used to calculate the electron density profiles the resolution obtained was not sufficient to distinguish any difference due to the presence of dolichol in the bilayer. This may be due to the way dolichol is distributed in the bilayer such that it does not contribute to coherent scattering in the region of low electron density when present in a proportion of 7.5 mole%.
To check whether the additional hydrocarbon of the dolichol was accommodated within the bilayer by an increased angle of tilt of the hydrocarbon chains of the phospholipid with respect to the bilayer plane an examination of the scattering intensity in the wide-angle region was undertaken. The results are presented in C as wide-angle X-ray scattering intensity profiles recorded at 22°C from dispersions containing between 0 and 10 moles% dolichol. This shows that the presence of increasing proportions of dolichol in DPPC results in an increasing angle of tilt of the hydrocarbon chains with respect to the bilayer normal as evidenced by the scattering intensity of the shoulder on the high-angle side of the peak of d-spacing 0.42 nm.
The effect of dolichol on the phase behaviour of saturated phosphatidylethanolamine
Aqueous dispersions of phosphatidylethanolamines are known to form lamellar gel and liquid-crystal phases as well as hexagonal-II phase at higher temperatures Citation[30]. Co-dispersions of dolichol C95 and DPPE were examined using synchrotron X-ray diffraction to investigate the effect of dolichol on the phase behaviour of saturated phosphatidylethanol-amine. The results are presented in . A shows a comparison of small-angle X-ray intensity patterns of dispersions of DPPE containing up to 20 mol% dolichol at 58°C and 70°C, respectively. The SAXS intensity patterns indicate that only lamellar phases are present at these temperatures. It can also be seen that the repeat spacing of the bilayers was the same regardless the proportion of dolichol in the mixture, i.e., 6.3 nm in gel phase (58°C) and 5.4 nm in liquid-crystal phase (70°C). It is noteworthy that with increasing proportions of dolichol C95 in the phospholipid the Bragg peaks become progressively broader suggesting that the multilamellar arrangement is becoming less ordered.
Figure 4. (A) Static small-angle X-ray diffraction patterns of dipalmitoylphosphatidylethanolamine containing 0, 1, 10 and 20 mol% dolichol C95 at 58°C (a) and 70°C, respectively. (B) Normalized X-ray scattering intensities of the wide-angle peaks centred at 0.43nm recorded from mixed aqueous dispersions of DPPE containing 1 (□);10 (○); 20 (Δ) mol% dolichol C95.
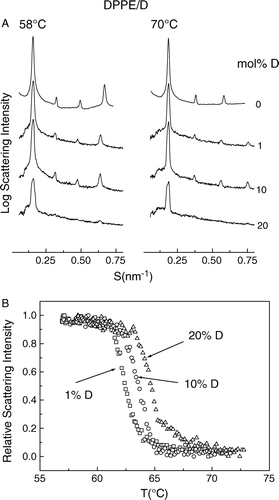
The effect of dolichol C95 on the gel to liquid-crystal phase transition of DPPE was determined from the X-ray scattering intensity profiles in the wide-angle region. Only single reflection peaks were observed in the wide-angle scattering region for all mixtures examined (data not shown). The normalized scattering intensity of the diffraction peak centred at 0.43 nm of phospholipid in the gel phase, corresponding to a hexagonal packing of the acyl chains of the phospholipid as a function of temperature, is presented in c. This shows that with increasing proportions of dolichol C95 in the phospholipid there is a progressive increase of the transition temperature from gel to liquid-crystal phase. Thus Tm increases from 62.5–65°C on increasing the proportion of dolichol C95 from 1–20 mole% B.
