Abstract
Gastrointestinal bacteria, like Escherichia coli, must remove bile acid to survive in the gut. Bile acid removal in E. coli is thought to be mediated primarily by the multidrug efflux pump, AcrB. Here, we present the structure of E. coli AcrB in complex with deoxycholate at 3.85 Å resolution. All evidence suggests that bile acid is transported out of the cell via the periplasmic vestibule of the AcrAB-TolC complex.
| Abbreviations | ||
| DDM | = | dodecyl-B-D-maltopyranoside |
| DOC | = | deoxycholate, E. coli, Escherichia coli |
| GFP | = | Green Fluorescent Protein |
| MIC | = | minimum inhibitory concentration |
| Ni-NTA | = | nickel-nitrilotriacetic acid |
| RND | = | resistance nodulator cell division |
| TEV | = | tobacco etch virus protease |
| IMAC | = | Immobilized Metal Affinity Chromatography |
Introduction
Gastrointestinal bacteria such as Escherichia coli must remove bile acid to survive in the gut Citation[1–3]. In E. coli the resistance nodulator cell division (RND) transporter AcrB works in concert with the outer membrane protein TolC, and a membrane fusion partner AcrA, to export various antibiotics in a proton-driven process Citation[4]. The AcrAB-TolC complex also removes bile acid Citation[2], Citation[3], Citation[5], a detergent like amphipathic molecule that makes up ∼ 1% of the fluid within the intestine. Bile acid significantly induces expression of the acrAB operon by acting on specific transcriptional activators Citation[6], Citation[7], and triggers expression of virulence genes to alter pathogenic behaviour Citation[8]. Deletion of the acrAB operon leaves cells highly sensitive to bile acid, with a 10-fold decrease in the minimum inhibitory concentration (MIC) to deoxycholate Citation[2]. The bile acid deoxycholate was found to be a direct transport competitor of the substrate fluorophospholipid in an AcrAB reconstituted system Citation[5]. The high-resolution structure of AcrB was first solved as a symmetrical homotrimer in 2002 Citation[9], and later as a physiological asymmetrical trimer Citation[10], Citation[11], Citation[12]. Each protomer is thought to represent an alternate single state of a three-step transport cycle designated loose (L), tight (T), and open (O) Citation[13]; (i) in the L state the periplasmic vestibule is open for substrate entry (ii) in the T state the vestibule tunnel is extended to include a hydrophobic binding pocket and (iii) in the O state, the periplasmic vestibule closes, and the central pore opens for substrate to exit. Most likely the sequential states occur by a rotating mechanism driven by proton translocation across the membrane-embedded portion of AcrB Citation[10–12].
Here, to confirm that AcrB is unequivocally required for bile acid efflux in E. coli we first constructed a single acrB deletion mutant and monitored the effect of bile acid to growing cells. To elucidate the export pathway for bile acid, we solved the structure of AcrB in complex with deoxycholate.
Methods and materials
Construction of AcrB deletion strain
The DacrB::KmR mutation from the Keio collection was moved into strain BL21(DE3) by bacteriophage P1-mediated transduction Citation[14]. After P1 transduction the BL21(DE3)DacrB::KmR mutant was checked by PCR as described previously Citation[14].
Bile acid growth assay
E. coli strains BL21(DE3) and BL21(DE3)▵ acrB::KmR were cultured at 37°C in 50 ml of Luria-Broth (LB) containing kanamycin 50 mg/ml in a 250 ml shaker flask set at 220 r.p.m. Densities of cells were monitored at 600 nm at regular time intervals. At OD600=0.7 – 0.8, a final concentration of 5 mM deoxycholate (Sigma, UK) was added and optical densities were continuously measured.
Purification of AcrB
AcrB forms a natural hexahistidine tag (i.e. AcrB forms a trimer and has 2 histidines at its very C-terminus), which binds to Ni-NTA resin in addition to targeted membrane protein-GFP-His8 fusions in our GFP-based pipeline Citation[15], in which targets are typically purified from BL21(DE3) derived membranes with buffer containing 20 mM Tris/HCl pH 7.5, 150 mM NaCl, and 1% dodecyl-β-D-maltopyranoside (DDM, Anatrace, USA). To isolate AcrB we collected protein that bound to the second Ni-NTA step in our standard purification pipeline in buffer containing 250 mM imidazole, 20 mM Tris/HCl pH 7.5, 150 mM NaCl, and 0.03% dodecyl-β-D-maltopyranoside. To separate AcrB from other contaminants and tags, AcrB was loaded onto a Superdex 200 10/300 (GE healthcare, UK) gel filtration column in 20 mM Tris/HCl pH 7.5, 0.1 M NaCl, and 0.03% DDM at a flow-rate of 0.4 ml/min. The protein peak corresponding to AcrB was collected and concentrated to 8 mg/ml.
