Abstract
The cytoplasmic C-terminus plays regulatory roles in the gating of many ion channels. However, lack of structural information on the C-terminus prevents the elucidation of how the C-terminal domain interacts with the gating machinery to exert its effects on the channel gating. In this report, we investigated the regulatory role of the C-terminus with functional study and structural modeling of a succession of C-terminal truncations of the Kv1.2 and Kv1.2427-KcsA112-160 chimeric channels. Functional study demonstrated a length-dependent shift of the activation curves for the C-terminal truncations of the Kv1.2 channel. Structural modeling indicated that the C-terminus of one subunit could dynamically interact with the S4–S5 linker of a neighboring subunit and the probability of interaction was dependent on the length of the C-terminal truncated Kv1.2 channels. In contrast, no length-dependent shift of the activation curve and probability of interaction between C-terminus and the neighboring S4–S5 linker were observed for the truncations of the Kv1.2-KcsA chimeric channel, suggesting that the native C-terminus of the Kv1.2 channel is essential for the interaction. Furthermore, surface plasmon resonance measurements indicated that there is direct interaction between the C-terminal domain and the S4–S5 linker of the Kv1.2 channel. These results imply that the dynamic interaction of the C-terminus with the S4–S5 linker from a neighboring subunit of the Kv1.2 channel provides a mechanism for its C-terminus to regulate the channel activation.
Introduction
Voltage-gated K+ (Kv) channels are essential for important physiological processes such as sensory transduction, action-potential generation and muscle contraction. Over the past decade, most structural and functional studies on the Kv channels have been centered on the transmembrane segments, whose conformational changes are essential for the channel gating Citation[1]. This has been highlighted by the crystallographic structure of the Kv1.2 channel, which revealed that motions of the S4 helices are transmitted to the S6 inner helix bundle (activation gate) via the S4–S5 linker helices to open the channel Citation[2], Citation[3]. On the other hand, many studies have shown that the cytoplasmic C-terminus also plays important regulatory roles in the channel gating Citation[4–8]. For example, it regulates the voltage dependence of channel activation, inactivation, and recovery from inactivation of the Kv4 channels Citation[8]. In Kv2.1 (drk-1) channels, the N- and C-terminal regions interact to regulate the channel gating processes Citation[9] and deletions of the C-terminus cause a hyperpolarizing shift of the voltage dependence of activation Citation[4]. However, lack of structural information on the C-terminus prevents the elucidation of how the C-terminal domain interacts with the gating machinery to exert its effects on the channel gating.
In this report, we provide evidence that the Kv1.2 C-terminus is intrinsically disordered and show that the C-terminus of the Kv1.2 channel regulates channel activation in a length-dependent manner. Furthermore, structural modeling suggests that the regulation is due to the dynamical interaction between the C-terminus and the S4–S5 linker from a neighboring subunit.
Materials and methods
Plasmid construction
The C-terminal truncated Kv1.2 channels were generated by introducing a stop codon after amino acid residues 465, 443, 428, 421 and 418, respectively, using cDNA encoding the rat Kv1.2 channel as a template. The Kv1.2-KcsA chimera were obtained through PCR-based mutagenesis. These constructs were subcloned into pcDNA3.1(-) vectors (Invitrogen) for expression in mammalian cells. The C-terminal domain (CTD) coding gene of the rat Kv1.2 channel (from His418 to Val499) was amplified from the pcDNA 3.0-kv1.2 and subcloned into pET28a (Novagen). The pET28a-CTD-sbp was constructed as follows: a streptavidin-binding peptide (SBP, MDEKTTGWRGGHVVEGLAGELEQLRAR LEHRARLEHHPQGQREP) was obtained by PCR from the plasmid pTAG2K. A linker peptide (Ser-Gly)5 coding sequence was inserted between CTD and sbp to generate CTD-SBP fusion protein. The orientation and reading frame of all constructs were confirmed by the DNA sequencing.
Cell culture and gene transfection in CHO-K1 cells
CHO-K1 cells were grown in a 37°C incubator with 5% CO2 humidified environment using Ham's F-12 nutrient mixture (Invitrogen, Co. Grand Island, NY, USA) supplemented with 10% fetal bovine serum. The cells were passaged twice weekly through exposure to 0.05% trypsin, 0.5 mM EDTA in PBS(-) solution (in mM): 137 NaCl; 3 KCl; 4 Na2HPO4; 2 KH2PO4 at pH 7.4. For gene transfection, the cells were transferred to poly-l-lysine (Sigma) coated glass coverslips. After cell density reached 50–70% confluence, pEGFP (Clontech, Palo Alto, CA, USA) was transiently coexpressed with the Kv1.2 channel gene at a ratio of 5:1 (weight /weight) using LipofectAMINE Plus(TM) reagent (Invitrogen). Cells were used for electrophysiological studies for 1–3 days after 24 h of the transfection.
