Abstract
The brush border of pig small intestine is a local hotspot for β-galactoside-recognizing lectins, as evidenced by its prominent labeling with fluorescent lectin PNA. Previously, galectins 3-4, intelectin, and lectin-like anti-glycosyl antibodies have been localized to this important body boundary. Together with the membrane glycolipids these lectins form stable lipid raft microdomains that also harbour several of the major digestive microvillar enzymes. In the present work, we identified a lactose-sensitive 14-kDa protein enriched in a microvillar detergent resistant fraction as galectin-2. Its release from closed, right-side-out microvillar membrane vesicles shows that at least some of the galectin-2 resides at the lumenal surface of the brush border, indicating that it plays a role in the organization/stabilization of the lipid raft domains. Galectin-2 was released more effectively from the membrane by lactose than was galectin-4, and surprisingly, it was also released by the noncanonical disaccharides sucrose and maltose. Furthermore, unlike galectin-4, galectin-2 was preferentially coimmunoisolated with sucrase-isomaltase rather than with aminopeptidase N. Together, these results show that the galectins are not simply redundant proteins competing for the same ligands but rather act in concert to ensure an optimal cross-linking of membrane glycolipids and glycoproteins. In this way, they offer a maximal protection of the brush border against exposure to bile, pancreatic enzymes and pathogens.
Introduction
The galectins are a family of soluble lectins containing a conserved carbohydrate-recognition domain (CRD) with binding affinity for -galactosides Citation[1–6]. The interaction between monosaccharides and the CRD is relatively weak and most galectins preferably bind glycoproteins and glycolipids containing repeating units of the N-acetyl-lactosamine, either as disaccharide units at the termini of complex N-glycans or as repeating units in a poly-N-acetyl-lactosamine chain on N- or O-glycans Citation[1], Citation[2], Citation[7].
The galectins are ubiquitous proteins and so far 15 distinct mammalian galectins have been identified. Commonly, they are involved in multiple functions in the cells, including cell adhesion, growth, proliferation, differentiation, migration, cytokine secretion, apical protein sorting, regulation of inflammatory response and apoptosis Citation[1–5], Citation[7], Citation[8]. Structurally, the galectines can be subdivided into three groups: Prototype galectins (galectin-1, -2, -5, -7, -10, -11, -13, -14 and -15) containing one CRD, chimera galectin (galectin-3) containing a proline- and glycine-rich domain connected to a CRD, and tandem-repeat galectins (galectin-4, -6, -8, -9 and -12) containing two CRDs Citation[1], Citation[2], Citation[9].
Whereas galectin-3, -4, -6, -7 and -9 are expressed along the entire gastrointestinal tract Citation[5], Citation[6], galectin-2 is mainly confined to the stomach and the small intestine Citation[3], Citation[9]. Here, it has been reported to enhance cell migration of enterocytes and thus improve wound healing Citation[10]. In addition, galectin-2 induces cell proliferation and inhibits the release of pro-inflammatory cytokines Citation[10], Citation[11]. These properties help controlling the inflammatory response and thus improve the condition, for instance during inflammatory bowel disease Citation[10], Citation[12].
The small intestinal brush border is specifically designed to act both as a high-throughput digestive/absorptive surface and as a protective permeability barrier against luminal pathogens Citation[13–15]. To accomplish these tasks, the microvillar membrane is organized into lipid rafts microdomains mainly due to its high content of glycolipids Citation[16], Citation[17]. Previously, the lectins galectin-3, galectin-4, and intelectin have been localized to the brush border Citation[8], Citation[18], Citation[19]. Together, these di- or multivalent lectins crosslink glycolipids and glycoproteins to form stable supramolecular complexes Citation[20]. Both galectin-3 and -4 have been implicated in the intracellular sorting of newly synthesized proteins destined for the apical surface Citation[8], Citation[21–23], and the extraordinary stability of these microdomains has been documented by their ability to form highly detergent resistant membranes (‘superrafts’) Citation[19]. Many of the major microvillar digestive hydrolases cluster into the microdomains and thereby minimize their loss to the gut lumen due to pancreatic proteolytic and lipolytic activities and exposure to bile salts Citation[24]. Unfortunately, a large number of pathogens specifically recognize glycolipids in their initial contact with a target epithelial cell and thus the glycolipid-based microdomains may serve as a portal for these Citation[25–29]. The invaded organism relies on secretion of antibodies as the first line of defence against such pathogens Citation[30], and ‘anti-glycosyl’ antibodies, i.e., antibodies induced in the host by a glycosyl antigen Citation[31], have previously been shown to be deposited in the intestinal brush border as guardians of the lipid rafts Citation[32].