The effect of dolichol on the phase behaviour of unsaturated phosphatidylethanolamine
To investigate the effect of dolichol on the structure and thermotropic phase behaviour of unsaturated phosphatidylethanolamine mixed aqueous dispersions of SOPE and dolichol C95 were examined in the temperature range 20–75°C using synchrotron X-ray diffraction methods. The structural properties of the pure hydrated phospholipid were recorded during thermal scans, and the SAXS/WAXS intensity profiles are shown in a and 5b, respectively. It can be seen that the phospholipid undergoes a gel to liquid-crystal (Lβ→Lα) phase transition at about 31°C. This is characterized by a decrease of the lamellar repeat spacing in the small-angle region (a) from 6.8 nm (20°C) to 5.6 nm (40°C) and a coincident change from a sharp symmetrical diffraction peak at 0.43 nm ( 20°C) in the wide-angle scattering region to a broad peak centred at 0.45 nm (40°C) (b). An inverted hexagonal phase (HII) was first observed at 58°C and this structure completely replaces the Lα phase on heating to 68°C.
Figure 5. Plots of successive SAXS (a) /WAXS (b) intensity profiles at 2° intervals versus reciprocal spacing as a function of temperature of a dispersion of SOPE recorded during temperature scans at 2°/min heating from 20–70°C. SAXS (c) /WAXS (d) intensity profiles recorded from a codispersion of 1 mol% dolichol C95 in SOPE during an identical heating scan from 20–74°C. Each diffraction pattern represents scattering accumulated in 5 seconds.
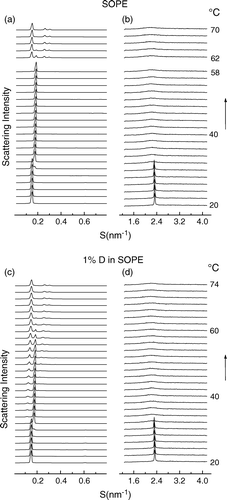
The effect of including 1 mole% dolichol C95 in the SOPE dispersion on SAXS/WAXS intensity profiles on heating from 20–74°C are shown in c and d, respectively. There was no significant difference in the gel to liquid-crystal phase transition temperature due to the presence of dolichol C95 in the phospholipid. However, an HII phase structure, assigned from the diffraction orders in a sequence 1:1/v3:1/v4, appeared in the SAXS intensity profile at a temperature of about 34°C, 24 degrees lower than the temperature where the HII phase of the pure SOPE dispersion appeared. Diffraction patterns characterising Lα and HII phases coexisted over a temperature range of about 36°. The coexisting Lα and HII phases were also observed in SOPE containing up to 20 mol% dolichol (data not shown), where non-lamellar phases were formed a few degrees above the Lβ → Lα phase transition, and which progressively replaced the Lα phase with increasing temperature. The coexistence of Lα and HII phases over a wide temperature range may be due to the segregation of dolichol into domains of HII phase and depletion from the other domains which retain their bilayer structure.
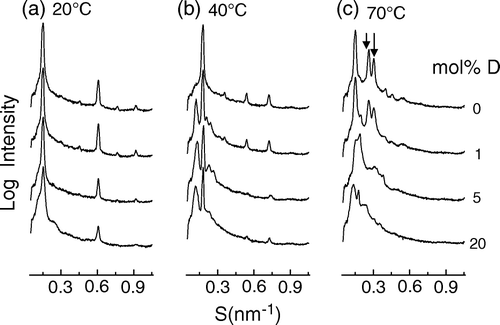
A comparison of static small angle X-ray diffraction profiles of aqueous dispersions of SOPE containing different amounts of dolichol at three different temperatures is presented in . It can be seen that at 20°C all of the mixtures were in lamellar configuration indexed by six-orders of a lamellar repeat, d = 6.72 nm (a), but 1 mol% dolichol induced non-lamellar structure at 40°C (d = 6.33 nm) at which temperature the pure phospholipid only showed a lamellar structure, d = 5.32 nm (b). At 70°C all dispersions had non-lamellar structures; pure phospholipid only showed HII phase (d = 6.70 nm), but the dispersions containing more than 1 mol% dolichols showed the mixture of HII (d = 6.30 nm) and cubic phases (c).
Figure 6. Small-angle X-ray scattering intensity patterns of mixed aqueous dispersions of SOPE containing 0; 1; 5 and 10 mol% dolichol C95 at three different temperatures. The profiles are plotted at the logarithm of intensity to emphasize the minor bands. Arrows indicate the 1/v3 and 1/v4 reflections from hexagonal-II phase.