Whole-cell patch clamp recordings
Voltage-clamp recordings were performed using the EPC-9 patch-clamp amplifiers (HEKA, Germany). Pipette and membrane capacitances were compensated automatically with the amplifier. To evoke the currents, the membrane potential was held at −100 mV and depolarized to +80 mV for 300 ms in a 20 mV increment. Currents were corrected offline for a linear leak current measured at −90 mV. A program package Pulse + Pulsefit (HEKA, Germany) was used for data acquisition and analysis. HANKS’ Balanced salts solution (HBSS, Sigma) was taken as the extracellular solution (in mM): 1.3 CaCl2, 0.8 MgSO4, 5.4 KCl, 0.4 KH2PO4, 136.9 NaCl, 0.3 Na2PO4, 10 d-glucose and 4.2 NaHCO3. The intracellular solution contained (in mM): 140 KCl, 2 MgCl2, 2 CaCl2, 1 EGTA, 2 Na2ATP, and 10 HEPES at pH 7.3. The peak current amplitude (I) at each test potential was converted into conductance (G) using the equation G=I/ (V−EK). The Nernst K+ equilibrium potential EK was calculated as −82 mV. The normalized conductance G was plotted against the test potential (V) and fitted to the single Boltzmann equation G/Gmax=1/ {1+exp [−(V−V1/2)/k]}, where Gmax is the maximum conductance, V1/2 is the voltage at half-maximal activation, and k is the slope factor. Fittings were calculated with the Origin program (OriginLab) using the non-linear regression method to obtain the best fit curve. All experiments were performed at room temperature (22–25°C).
Disorder prediction
Intrinsic disorder regions of the channels were predicted by VSL2P predictor (http://www.ist.temple.edu/disprot/predictorVSL2.php) with default parameters Citation[10]. Other predictors, including IUPred Citation[11] (http://iupred.enzim.hu/), FoldIndex Citation[12] (http://bip.weizmann.ac.il/fldbin/findex) and GlobPlot Citation[13] (http://globplot.embl.de) were also applied to verify the VSL2 prediction.
Structural modeling of the C-terminus of the Kv1.2 channel
A coarse-grained representation of the C-terminus chain was used in which only backbone heavy atoms were explicitly modeled. Side chains were represented by a single ‘centroid’ dummy atom that was calculated from 6000 already known structures distributed in PDB Citation[14]. Bond lengths and angles are kept fixed with residue type-dependent values from CHARMM22 topology parameters Citation[15], while the Φ, Ψ and ω torsion angles were given the freedom to move. A triplet local conformational energy and specific amino acid information Citation[16] were used in the energy function.
In the modeling, Kv1.2 TM-T1 tetramer was used as the basic framework, which was extracted from X-RAY Kv1.2-β2 subunit complex Citation[2] whose C-terminal structure is missing. The procedure of modeling was based on Rosetta Citation[17] that assembles local fragments into protein-like structures iteratively by a Monte Carlo search. For each truncated constructs, 34 repeated simulations were carried out. The protocol was implemented on a Linux cluster of dual 2.8G Hz CPU machines with 2 GB of RAM, and all simulations were distributed to 34 CPUs at a cost of ∼ 20 h/CPU.
Expression and purification of CTD-SBP fusion protein
To produce CTD-SBP fusion protein, pET28a-CTD-sbp construct was transformed into E. coli BL21 (DE3) cells, which were cultured in LB medium containing 35 µg/ml kanamycin at 37°C and induced with 0.4 mM IPTG upon OD600 nm of 0.6. After 16 h induction, the cultures were harvested by centrifugation at 4000 rpm for 30 min and lysed by ultrasonication. The lysates were centrifuged at 16,000 rpm for 30 min at 4°C to remove the cell fragments. The supernatant was collected and loaded onto a Ni2+-NTA column (Amersham). Then, the fusion protein was purified by gradient imidazole elutions. Its purity was analyzed by sodium dodecyl sulfate-polyacryl (SDS-PAGE). The purified protein was stored in buffer containing 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.025 mM EDTA, 5 mM dithiothreitol (DTT) and 20% glycerol at −70°C until use.