Structure determination of AcrB in complex to bile acid
Native AcrB crystals were grown from mixing equal volumes of a protein solution containing 8 mg/ml of AcrB in 20 mM Tris/HCl pH 7.5, 150 mM NaCl, 0.03% DDM and 0.01% DM and reservoir solution containing 10% PEG 4000, 0.1 M HEPES pH 7.5, 0.1 M ammonium sulphate and 22% v/v glycerol (MemGold, Molecular dimensions, UK) [16] . Crystals appeared within two days and grew to final size within a week. Crystals were soaked with 50 mM sodium deoxycholate (Sigma, UK) for one month prior to data collection. The crystals were flash frozen in liquid nitrogen prior to data collection. A full data set was collected to 3.85 Å resolution at beamline I04 at Diamond Light Source ID14-4 at ESRF () at 100K. The data were integrated with XDS Citation[17], scaled with SCALA Citation[18] and phased by molecular replacement using Phaser Citation[19] from the CCP4 suite of crystallography programs. PDB file 1 1IWG (native AcrB) Citation[9] was used for molecular replacement. The structure was refined using REFMAC Citation[20]. Model building and electron density inspection was carried out in COOT Citation[21]. Figures were prepared with PYMOL Citation[22] and CCP4mg Citation[23]. summarizes the data collection and refinement statistics for the AcrB-deoxycholate complex. Coordinates and structure factors have been deposited in the RCSB Protein Data Bank with PDB ID code 2W1B.
Table I. Data collection and refinement statistics.
Results and discussion
Single acrB E. coli deletion strain is hypersensitive to bile acid
A single acrB knockout E. coli strain was constructed to complement previous growth experiments measured in an acrAB operon deletion mutant strain Citation[2].
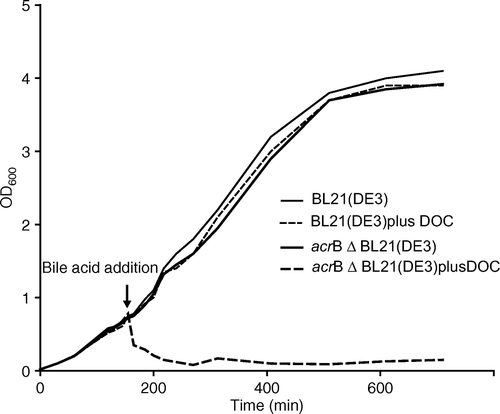
Cells were grown to mid-log phase and challenged with the bile acid deoxycholate. As shown in , cells continue to grow in the presence of 5 mM bile acid in a wild-type E. coli strain (thin dashed line), but growth is severely impaired in the acrB deletion strain (thick-dashed line). Within two hours the cell density is declined to almost background levels, OD600=0.08. As expected, there was some recovery of cell growth in the acrB deletion strain, reaching an OD600 of 0.4 after 12 h. Putative RND transporter genes mdtABC, are noted to form some limited resistance against bile acid in an acrAB deletion strain Citation[24], Citation[25]. Nevertheless, recovery of cell growth was minimal as monitored for 24 h, and cells failed to grow in freshly inoculated media (results not shown).
Figure 1. Comparison of growth of an E. coli wild-type strain with an acrB deletion strain in the presence of deoxycholate (DOC). thin line, BL21(DE3) in absence of bile acid; thin dashed line, BL21(DE3) with 5 mM addition of deoxycholate; thick line, BL21(DE3)ΔacrB::KmR in absence of bile acid, thick dashed-line, BL21(DE3)ΔacrB::KmR with 5 mM addition of deoxycholate.
To understand the transport mechanism for bile acid, we purified and crystallized native AcrB in the presence of deoxycholate.