In the present study galectin-2 was identified as yet another lectin present in the enterocyte brush border. On the basis of their different ligand-binding properties, we propose that galectin-2 acts in concert with other lectins and lectin-like antibodies to help organizing and stabilizing the lipid raft microdomains of this exposed body boundary.
Materials and methods
Materials
Goat anti-galectin-2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), rabbit anti-galectin-2 from Atlas Antibodies (Stockholm, Sweden), rabbit antibodies to intestinal alkaline phosphatase from Biogenesis (Poole, UK), and rabbit antibodies to galectin-4, aminopeptidase N and sucrase-isomaltase were previously described Citation[33–35]. Secondary Alexa 488/594-conjugated antibodies and Alexa Fluor 488-conjugated to the lectin PNA were from Molecular Probes (Eugene, OR, USA). Pig small intestines were surgically obtained from anesthetized animals by licensed staff of the Department of Experimental Medicine, the Panum Institute, Copenhagen, Denmark.
Subcellular fractionation
Microvillar right-side-out membrane vesicles were prepared from pig small intestine by the divalent cation precipitation method Citation[36]. Briefly, mucosal scrapings were homogenized in a manually operated Potter-Elvehjem homogenizer in 2 mM Tris-HCl and 50 mM mannitol (pH 7.1) containing 10 µg/ml aprotinin and leupeptin. The homogenate was cleared by centrifugation at 500 g, 5 min. MgCl2 was added to the supernatant to a final concentration of 10 mM and left on ice for 10 min. The preparation was then centrifuged at 1500 g, 10 min, to pellet intracellular and basolateral membranes. The supernatant was collected and centrifuged at 48,000 g, 30 min, to obtain a pellet of microvillar membrane vesicles and a supernatant of soluble proteins.
For preparation of a detergent resistant fraction (DRF, containing detergent resistant membranes [DRMs] and microvillar cytoskeletal proteins), the microvillar vesicles were resuspended in 25 mM HEPES containing 150 mM NaCl (pH 7.1) and extracted with 1% Triton X-100 for 10 min on ice. The preparation was centrifuged at 20,000 g, 30 min, to pellet the DRF.
Immunoisolation
100 µl samples of antibodies to aminopeptidase N or sucrase-isomaltase were each bound to ∼10 µl protein A-Sepharose resuspended in 100 µl 25 mM HEPES-HCl, 150 mM NaCl, pH 7.1, by incubation for 2 h at room temperature. Afterwards, the protein A-Sepharose was washed three times with 1 ml buffer before use. DRF, prepared as described above, were resuspended in 1 ml 25 mM HEPES-HCl, 150 mM NaCl, pH 7.1, containing 1% Triton X-100, and incubated for 10 min at room temperature. After centrifugation at 20,000 g, 30 min, 200 µl samples of the supernatant were incubated with protein A-Sepharose-coupled antibodies to aminopeptidase N or sucrase-isomaltase for 1 h at room temperature. After incubation, the protein A-Sepharose was washed rapidly three times with 1 ml buffer before electrophoresis.