Discussion
Although dolichols are relatively minor components of cell membranes they are widely distributed in nature. They are known to have important functions related to the formation of polysaccharides and biosynthesis of glycoproteins Citation[31]. Dolichol is believed to be an intermediate in N-glycosylation of nascent polypeptides which are cotranslationally glycosylated in a reaction catalysed by oligosaccharyl transferase in the lumen of the endoplasmic reticulum. The dolichol-P-P product is dephosphorylated in two steps by dolichyl pyrophosphate phosphatase and a second phosphatase to produce dolichol. Dolichol is significantly less polar than its phosphorylated derivatives and can readily traverse the membrane where it is again phosphorylated on the cytoplasmic surface of the endoplasmic reticulum preparatory to subsequent glycosylation Citation[32].
To study the effect of dolichol on the structure and phase behaviour of membranes, we examined bilayers of phospholipids DPPC, DPPE and SOPE using simultaneous synchrotron X-ray diffraction and differential scanning calorimetry. The results indicate that the effect of dolichol on the phase behaviour of the model membranes depends on the phospholipid class and particular molecular species of phospholipid with which it is co-dispersed. In bilayers of DPPC, dolichol tends to partition into a domain that does not perturb the phase transition behaviour of the phospholipid. In phosphatidylethanolamine bilayers, however, dolichol tends to stabilize the bilayers by shifting the temperature of the gel to liquid-crystal phase transition to higher temperatures. By contrast, the presence of only 1 mole% dolichol C95 in an unsaturated molecular species of phosphatidylethanolamine results in a marked decrease in the temperature of onset of transition to hexagonal-II phase and appearance of other non-lamellar phases.
Remarkably, the main transition, the patterns of ripple and metastable ripple structures of DPPC bilayers remained unchanged in the presence of up to 10 mol% of dolichol, suggesting that dolichols do not distribute homogeneously in the DPPC bilayers. Similar effects of other polyprenyl compounds like ubiquinones on the phase behaviour of DPPC have been reported Citation[33]. Because dolichol C95 assumes an S-form, and has a trans-trans-polycis conformation in biological membranes Citation[34–36] and has an amphipathic balance favouring a non-polar environment the evidence is consistent with the existence of dolichols in the form of clusters in the central plane of phospholipid bilayers. The same phase behaviour was observed in co-dispersions of dolichol and dimyristoylphosphatidylcholine mixtures Citation[37]. In these studies the possibility that dolichols were not co-dispersed with the phospholipid was discounted because dolichol co-eluted together with liposomes during gel filtration through Sepharose 4B. This is consistent with the present observation that the stacking order of DPPC bilayers is reduced in proportion to the amount of dolichol in the mixture. Interestingly, it was reported that the enthalpy of the pre-transition and main transition of DMPC was increased by the presence of dolichol but that dolichol had no significant effect on these parameters for DPPC bilayers Citation[37]. Other evidence that dolichol is incorporated into DPPC bilayers is that the presence of 10 mol% dolichol caused significant broadening of the X-ray diffraction peaks () of DPPC multilamellar liposomes; such an effect could not be explained by the presence of dolichol in a separate aqueous domain.
The absence of any effect of dolichol on the gel to liquid-crystal phase transition behaviour of DPPC is not consistent with models that intercalate dolichol between the hydrocarbon chains of the phospholipid in gel phase Citation[20]. Isoprenoid compounds with shorter hydrocarbon substituents in which the amphipathic balance is more favourable for hydrophilic affinity are known to intercalate between the hydrocarbon chains of the phospholipids and perturb the phase transition behaviour Citation[38]. The reason that dolichol C95 does not perturb the main and pre transitions of DPPC may be explained by the phase separation of the dolichol into the central region of the bilayers as the phospholipid is cooled from the liquid-crystal to the gel phase. Since the volume of hydrocarbon increases in the presence of dolichol it may be expected that the thickness of the bilayers would increase. This is clearly not the case as seen by the calculated electron density profiles of DPPC compared with phospholipid containing 7.5 mole% dolichol C95. A similar finding has been reported from neutron scattering studies of co-dispersions of 5 mole% ubiquinone-10 co-dispersed with DMPC Citation[39]. It may be assumed that the additional hydrocarbon is accommodated by an increased tilting of the hydrocarbon chains or, in the case of phospholipid in liquid-crystal phase, by an increase in area occupied by the phospholipid at the aqueous interface.