Synthesis of the S4–S5 linker peptide of the Kv1.2 channel
The S4–S5 linker of the Kv1.2 channel (RHSKGLQILGQTLKASMREL) was synthesized and purified (SBS Genetech, Beijing). The purity of the synthetic peptide was analyzed with HPLC (Knauer) and LC/MSD (VL) TRAP (Agilent).
Surface plasmon resonance (SPR) measurements
SPR measurements were carried out using the BIAcore 3000 (BIAcore AB, Uppsala, Sweden) at 25°C. The running buffer (mM): 300 NaCl (pH 8.0), 2.7 KCl, 10 Na2HPO4, 2 KH2PO4 and 0.005% (v/v) Tween 20 was vacuum filtered before use. 2600 RU CTD-SBP was immobilized on one flow cell of streptavidin-modified chip via the SBP-strepavidin interaction Citation[18]. Another blank flow cell without CTD-SBP immobilization was used as a real-time reference for nonspecific events. Before measurement, the sensor chip was equilibrated with running buffer until the baseline was stable. Running buffer containing 10 µg/ml and 20 µg/ml concentrations of the S4–S5 linker peptide were injected into the CTD-SBP binding cells and the blank cell at a flow rate of 20 µl/min for 2 min. The sensor chip surface was regenerated with 15 mM NaOH for 30 sec followed by 1 min running buffer injection. All data were analyzed using BIAevaluation 4.1 software to obtain corrected sensorgrams.
Statistical analysis
Data were expressed in mean and standard error of mean (mean±SEM) throughout the text. One-way analysis of variance (ANOVA) was used to detect statistical significance. Statistical significance was set at p<0.05.
Results
Effect of C-terminal truncations on the channel activation
To study effect of C-terminus on the channel activation, we constructed five sequential truncations: Kv1.2465stop, Kv1.2443stop, Kv1.2428stop, Kv1.2421stop and Kv1.2418stop, which removed the distal (Kv1.2465stop), about half (Kv1.2443stop), the most (Kv1.2428stop) and the whole (Kv1.2421stop and Kv1.2418stop) part of the C-terminus of the Kv1.2 channel (A). Functional K+ channels were observed for all constructs except Kv418stop (B and ). They displayed significantly altered activation properties. Their activation curves gradually shifted to the left with the mid-point of channel activation voltage (V1/2) value of 17.5±1.9 mV for the wild type, 13.5±1.8, 6.1±4.0,−0.2±3.9 and−1.8±2.9 mV for Kv1.2465stop, Kv1.2443stop, Kv1.2428stop, and Kv1.2421stop, respectively (A, 2B). These observations indicate that the Kv1.2 channel activation is correlated with the length of the truncated C-terminus, with a greater truncation resulting in a more leftward shift of the activation curve, suggesting that the C-terminus plays a regulatory role in the activation of the Kv1.2 channel. This is consistent with deletions of the C-terminus of another K+ channel, Kv2.1 (drk-1), which cause a leftward shift of the voltage dependent activation as well Citation[4].
Figure 1. Transmembrane topology and current traces for the channels. (A) Polypeptide chain topology of the Kv1.2 channel with sequence of its S6 inner helix and C-terminus (NP_037102). The six cylinders correspond to the six transmembrane segments of the channel. The intrinsically disordered C-terminus is in grey. Arrow heads indicate the five truncation sites. I: Kv1.2465stop, II: Kv1.2443stop III: Kv1.2428stop IV: Kv1.2421stop V: Kv1.2418stop. Diagram above indicates secondary structural motifs in the Kv1.2 channel (PDB ID: 2A79). (B) Representative current traces for the wild type Kv1.2, Kv1.2465stop, Kv1.2443stop p, and Kv1.2418stop channels, respectively. The currents were evoked by stepping membrane potential from −100 to +80 mV in a step of 20 mV.
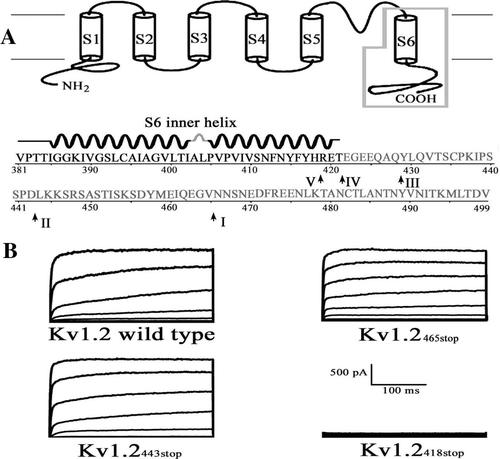
Figure 2. Effect of C-terminal truncations on the channel activation. (A) Activation curves of the wild-type and truncated Kv1.2 channels. Smooth curves through the data represent fits to the mean values with the Boltzmann function. (B) Statistics on the V1/2 values the channels. Times of each experiment are indicated in the parenthesis. Significant difference was compared between wild type and each of the mutant channels. *: p<0.05. **: p<0.01.