SDS-PAGE and immunoblotting
SDS-PAGE in 15% gels was performed as described previously Citation[37]. After electrophoresis and electrotransfer of proteins onto Immobilon PVDF membranes, immunoblotting was performed with antibodies to galectins-2 and -4. The immunoblots were developed by electrochemiluminescence using Amersham ECL Western blotting detection reagents according to a protocol supplied by the manufacturer (GE Healthcare limited UK, Little Chalfont, Buckinghamshire, UK). After immunoblotting, total protein was visualized by staining with Coomassie brilliant blue R250 (0.2% dissolved in a ethanol/H2O/acetic acid mixture [50:43:7]) for 1 min, followed by destaining in ethanol/H2O/acetic acid mixture (50:43:7) for 20 min.
MALDI-TOF analysis
A ∼14 kDa band from a SDS-PAGE in a 15% gel, visualized after electrotransfer onto an Immobilon PVDF membrane and protein staining, was excised and submitted to commercial MALDI-TOF analysis (Alphalyse, Odense, Denmark). The analysis resulted in identification of three peptides matching the amino acid residues 5–16, 42–60 and 97–108 of Galectin-2 (MW: 13790 Da.)
Fluorescence microscopy
Small pieces of pig jejunal mucosa were excised and fixed in 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.2 for 2 h at 4°C. After washing in the same buffer the explants were dehydrated and embedded in paraffin. Sections of 6 µm were cut in a Leica RM 2165 microtome. The expression of galectin-2 and galectin-4 was studied by double immunofluorescence labelling. After rehydration the paraffin sections were incubated with goat anti-galectin-2 for 2 h, followed by incubation with anti-goat Alexa 594-conjugated antibodies for 2 h. Hereafter the same labeling procedure was performed with a rabbit antibody to galectin-4 and an anti-rabbit Alexa 488-conjugated antibody. Control experiments were performed in parallel with omission of the primary antibodies. In other experiments, paraffin sections were incubated with Alexa Fluor 488-conjugated lectin PNA. The labeled sections were mounted in antifade mounting medium (DAKO, Glostrup, Denmark) and finally examined with a Leica DM 4000 B microscope equipped with a Leica DC 300 FX camera.
Results
Localization of ligands for β-galactosyl specific lectin
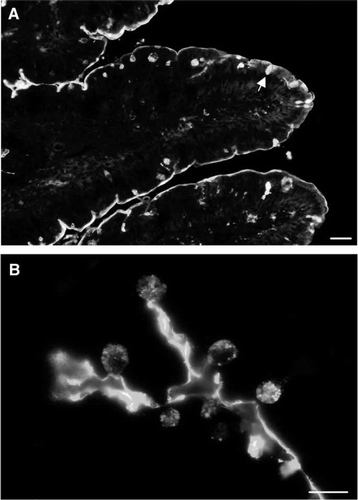
Lectin PNA specifically recognizes terminal galactose residues Citation[38]. It is therefore suitable for visualizing -galactosides on tissue sections and thus to demonstrate the location of possible ligands for endogeneous β-galactosyl specific lectins in the small intestine Citation[39]. A shows that the labelling of lectin PNA in sections of small intestine was mainly confined to the brush border of the enterocytes, highlighting that the lumenal surface is indeed a hotspot for this type of carbohydrate recognizing proteins. At higher magnification (B), a distinct labelling of the secretory granules of goblet cells was also seen.
Figure 1. Localization of lectin PNA by fluorescence microscopy. (A) A distinct staining of the brush border surface is seen along the villi. In addition, goblet cells (marked by an arrow) appear as strongly labeled dots. (B) A higher magnification image of the villus shows strong labeling of the secretory granules of goblet cells. Bars: 25 µm (A), 15 µm (B). Published in colour in the online version.