Isolation of AcrB
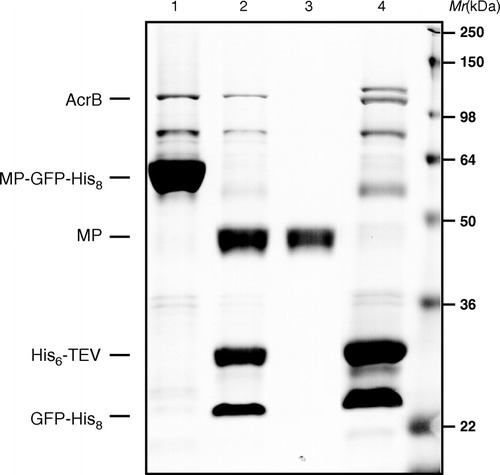
AcrB forms a natural hexahistidne tag due to the fact the last two residues are histidines and it oligomerizes into a trimer. AcrB is difficult to remove from IMAC purifications of your target protein, and is considered a problem for structural biologists as it crystallizes at only 5% of total purified membrane protein Citation[26]. One approach to avoid this problem is to cleave off the His-tag with a His-tagged site-specific protease and then to re-bind cleaved material. This is our standard approach for membrane protein purification in our laboratory as we work with a GFP-His8 fusion tag that is cleaved and removed prior to crystallization Citation[15]. Thus, we took advantage of this and recovered AcrB by eluting re-bound material from a second IMAC step, i.e. contaminants, GFP-His8 and His6-TEV protease (). AcrB was further purified by size exclusion chromatography and concentrated to 8 mg/ml for crystallization.
Figure 2. Fractions of a standard membrane protein-GFP-His8 purification were loaded onto a 12% SDS-polyacrylamide gel and then stained with Coomassie to illustrate isolation of native AcrB. (1) MP-GFP-His8 elution from first IMAC; (2) overnight His6-TEV protease digested material; (3) flow-through of second IMAC step; and (4) elution of bound material from second IMAC (bands corresponding to AcrB, MP-GFP-His8, His6-TEVp, and MP are as indicted on gel).
Biding site of deoxycholate
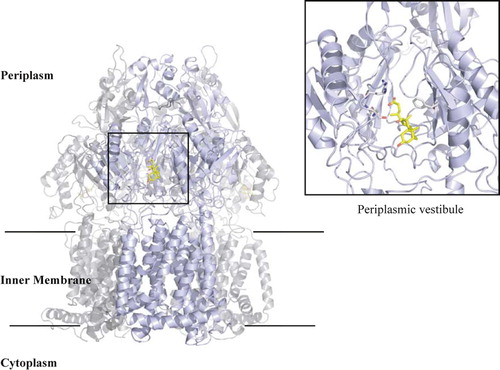
AcrB is a trimer with an overall ‘jellyfish’ shape’ composed of a central transport proton-dependant pump cavity, vestibule, and pore funnel Citation[9] (). After molecular replacement using the native AcrB structure and some model building of the overall structure, 2Fo-Fc and Fo-Fc electron density maps were calculated. Although native AcrB without ligand diffracts to 3.2 Å resolution in this preparation, bile acid complex crystals diffract only to 3.85 Å. This is typical for AcrB complexes with almost all obtained at lower than 3.5 Å resolution Citation[27], Citation[28].
Figure 3. Overall structure of AcrB trimer. The bound deoxycholate in the periplasmic vestibule is shown in yellow stick. Boxed area is a close-up view of the vestibule site with the deoxycholate and side chains that are involved in the binding shown in grey stick.
Inspection of the periplasmic binding cleft reveals positive density for bile acid, b. Binding of deoxycholate to the periplasmic cavity there were no observed conformational changes, as the structure can be superimposed with the other published substrate-bound structures with an rmsd of 1.06 Å over 960 Ca atoms. The binding site for deoxycholate is in a similar position to the published complexes Citation[27] rhodamine 6G Citation[27], ethidium bromide Citation[27], Phe-Arg-β-naphthylamide Citation[27], nafcillin Citation[27], and ciprofloxacin Citation[27] despite their molecular diversity (). The hydroxyl group of C-13 of the steroid ring of deoxycholate forms hydrogen bonds to Ser715, 2.51 Å, and carbon atoms C-17, C-20, C-21 and C-22 pack by van der Waals interactions to Phe664 (c). mutational analysis of these vestibule ligand binding residues showed that the transport activity of structurally different substrates was compromised in most cases Citation[27]. The only mutation to affect (almost) all substrates was the Phe664 to Ala mutant. Phe664 is clearly an important hydrophobic residue for substrate binding, and likewise for deoxycholate, coordinates most interactions. The C-13 OH-coordination with Ser715 is novel, as previous complex structures have no hydrogen bonding to their substrate. Although, the Ser715Ala mutant was noted to have no effect on broad activity, Ser715 could be a substrate-specific residue for deoxycholate. Indeed, this is the first AcrB structure with a naturally occurring substrate.