Intrinsically disordered C-terminus of the Kv1.2 channel
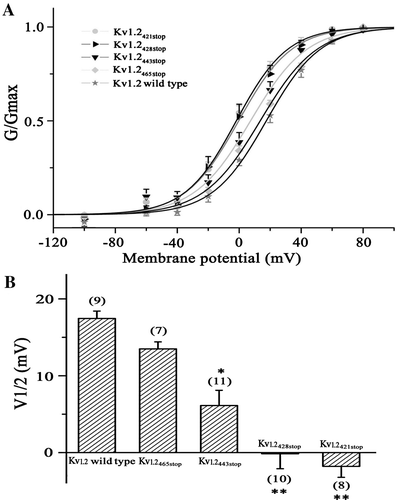
To get insight into structural property of the C-terminus, we made a disordered prediction on the full length of the Kv1.2 channel using disorder predictor VSL2 with default parameters Citation[10]. The C-terminus of the Kv1.2 channel was predicted to be intrinsically disordered (). This is in agreement with the lack of electron density at the C-terminus of the Kv1.2 channel as revealed by x-ray crystallography Citation[2], Citation[19] and the findings that the most of Kv channels contain intrinsically disordered C-terminal segments Citation[20], Citation[21]. Besides, the predictor also correctly predicted six and two ordered transmembrane segments for the Kv1.2 channel Citation[2] and the KcsA channel Citation[22]. Moreover, several other predictors, including IUPred Citation[11], FoldIndex Citation[12] and GlobPlot Citation[13] also predicted that the C-terminus of the Kv1.2 and KcsA channels has high tendency of intrinsically disorder (data not shown), suggesting that the prediction is independent of the predictors and thus reliable. The intrinsically disordered domains generally have high conformational flexibility Citation[23]. Therefore, we inferred that the intrinsically disordered C-terminus of a bacterial KcsA (KcsA112-160, B) might also be able to regulate the Kv1.2 channel due to its conformational flexibility conferred by the intrinsically disordered nature of the C-terminus. To test this, the C-terminus of the Kv1.2 channel from positions 427 was replaced by the C-terminus of the bacterial KcsA channel. The V1/2 were similar for Kv1.2427stop truncated and Kv1.2427-KcsA112-160 chimeric channels, seemingly suggested that the KcsA C-terminus has no effect on the Kv1.2 activation. However, progressive deletions of the Kv1.2427-KcsA112-160 chimeric channel changed the activation of the channel with about 15 mV rightward shift in the V1/2 values when 15, 23 and 38 amino acids of KcsA C-terminus in the chimeric channels were deleted, while more deletions (42–48 residues) made the V1/2 value returned to the value of Kv1.2427-KcsA112-160 chimeric channel (). These results suggested that the C-terminus of the bacterial channel could regulate the function of the eukaryotic channel in spite of their fundamentally different amino acid sequences, implying the flexibility and thus the intrinsically disordered nature of the C-terminus. However, no length-dependent shift of the activation curve as that of the Kv1.2 channel was observed for the truncations of the Kv1.2-KcsA chimeric channel.
Figure 3. Intrinsically disordered C-terminus of KcsA and Kv1.2 channels. (A) VSL2 prediction score (disorder probability) on the Kv1.2 channel. The six grey bars labeled S1 to S6 correspond to the six transmembrane segments of the channel. (B) VSL2 prediction score on the KcsA channel. The two grey bars labeled TM1 and TM2 correspond to the two transmembrane segments of the channel. The C-terminus is marked as the black bar. Residues with prediction values above 0.5 are interpreted as predicted disordered.