Identification of galectin-2 as a detergent-resistant microvillar membrane protein
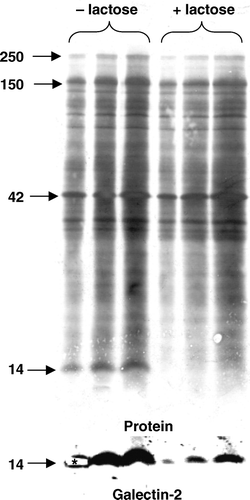
In a search for novel -galactoside-binding proteins localized to the enterocyte brush border, a detergent resistant fraction (DRF) of microvillar membranes, containing detergent resistant membranes (DRMs) and some microvillar cytoskeletal proteins, was prepared and incubated in the presence or absence of 0.1 M lactose before analysis by SDS-PAGE. shows that a distinct band of ∼14 kDa was released from the DRF by lactose, suggesting it to be a β-galactosyl specific lectin. A subsequent MALDI-TOF analysis of the excised band resulted in identification of three peptides matching sequences of galectin-2. Finally, immunoblotting of the membrane with a goat antibody to galectin-2 (), as well as with a rabbit anti-galectin-2 antibody (data not shown), confirmed the identification of the excised 14-kDa band.
Figure 2. Identification of galectin-2 in a DRF prepared from microvillar membranes. A microvillar DRF was prepared as described in Methods, resuspended in 25 mM HEPES-HCL, 150 mM NaCl, pH 7.1, and incubated for 30 min at room temperature in the absence or presence of 0.1 M lactose. After incubation, the DRF was centrifuged at 20,000 g for 30 min, and the pellets (in three different loadings) were analyzed by SDS-PAGE, followed by transfer onto an immobilon PVDF membrane. Protein staining revealed a distinct lactose-sensitive band at ∼14 kDa. This band was carefully excised from the gel track furthest to the left and identified by MALDI-TOF analysis as galectin-2. The identification was verified by immunoblotting with an antibody to galectin-2. (The part of the membrane excised for MALDI-TOFF analysis is marked by an asterisk on the immunoblot image). Molecular weight values are indicated by arrows.
Subcellular localization of galectin-2
The subcellular localization of galectin-2 was investigated by fractionation of pig jejunal mucosa into microvillar membrane vesicles, Mg2+-precipitated (basolateral and intracellular) membranes and soluble proteins. A shows that the protein was distinctly present in the microvillar membranes, but galectin-2 were also detected in the Mg2+-precipitated membrane fraction and the soluble fraction. Furthermore, like the lipid raft marker alkaline phosphatase Citation[19], galectin-2 was enriched in the DRF.
Figure 3. Subcellular localization of galectin-2. (A) SDS-PAGE of Mg2+-precipitated (basolateral and intracellular) membranes (Mg), soluble proteins (Sol), microvillar membranes (Mic), and DRF prepared from jejunal mucosa, as described in Methods. After electrophoresis, alkaline phosphatase (AP, 67 kDa) and galectin-2 (Gal-2) were visualized by immunoblotting and total protein by staining with Coomassie Brilliant Blue. Molecular weight values are indicated by arrows. (B) Samples of a right-side-out microvillar membrane vesicle fraction were resuspended in 25 mM HEPES-HCl, 150 mM NaCl, pH 7.1, containing 0 mM, 10 mM or 50 mM of lactose, respectively. After incubation for 30 min at room temperature the samples were centrifuged at 20,000 g for 30 min. The pellets and supernatants were subjected to SDS/PAGE, followed by immunoblotting with an antibody to galectin-2.
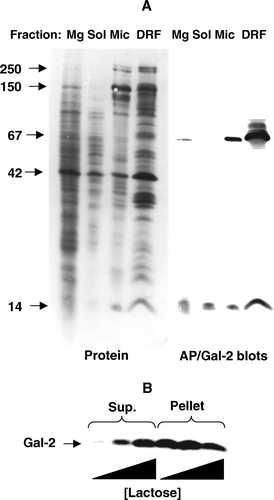
Microvillar membranes prepared by the divalent cation precipitation method are closed, right-side-out membrane vesicles Citation[18]. B shows that a brief incubation with as little as 10 mM lactose released substantial amounts of galectin-2 from the microvillar vesicles. This experiment demonstrates that at least some of the microvillar galectin-2 is located at the outer surface of the vesicles and therefore is deposited at the lumenal side of the brush border in situ.