Figure 4. Binding of deoxycholate in the periplasmic vestibule of AcrB. (a) Electron density for native AcrB (purple), (b) deoxycholate bound prior to adding the ligand (light blue), (c) deoxycholate density after including it in the refinement (dark blue). The 2Fo-Fc maps are contoured at 1.0σ and deoxycholate (pink, contoured at 1.0σ) are shown for clarity, calculated after some model building (prior to deoxycholate being built). Some of the vestibule amino acid side chains are shown. Deoxycholate (yellow sticks) is included for reference. Nitrogen atoms are shown in blue and oxygen in red.
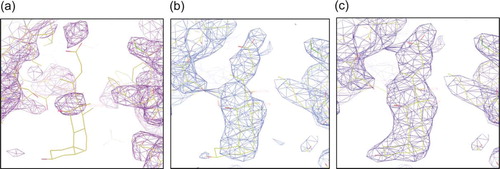
Figure 5. Alignment of deoxycholate (yellow) with ethidium (light orange), Phe-Arg-β-naphthylamide (light blue), and ciprofloxacin (pink). The residues involved in the binding of deoxycholate are shown as grey sticks. Arg717 is only shown as a reference since there is no direct contact with the deoxycholate.
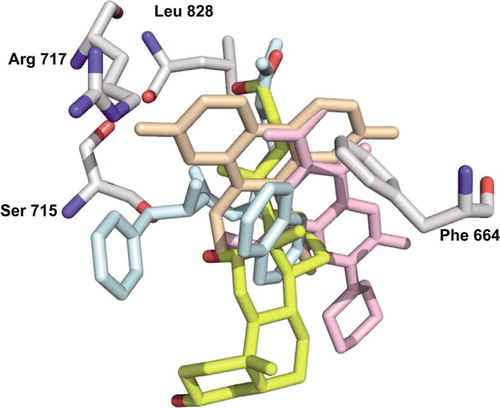
In contrast to foreign compounds rhodamine 6G, ethidium, dequalinium, and ciprofloxacin, which bind to the periplasmic vestibule and the top of the lipid-interface at the central cavity Citation[28], deoxycholate binds to the vestibule only. We suggest that the later interaction site is the biologically relevant interaction site, because it is in vectorial alignment to the binding site in the extended vestibule conformation (T).
The final electron density maps show positive density between the carboxyl group of C-23 and Leu828. A water molecule or sodium ion could be modelled and coordinated in the density. We prefer the latter as it would provide a counter-ion to deoxycholate; however, the resolution is insufficient to be certain. Interestingly, there is no density for YajC as observed in a recent native AcrB structure Citation[29], perhaps due to the differences in culture conditions and/or strain background used.
Transport state of deoxycholate-bound AcrB
The AcrB-deoxycholate complex was crystallized in the rhombohedral space-group, H32, all molecules are symmetrical, with the three-fold crystallographic axis running down the centre of the trimer. Under these conditions, only the L state of AcrB is observed. This state is either a crystal artefact, in which the loose conformation is preferentially crystallized, or a true physiological state in which all three vestibules are open Citation[13]. Detergents are also substrates for AcrB Citation[13], but in our native AcrB structure there is no apparent density that could indicate the binding of detergent molecules in these regions (a).
Concluding remarks
This is the first AcrB structure with a substrate that E. coli has had to evolve to continuously remove for its survival. It is apparent that deoxycholate is mainly exported by AcrB, and gains entry from the periplasmic space. Entry from the periplasm makes sense, slow passive diffusion into the cytoplasm would lead to high levels of bile acid, ∼ 1 mM is reported to be enough to compromise membrane integrity Citation[30]. This entry site is an attractive area for rational drug design. Indeed, the pathogenic bacterium Campylobacter jejuni fails to colonize the chicken gastrointestinal tract when it lacks a component of the homologous AcrAB efflux system Citation[1].