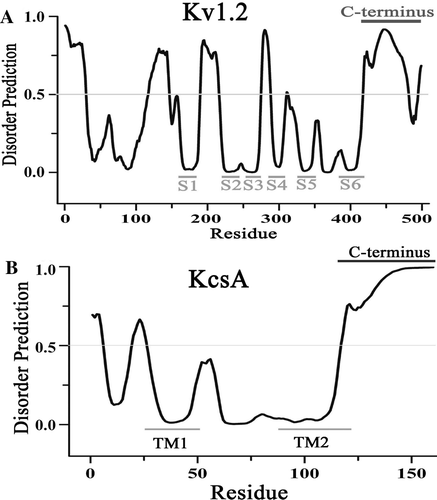
Figure 4. KcsA C-terminal truncations on the activation of the Kv1.2-KcsA112-160 chimeric channel. (A) Steady-state activation curves for the channel of Kv1.2427- KcsA112-160 and its counterpart with deletions of 15 (Kv1.2427- KcsA-15aa), 38 (Kv1.2427- KcsA-38aa) and 42 (Kv1.2427- KcsA-42aa) amino acids on the C-terminus. The normalized conductance (G/Gmax) is plotted against the membrane potential and fitted with the Boltzmann equation. (B) Statistics on the V1/2 values of Kv1.2427-KcsA112-160 and its counterparts with deletions of different number of amino acids. Significant difference was compared between Kv1.2427-KcsA112-160 and each of the deleted mutant channels.
Structural modeling of the C-terminal domains
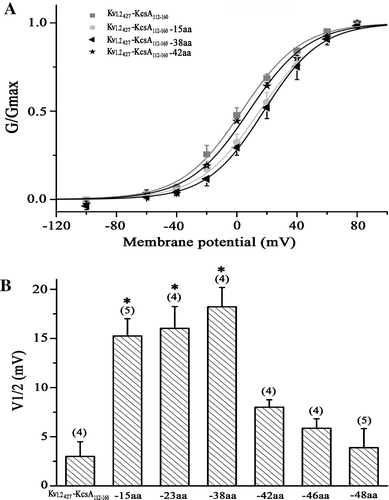
To get insight into the structural information, we did structural modeling on the C-terminal domain of the channels. The above observation indicated that C-terminal truncations of the Kv1.2 channel, in spite of removing the distal (Kv1.2465stop), about half (Kv1.2443stop) and the whole (Kv1.2421stop) part of the C-terminus, all altered the activation of the channel (). How might these different sized C-termini interact with the gating machinery to account for their effects on the channel activation? To address this question, we need to build their structural models to look into their possible interactions with the gating machinery as the C-terminus is missing in the resolved crystallography structure of the Kv1.2 channel. Given the high flexibility conferred by its intrinsically disordered nature Citation[23–25], we modeled the C-terminus as a dynamical ensemble of conformations using an ab initio structure prediction approach. illustrated that each C-terminus populates as an ensemble of inter-converting low-energy conformers in equilibrium, which results in changes in the close distance between individual conformer and the S4–S5 linker. It was observed that, on the contact interface between the C-terminus and the S4–S5 linker, basic residues on the S4–S5 linker might interact electrostatically with acid and hydroxyl residues on the C-terminus. And the probability of interaction between the conformers and the S4–S5 linker decreases in a length-dependent manner: 78.9% for the wild type Kv1.2, 61.9% for Kv1.2465stop, 41.7% for Kv1.2443stop and only 3.0% for Kv1.2428stop channels. This phenomenon correlates well with the length dependent leftward shift of the activation curves of the Kv1.2 channel.
Figure 5. Structural modeling of the different sized C-termini of the Kv1.2 and Kv1.2-KcsA chimeric channel. (A) Side view of the full-length Kv1.2 channel (PDB ID 2A79) with uniquely colored subunits. Two representative conformers from the ensemble of the C-termini are colored in blue and gray for the full-length Kv1.2 channel. The S6 inner helix, S4-S5 linker and N-terminus around the simulated C-terminus (blue and grey of the red subunit) and conductive pore are labeled. Green lines mark the approximate boundaries of extracellular (E) and intracellular (I) membrane. (B–D) An enlarged stereoview to illustrate the relationship between the neighboring S4-S5 linker and the ensemble of the 34 C-terminal conformers for wild type (B), Kv1.2465stop (C), and Kv1.2443stop (D). (E) An enlarged stereoview to illustrate the relationship between the N-terminus and the ensemble of the 34 C-terminal conformers for the Kv1.2-KcsA chimeric channel with 48 amino acid residues of KcsA C-terminus. The closest distance between them is shown by red numbers. Residues on the blue conformer of the C-terminus and the S4-S5 linker are highlighted with bright orange ribbons to show that the distance between their paired Cα atoms is within 10 Å. The S4-S5 linker and the end of the S6 inner helix are shown as bright and dim orange ribbons. The structure was generated with Visual Molecular Dynamics Citation[29]. This Figure is reproduced in colour in Molecular Membrane Biology online.