Localization of galectin-2 and -4 by immunofluorescence microscopy
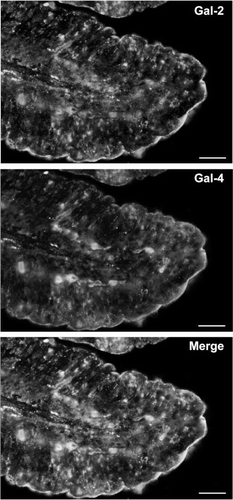
The location of galectin-2 in the brush border membrane of the enterocytes was further confirmed by immunofluorescence microscopy. As shown in galectin-2 was mainly located in the brush border, and in addition a weak intracellular labeling was seen, but galectin-2 was not detected in the goblet cells. A similar localization of galectin-2 was observed using a rabbit anti-galectin-2 (data not shown). As previously shown Citation[30], Citation[33], galectin-4 was present in the brush border, and the merged image shows extensive co-localization of the two galectins in this membrane ().
Figure 4. Localization of galectin-2 and galectin-4 in the jejunum by immunofluorescence microscopy. Double labeling for galectin-2 and galectin-4 on paraffin sections was performed as described in Methods. Labeling for both galectins is seen along the brush border of the villi. Extensive, but not complete colocalisation of the two galectins is evident in the merged micrograph. Bars, 25 µm. Published in colour in the online version.
Ligand-binding properties of microvillar galectin-2 and -4
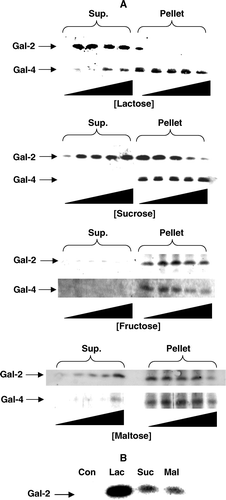
A shows that galectin-2 was much more efficiently released from the DRF by lactose (10–100 mM) than was galectin-4. Thus, 10 mM lactose was sufficient to fully displace galectin-2 from the membranes whereas galectin-4 was only partially displaced by lactose at 100 mM. In addition, galectin-2, but not galectin-4, could be released from the DRF by increasing concentrations (0.25–1.5 M) of sucrose or maltose, whereas within a similar concentration range, fructose failed to release galectin-2 as well as galectin-4 (A). To our knowledge, galectin-2 (or any other member of the galectin family) has not previously been observed to interact with glycans other than β-galactosides. As shown in B, like lactose, both sucrose and maltose also released galectin-2 from right-side-out microvillar vesicles, albeit less efficiently. This indicates that the use of detergent for preparing the DRF does not per se make the ligand/galectin-2 binding susceptible to either sucrose or maltose.
Figure 5. Release of microvillar galectin-2 and galectin-4 by different carbohydrates. (A) 50 µl samples of a microvillar DRF, prepared as described in Methods, were resuspended in 25 mM HEPES-HCl, 150 mM NaCl, pH 7.1, and incubated for 30 min at room temperature in the presence of 0 mM, 10 mM, 25 mM, 50 mM or 100 mM lactose, or 0 M, 0.25 M, 0.50 M, 1.0 M or 1.5 M of sucrose, fructose or maltose, respectively. After incubation, the samples were centrifuged at 20,000 g, 30 min, and the supernatant and pellet fractions subjected to SDS/PAGE and immunoblotting for galectins 2-and 4. (B) Samples of a right-side-out microvillar membrane vesicle preparation were resuspended in 25 mM HEPES-HCl, 150 mM NaCl, pH 7.1, in the absence of disaccharides (Con) or in the presence of 50 mM lactose (Lac), 0.5 M sucrose (Suc) or 0.5 M maltose (Mal). After incubation for 30 min at room temperature, the samples were centrifuged at 20,000 g for 30 min, and the supernatants collected and subjected to SDS/PAGE, followed by immunoblotting with an antibody to galectin-2.