The effect of the presence of dolichol C95 on the lamellar phase transitions of DPPC differs from that of DPPE and SOPE in that there is an increase in the lamellar gel to liquid-crystal phase transition temperature of the ethanolamine phosphatides. Thus increasing the proportion of dolichol C95 up to 20 mol% resulted in an increase in the lamellar gel to liquid-crystal phase transition temperature from 63–65°C for DPPE, and from 31–33°C for SOPE. This could be due to the differences in sizes of the hydrated phosphorylcholine and phosphorylethanolamine head groups Citation[40]. The volumes of the anhydrous headgroups of choline and ethanolamine phosphatides are 0.324 nm3 and 0.243 nm3, respectively Citation[41]. The corresponding areas occupied by the phospholipid at the bilayer interface are 0.52 nm2 and 0.42 nm2 for DPPC and DPPE, respectively Citation[42]. The tighter packing of the phosphatidylethanolamines resulting in stronger van der Waals cohesive forces results in an elevation of the lamellar gel to liquid-crystal phase transition compared to the phosphatidylcholines and the additional cohesive forces in the presence of dolichol C95 may be the reason for the additional upshift in phase transition temperature of the mixtures. It may be noted that while the long axis of dolichol is found to be oriented parallel to the bilayer plane there is a considerable perturbation of the fatty acids in contact with dolichol so as to create increased hydrocarbon packing density in some domains and lowered density in others.
The effect of dolichol C95 on unsaturated molecular species of phosphatidylethanolamines is consistent with earlier reports of dolichol codispersed with dioleoylphosphatidylethanolamine Citation[37] and the known behaviour of alkanes Citation[43] and squalene Citation[44] on lowering the lamellar to hexagonal-II phase transition temperature. The propensity to induce non-lamellar structure depends on the length of the alkane. Thus short-chain but not long-chain alkanes alter the curvature and bending modulus of the monolayers comprising the bilayers Citation[45]. One consistent feature of the interaction of dolichol C95 with SOPE is that although the appearance of hexagonal-II structure in the dispersion occurs at a significantly lower temperature than in the absence of dolichol the non-lamellar phase does not completely replace the lamellar phase until the temperature of Th of the pure phospholipid is reached. This coexistence of lamellar and non-lamellar structures over a wide temperature range implies that the dolichol is not randomly distributed in the central domain of the bilayers but is enriched in domains of non-lamellar structure. This effect may explain the drastic effects of alkanes on the structure and stability of the membranes of living cells Citation[46].
Acknowledgements
Financial support was provided by the Human Frontier Science Program (RGP0016/2005C) and beamtime for the experiments was awarded by the Daresbury Laboratory. X.W. was supported by Chang Jiang Scholars Program. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Murgolo NJ, Patel A, Stivala SS, Wong TK. The conformation of dolichol. Biochemistry 1989; 28: 253–260
- Zhou GP, Troy FA. Characterization by NMR and molecular modeling of the binding of polyisoprenols and polyisoprenyl recognition sequence peptides: 3D structure of the complexes reveals sites of specific interactions. Glycobiology 2003; 13: 51–71
- Orlowski J, Machula K, Janik A, Zdebska E, Palamarczyk G. Dissecting the role of dolichol in cell wall assembly in the yeast mutants impaired in early glycosylation reactions. Yeast. 2007; 24: 239–252