![Figure 5. Structural modeling of the different sized C-termini of the Kv1.2 and Kv1.2-KcsA chimeric channel. (A) Side view of the full-length Kv1.2 channel (PDB ID 2A79) with uniquely colored subunits. Two representative conformers from the ensemble of the C-termini are colored in blue and gray for the full-length Kv1.2 channel. The S6 inner helix, S4-S5 linker and N-terminus around the simulated C-terminus (blue and grey of the red subunit) and conductive pore are labeled. Green lines mark the approximate boundaries of extracellular (E) and intracellular (I) membrane. (B–D) An enlarged stereoview to illustrate the relationship between the neighboring S4-S5 linker and the ensemble of the 34 C-terminal conformers for wild type (B), Kv1.2465stop (C), and Kv1.2443stop (D). (E) An enlarged stereoview to illustrate the relationship between the N-terminus and the ensemble of the 34 C-terminal conformers for the Kv1.2-KcsA chimeric channel with 48 amino acid residues of KcsA C-terminus. The closest distance between them is shown by red numbers. Residues on the blue conformer of the C-terminus and the S4-S5 linker are highlighted with bright orange ribbons to show that the distance between their paired Cα atoms is within 10 Å. The S4-S5 linker and the end of the S6 inner helix are shown as bright and dim orange ribbons. The structure was generated with Visual Molecular Dynamics Citation[29]. This Figure is reproduced in colour in Molecular Membrane Biology online.](/cms/asset/ad0459d5-602e-4b24-bd7f-fe3283b562c4/imbc_a_371644_f0005_b.jpg)
In contrast to the effect of the C-terminus on the length-dependent shift of the activation curve of the Kv1.2 channel, altering the C-terminus of the Kv1.2-KcsA chimera did not cause any length-dependent shift for the Kv1.2-KcsA chimeric channel. To get possible structural information on the different effects, we carried out structural modeling on the KcsA C-terminus of the Kv1.2-KcsA chimera. The result indicated that there was no interaction between the KcsA C-terminus and the S4–S5 linker of the Kv1.2-KcsA chimeric channels. In contrast, there are interactions between C- and N-terminus for the Kv1.2-KcsA chimeric channel (E). However, this interaction is not dependent on the length of the truncations for both the chimeric and the Kv1.2 channels. The probability of interaction between KcsA C-terminus of the chimeric channel and its N-terminus was approximately 50%, much higher than that of the Kv1.2 channel (ca. 16%).
Interaction between the C-terminus and the S4–S5 linker of the Kv1.2 channel
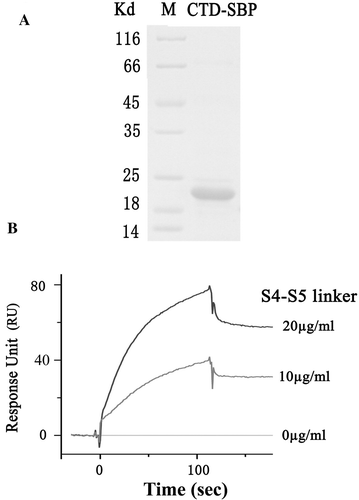
The structural modeling indicated that the C-terminus of one subunit could interact with the S4–S5 linker of a neighboring subunit in the Kv1.2 channel. To confirm this directly, we measured the interaction between these two regions using SPR technique. The purified CTD-SBP fusion protein was analyzed by SDS-PAGE (A). To carry out SPR measurement, the CTD of Kv1.2 channel was immobilized on the surface of a streptavidin-modified chip via SBP. SPR sensorgram showed that the RU value was increased in a concentration dependent manner when the running buffer containing the S4–S5 linker (B). This result indicated that the C-terminal domain could directly interact with the S4–S5 linker of the Kv1.2 channel.
Figure 6. SPR measurement of the interaction between the C-terminal domain and the S4-S5 linker of the Kv1.2 channel. (A) SDS-PAGE analysis of the purified CTD-SBP fusion protein (sample size: 5 µl). B) SPR sensorgram showing the interaction between the CTD and the S4-S5 linker of the Kv1.2 channel. CTD-SBP immobilization level: 2600 RU; injected samples: the S4-S5 linker peptide with the indicated concentrations (flowing for 120 sec).