Acknowledgements
We would like to thank Dr Alexander Cameron for useful discussions. D.D was a recipient of an EMBO long-term fellowship. K.B is a RCUK Fellow. Funded by the Biotechnology and Biological Science Research Council (BBSRC) Membrane Protein Structure Initiative (to S.I.), the Swedish Research Council and the Center of Biomembrane Research through the Swedish Foundation of Strategic Research (to J.W.-d. G.). Data were collected at beam-line I04 at Diamond Light Source and ID14-4 at ESRF (France). We would also like to acknowledge the Membrane Protein Lab. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun 2003; 71: 4250–4259
- Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol 1995; 16: 45–55
- Thanassi DG, Cheng LW, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol 1997; 179: 2512–2518
- Tikhonova EB, Zgurskaya HI. AcrA, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J Biol Chem 2004; 279: 32116–32124
- Zgurskaya HI, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc Natl Acad Sci USA 1999; 96: 7190–7195
- Nikaido E, Yamaguchi A., Nishino K. AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J Biol Chem. 2008; 283: 24245–24253
- Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol Microbiol 2003; 48: 1609–1619
- Malik-Kale P, Parker CT, Konkel ME. Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression. J Bacteriol 2008; 190: 2286–2297
- Murakami S, Nakashima R, Yamashita E, Yamaguchi A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 2002; 419: 587–593
- Sennhauser G, Amstutz P, Briand C, Storchenegger O, Grutter MG. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol 2007; 5: e7
- Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 2006; 313: 1295–1298
- Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 2006; 443: 173–179
- Murakami S. Multidrug efflux transporter, AcrB-the pumping mechanism. Curr Opin Struct Biol 2008; 18: 459–465
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2006; 2: 2006–2008
- Drew D, Lerch M, Kunji E, Slotboom DJ, de Gier JW. Optimization of membrane protein overexpression and purification using GFP fusions. Nat Methods 2006; 3: 303–313
- Newstead S, Ferrandon S, Iwata S. Rationalizing alpha-helical membrane protein crystallization. Protein Sci 2008; 17: 466–472
- Kabsch WJ. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst 1993; 21: 916–924
- Evans PR. 1993. Data reduction. Proceedings of CCP4 Study Weekend, 1993, on Data Collection & Processing, 114–122.
- McCoy AJ Grosse-Kunstleve RW Adams PD Winn MD Storoni LC Read RJ. 2007. Phaser crystallographic software. J Appl Cryst 658–674.
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 1997; 53: 240–255
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004; 60: 2126–2132
- DeLano WL The PyMOL Molecular Graphics System. San CarlosCA, USA: DeLano Scientific LLC.
- Potterton L, McNicholas S, Krissinel E, Gruber J, Cowtan K, Emsley P, Murshudov GN, Cohen S, Perrakis A, Noble M. Developments in the CCP4 molecular-graphics project. Acta Crystallogr D Biol Crystallogr 2004; 60: 2288–2294
- Baranova N, Nikaido H. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J Bacteriol 2002; 184: 4168–4176
- Nagakubo S, Nishino K, Hirata T, Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J Bacteriol 2002; 184: 4161–4167
- Veesler DBS Cambillau C Sciara G. 2008. There is a baby in the bath water: AcrB contamination is a major problem in membrane-protein crystallization. Acta Crystallographica Section F F64:880–885.
- Yu EW, Aires JR, McDermott G, Nikaido H. A periplasmic drug-binding site of the AcrB multidrug efflux pump: a crystallographic and site-directed mutagenesis study. J Bacteriol 2005; 187: 6804–6815
- Yu EW, McDermott G, Zgurskaya HI, Nikaido H, Koshland DE, Jr. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 2003; 300: 976–980
- Tornroth-Horsefield S, Gourdon P, Horsefield R, Brive L, Yamamoto N, Mori H, Snijder A, Neutze R. Crystal structure of AcrB in complex with a single transmembrane subunit reveals another twist. Structure 2007; 15: 1663–1673
- Hofmann M, Zgouras D, Samaras P, Schumann C, Henzel K, Zimmer G, Leuschner U. Small and large unilamellar vesicle membranes as model system for bile acid diffusion in hepatocytes. Arch Biochem Biophys 1999; 368: 198–206