- Kelleher DJ, Banerjee S, Cura AJ, Samuelson J, Gilmore R. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J Cell Biol 2007; 177: 29–37
- Krag SS. The importance of being dolichol. Biochem Biophys Res Commun 1998; 243: 1–5
- Ward WC, Guan Z, Zucca FA, Fariello RG, Kordestani R, Zecca L, Raetz CRH, Simon JD. Identification and quantification of dolichol and dolichoic acid in neuromelanin from substantia nigra of the human brain. J Lipid Res 2007; 48: 1457–1462
- Van Duijn G, Valtersson C, Chojnacki T, Verkleij AJ, Dallner G, De Kruijff B. Dolichyl phosphate induces non-bilayer structures, vesicle fusion and transbilayer movement of lipids: A model membrane study. Biochim Biophys Acta 1986; 861: 211–223
- Chojnacki T, Dallner G. The biological role of dolichol. Biochem J 1988; 251: 1–9
- Parentini H, Cavallini G, Donati A, Gori Z, Bergamini E. Accumulation of dolichol in older tissues satisfies the proposed criteria to be qualified a biomarker of aging. J Gerontol Ser A-Biol Sci Med Sci 2005; 60: 39–43
- Bergamini E, Bizzarri R, Cavallini G, Cerbai B, Chiellini E, Donati A, Gori Z, Manfrini A, Parentini I, Signori F, Tamburini I. Ageing and oxidative stress: A role for dolichol in the antioxidant machinery of cell membranes?. J Alzheimers Dis 2004; 6: 129–135
- Guan ZZ, Soderberg M, Sindelar P, Prusiner SB, Kristensson K, Dallner G. Lipid composition in scrapie-infected mouse brain: Prion infection increases the levels of dolichyl phosphate and ubiquinone. J Neurochem 1996; 66: 277–285
- Kumar AR, Kurup PA. Hypothalamic digoxin, hemispheric chemical dominance and sarcoidosis. Acta Neuropsychiat 2004; 16: 160–168
- Wong TK, Decker GL, Lennarz WJ. Localization of dolichol in the lysosomal fraction of rat liver. J Biol Chem 1982; 257: 6614–6618
- Keller RK, Arnold TP, Fliesler SJ. Formation of 7-dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith-Lemli-Opitz syndrome. J Lipid Res 2004; 45: 347–355
- McCloskey MA, Troy FA. Paramagnetic isoprenoid carrier lipids. 1. Chemical synthesis and incorporation into model membranes. Biochemistry 1980; 19: 2056–2060
- McCloskey MA, Troy FA. Paramagnetic isoprenoid carrier lipids. 2. Dispersion and dynamics in lipid membranes. Biochemistry 1980; 19: 2061–2066
- Deropp JS, Troy FA. Chemical synthesis and H-2 NMR investigations of poly isoprenols – dynamics in model membranes. Biochemistry 1984; 23: 2691–2695
- Deropp JS, Troy FA. H-2 NMR investigation of the organization and dynamics of polyisoprenols in membranes. J Biol Chem 1985; 260: 5669–5674
- Knudsen MJ, Troy FA. Nuclear magnetic-resonance studies of polyisoprenols in model membranes. Chem Phys Lipids 1989; 51: 205–212
- Zhou GP, Troy FA. NMR study of the preferred membrane orientation of polyisoprenols (dolichol) and the impact of their complex with polyisoprenyl recognition sequence peptides on membrane structure. Glycobiology 2005; 15: 347–359
- Cunningham BA, Bras W, Lis LJ, Quinn PJ. Synchrotron X-ray studies of lipids and membranes – a critique. J Biochem Biophys Meth 1994; 29: 87–111
- Spink CH. Differential scanning calorimetry. Biophysical tools for biologists: Vol 1. In Vitro Techniques 2008; 84: 115–141
- Boulin C, Kempf R, Koch MHJ, McLaughlin SM. Data appraisal, evaluation and display of synchrotron radiation experiments – hardware and software. Nucl Instr Meth Phys Res Section A. Q-Accelerators Spectrometers Detectors and Associated Equipment 1986; 249: 399–407
- Bigi A, Dovigo L, Koch MHJ, Morocutti M, Ripamonti A, Roveri N. Collagen structural organisation in uncalcified and calcified human anterior longitudinal ligament. Connective Tiss Res 1991; 25: 171–179