Discussion
In the present study, we investigated the role of the C-terminus of the voltage-gated Kv1.2 channel using a combination of functional analysis and structural modeling. Functional study showed that alteration in the length of the C-terminus shifted the voltage dependence of the Kv1.2 channel activation. Structural modeling indicated that the C-terminus interacts with the S4–S5 linker of a neighboring subunit in a length-dependent manner as well. In contrast, no such correlation was observed for the Kv1.2-KcsA chimeric channel, even though the C-terminus of the KcsA channel could regulate the function of the Kv1.2 channel. Furthermore, the SPR sensorgram indicated that the C-terminus could directly interact with the S4–S5 linker. These results imply that the interaction between the C-terminus and the neighboring S4–S5 linker might be a key factor for the length-dependent shift of the activation curve. And the native C-terminus of the Kv1.2 channel might play an important role in the interaction between the C-terminus and the S4–S5 linker in the Kv1.2 channel. Taking together, we argue that the C-terminus regulates activation of the Kv1.2 channel in a length-dependent manner possibly by interaction with the S4–S5 linker of a neighboring subunit. In addition, structural modeling shows that there are interactions between C- and N-terminus for both Kv1.2 and the Kv1.2-KcsA chimeric channel and this interaction is not dependent on the length of the truncations. The probability of interaction between KcsA C-terminus of the chimeric channel and its N-terminus is much higher than that of the Kv1.2 channel. These results implied that the dynamic interaction between N- and C-terminus may play an important role in regulating the activation of the chimeric channel. Such interaction between C- and N-terminus of the channel has been shown for the Kv2.1 channel by biochemical experiment Citation[9]. Our studies have focused on the regulatory effect of C-terminus on the Kv1.2 channel activation and it may be that the mechanism of C-terminus regulation in other Kv channel is different. Therefore, we are aware that the length-dependence might be only part of the story and further studies are necessary to give more convincing evidence. Nevertheless, this study, for the first time, proposes a mechanism for the C-terminus in regulation of the voltage-dependent channel gating.
This model seems plausible because the S4–S5 linker has been shown to play an important role in the channel gating. It has been suggested that there is a direct interaction between the S4–S5 linker and the end of the S6 inner helix Citation[26–28], which forms the main gate of the channel Citation[2]. The recently solved crystal structure of the voltage-gated Kv1.2 channel reveals that the S4–S5 linker forms an amphipathic α-helix running parallel to the cytoplasmic interface and is positioned over the C-terminal end of the S6 inner helix from the same subunit. This makes the S4–S5 linker become the structural link that couples voltage-sensor motions to the gate of the same subunit of the channel Citation[2], Citation[3]. Recently, it has been indicated that the Kv2.1 channel gating is strongly influenced by specific interactions between the N- and C-terminal domains Citation[9]. However, this observation also did not show how the C-terminus interacts with the gating machinery in order to exert its effect on the function of the channel. Our structural modeling suggests that in addition to the interaction between the S6 inner helix and the S4–S5 linker, there is an interaction between the S4–S5 linker of one subunit and the C-terminus of the other subunit. The C-terminus is located at the cytoplasmic side, so it is highly likely that it doesn't play any role in sensing voltage changes directly. Our results from both structural modeling and SPR measurement showed that the C terminus could directly interact with the S4–S5 linker. Hence, the C-terminus might sense changes in the S4–S5 linker, which has been show to be able to sense the movement of the voltage sensor Citation[2], Citation[3].
In summary, by using a combination of functional analysis and structural modeling, this study suggests that the C-terminus regulates the activation of the Kv1.2 channel activation in a length-dependent manner possibly by interaction with the S4–S5 linker of a neighboring subunit.
Acknowledgements
We thank Dr Y.N. Jan for providing Kv1.2 channel cDNA, Dr. D.S. Wilson for providing pTAG2K plasmid, Ms Y.Y. Chen for assistance of SPR measurement. This work was supported by the National Basic Research Program of China (2005CB522804, 2006CB911003 to ZQ; 2006CB911002, 2009CB918503 to TJ), the National Science Foundation of China (30570428, 30599432 to TJ; 30670443 to LB) and the Chinese Academy of Science Foundation (No.KSCX1-YW-R-63 to LB). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Yellen G. The moving parts of voltage-gated ion channels. Quart Rev Biophys 1998; 31: 239–295
- Long SB, Campbell EB, MacKinnon R. Crystal structure of a mammalian voltage-dependent Shaker Family K+ Channel. Science 2005; 309: 897–903
- Long SB, Campbell EB, MacKinnon R. Voltage sensor of Kv1.2: structure basis of electromechanical coupling. Science 2005; 309: 903–908
- VanDongen AM, Frech GC, Drewe JA, Joho RH, Brown AM. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron 1990; 5: 433–443
- Marten I, Hoshi T. Voltage-dependent gating characteristics of the K+ channel KAT1 depend on the N and C termini. Proc Natl Acad Sci USA 1997; 94: 3448–3453
- Aydar E, Palmer C. Functional characterization of the C-terminus of the human ether-a-go-go-related gene K+ channel (HERG). J Physiol (London) 2001; 534: 1–14
- Loukin SH, Lin J, Athar U, Palmer C, Saimi Y. The carboxyl tail forms a discrete functional domain that blocks closure of the yeast K+ channel. Proc Natl Acad Sci USA 2002; 99: 1926–1930
- Hatano N, Ohya S, Muraki K, Clark RB, Giles WR, Imaizumi Y. Two arginines in the cytoplasmic C-terminal domain are essential for voltage-dependent regulation of A-type K+ current in the Kv4 channel subfamily. J Biol Chem 2004; 279: 5450–5459
- Ju M, Stevens L, Leadbitter E, Wray D. The roles of N- and C-terminal determinants in the activation of the Kv2.1 potassium channel. J Biol Chem 2003; 278: 12769–12778
- Peng K, Radivojac P, Vucetic S, Dunker AK, Obradovic Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinformatics. 2006; 7: 208–225
- Dosztanyi Z, Csizmok V, Tompa P, Simon I. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 2005; 21: 3433–3434
- Prilusky J, Felder CE, Zeev-Ben-Mordehai T, Rydberg EH, Man O, Beckmann JS, Silman I, Sussman JL. FoldIndex: a simple tool to predict whether a given protein sequence is intrinsically unfolded. Bioinformatics 2005; 21: 3435–3438
- Linding R, Russell RB, Neduva V, Gibson TJ. GlobPlot: exploring protein sequences for globularity and disorder Nucleic Acids Res 2003; 31: 3701–3708
- Berman HM, Westbrook J, Feng Z, Gilliland G., Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res 2000; 28: 235–242
- MacKerell AD, Jr, Zhao L, Wu A, Bi L, Liu P, Zhang X, Jiang T, et al. All-atom empirical potential for molecular modeling and dynamics Studies of proteins. J Phys Chem B 1998; 102: 3586–3616
- Yang JS, Chen WW, Skolnick J, Shakhnovich EI. All-atom ab initio folding of a diverse set of proteins. Structure 2007; 15: 53–63
- Rohl CA, Strauss CE, Misura KM, Baker D. Protein structure prediction using Rosetta. Methods Enzymol 2004; 383: 66–93
- Li YJ, Bi LJ, Zhang XE, Zhou YF, Zhang JB, Chen YY, Li W, Zhang ZP. Reversible immobilization of proteins with streptavidin affinity tags on a surface plasmon resonance biosensor chip. Anal Bioanal Chem 2006; 386: 1321–1326
- Long SB, Tao X, Campbell EB, Mackinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 2007; 450: 376–382
- Magidovich E, Fleishman SJ, Yifrach O. Intrinsically disordered C-terminal segments of voltage-activated potassium channels: a possible fishing rod-like mechanism for channel binding to scaffold proteins. Bioinformatics 2006; 22: 1546–1550
- Magidovich E, Orr I, Fass D, Abdu U, Yifrach O. Intrinsic disorder in the C-terminal domain of the Shaker voltage-activated K+ channel modulates its interaction with scaffold proteins. Proc Natl Acad Sci USA 2007; 104: 13022–13027
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 1998; 280: 69–76
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 2005; 6: 197–208
- Fink AL. Natively unfolded proteins. Curr Opin Struct Biol 2005; 15: 35–41
- Mittag T, Forman-Kay JD. Atomic-level characterization of disordered protein ensembles. Curr Opin Struct Biol 2007; 17: 3–14
- Lu Z, Klem AM, Ramu Y. Ion conduction pore is conserved among potassium channels. Nature 2001; 413: 809–813
- Lu Z, Klem AM, Ramu Y. Coupling between voltage sensors and activation gate in voltage-gated K+ channels. J Gen Physiol 2002; 120: 663–676
- Ferrer T, Rupp J, Piper DR, Tristani-Firouzi M. The S4-S5 linker directly couples voltage sensor movement to the activation gate in the human ether-a’-go-go-related gene (hERG) K+ channel. J Biol Chem 2006; 281: 12858–12864
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph 1996; 14: 33–38