- Addink EJ, Beintema J. Polymorphism of crystalline polypropylene. Polymer. 1961; 2: 185–193
- Torbet J, Wilkins MHF. X-ray diffraction studies of lecithin bilayers. J Theoret Biol 1976; 62: 447–458
- Franks NP, Lieb WR. Structure of lipid bilayers and the effects of general-anaethetics – X-ray and neutron-diffraction study. J Mol Biol 1979; 133: 469–500
- McIntosh TJ, Simon SA. Hydration force and bilayer deformation – a re-evaluation. Biochemistry 1986; 25: 4058–4066
- Yu ZW, Quinn PJ. Phase-stability if hosphatidylcholines in dimethylsulfoxide solutions Biophys J 1995; 69: 1456–1463
- Rappolt M, Hodzic A, Sartori B, Ollivon M, Laggner P. Conformational and hydrational properties during the L(beta)- to L(alpha)- and L(alpha)- to H(II)-phase transition in phosphatidylethanolamine. Chem Phys Lipids 2008; 154: 46–55
- Shang J, Gao NG, Kaufman RJ, Ron D, Harding HP, Lehrman MA. Translation attenuation by PERK balances ER glycoprotein synthesis with lipid-linked oligosaccharide flux. J Cell Biol 2007; 176: 605–616
- Rush JS, Gao N, Lehrman MA, Waechter CJ. Recycling of dolichyl monophosphate to the cytoplasmic leaflet of the endoplasmic reticulum after the cleavage of dolichyl pyrophosphate on the lumenal monolayer. J Biol Chem 2008; 283: 4087–4093
- Katsikas H, Quinn PJ. The distribution of ubiquinone-10 in phospholipid-bilayers – a study using differential scanning calorimery. Eur J Biochem 1982; 124: 165–169
- Burgos J, Morton RA, Pennock JF, Hemming FW. Dolichol – a naturally-occurring C100isoprenoid alcohol. Biochem J 1963; 88: 470–476
- Adair WL, Robertson S. Absolute-configuration of dolichol. Biochem J 1980; 189: 441–445
- Chojnacki T, Palamarczyk G, Jankowski W, Krajewskarychlik I, Szkopinska A, Vogtman T. The enzymatic formation of dolchyl phosphate mannose from C-3 enantomeric dolichyl phosphates. Biochim Biophys Acta 1984; 793: 187–192
- Valtersson C, Vanduyn G, Verkleij AJ, Chojnacki T, Dekruijff B, Dallner G. The influence of dolichol, dolichol esters and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J Biol Chem 1985; 260: 2742–2751
- Katsikas H, Quinn PJ. The polyisoprenoid chain-length influences the interaction of ubiquinones with phospholipid-bilayers. Biochim Biophys Acta 1982; 689: 363–369
- Hauss T, Dante S, Haines TH, Dencher NA. Localization of coenzyme Q(10) in the center of a deuterated lipid membrane by neutron diffraction. Biochim Biophys Acta-Bioenerg 2005; 1710: 57–62
- Damodaran KV, Merz KM. A comparison of DMPC-based and DLPE-based lipid bilayers. Biophys J 1994; 66: 1076–1087
- Small DM. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res 1967; 8: 551–558
- Marra J. Direct measurements of attractive van der Waals and adhesion forces between uncharged lipid bilayers in aqueous-solutions. J Colloid Interface Sci 1986; 109: 11–20
- Leventis R, Fuller N, Rand RP, Yeagle PL, Sen A, Zuckermann MJ, Silvius JR. Molecular-organization and stability of hydrated dispersions of headgroup-modified phosphatidylethanolamine analogs Biochemistry 1991; 30: 7212–7219
- Lohner K, Degovics G, Laggner P, Gnamusch E, Paltauf F. Squalene promotes the formation of nonbilayer stuctures in phospholipid model membranes. Biochim Biophys Acta 1993; 1152: 69–77
- Chen Z, Rand RP. Comparative study of the effects of several n-alkanes on phospholipid hexagonal phases. Biophys J 1998; 74: 944–952
- Urbina P, Alonso A, Contreras FX, Goni FM, Lopez DJ, Montes LR, Sot J. Alkanes are not innocuous vehicles for hydrophobic reagents in membrane studies. Chem Phys Lipids 2006; 139: 107–114