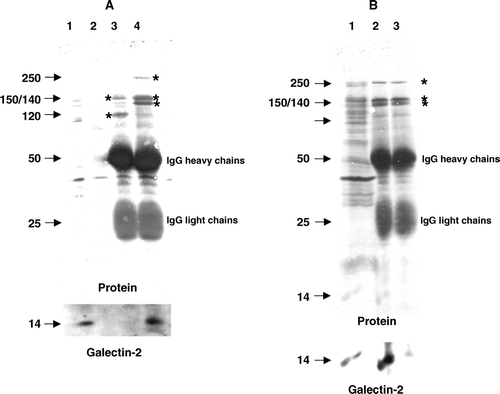
A shows an immunoisolation from solubilised microvillar DRF of two of the major lipid raft-associated brush border enzymes, aminopeptidase N and sucrase-isomaltase. Subsequent immunoblotting revealed that galectin-2 was coimmunoisolated with the latter, but not the former, of these two heavily glycosylated brush border enzymes. In contrast to this finding, galectin-4 has previously been shown to coimmunopurify with aminopeptidase N Citation[33]. Furthermore, coimmunoisolation of galectin-2 with sucrase-isomaltase was abolished in the presence of 50 mM lactose, indicating that the two proteins associate via the CRD of galectin-2 (B). Taken together, the above experiments show that the two galectins differ substantially in their preference of ligand association at the brush border membrane.
Figure 6. Coimmunopurification of galectin-2 with sucrase-isomaltase. (A) Aminopeptidase N and sucrase-isomaltase were immunoisolated from Triton X-100-solubilized DRF as described in Methods. After SDS/PAGE, galectin-2 was visualized by immunoblotting before total protein was stained with Coomassie Brilliant Blue. Lane 1; solubilized DRF, lane 2; protein A-Sepharose (control), lane 3; aminopeptidase N-coupled protein A-Sepharose, lane 4; sucrase-isomaltase-coupled protein A-Sepharose. The 150 and 120 kDa subunits of aminopeptidase N are seen in lane 3, and the 250 kDa single-chain sucrase-isomaltase, 150 kDa isomaltase and 140 kDa sucrase are seen in lane 4 (all marked by asterisks). (B) DRFs were solubilized by Triton X-100 as described in Methods and incubated for 30 min at room temperature in the absence or presence of 50 mM lactose before immunoisolation of sucrase-isomaltase. After SDS/PAGE, immunoblotting for galectin-2 was performed before staining for protein. (Asterisks mark the bands of sucrase-isomaltase.) Lane 1; solubilized DRF, lane 2; sucrase-isomaltase immunoisolated in the absence of lactose, lane 3; sucrase-isomaltase immunoisolated in the presence of lactose.
Discussion
A brief wash of microvillar membrane vesicles with lactose has previously been shown to release substantial amounts of lipid raft-associated brush border enzymes such as aminopeptidase N and alkaline phosphatase Citation[19]. This observation indicates that the integral membrane proteins after proteolytic or lipolytic cleavage of their membrane anchor by pancreatic enzymes remain at the luminal surface due to cross-linking by lectins. Given the very short half-life (6–8 h) of intestinal brush border enzymes Citation[40], lectins thus serve an important physiological function in prolonging the functional localization of the digestive enzymes in their harsh working environment.
In the present work galectin-2 was identified as a prominent component of the DRF of the enterocyte brush border. As expected for a member of the galectin family, galectin-2 showed affinity towards -galactosides Citation[1], as demonstrated by its rapid release from the DRF by lactose. In addition, release by lactose from closed, right-side-out microvillar membrane vesicles showed that galectin-2, at least to some extent, resides on the lumenal side of the brush border, much like its family member galectin-4. A weak intracellular staining by immunofluorescence microscopy and the presence of a relatively weak band in immunoblotting of the Mg2+-precipitated fraction together imply that galectin-2, like galectin-4, is also present in intracellular compartments of the enterocytes. It thus becomes secreted by a ‘non-classical’ mechanism, i.e., not mediated by an N-terminal signal sequence Citation[41]. The exact mode of non-classical secretion has been elusive for a long time, but it has been reported that a functional CRD is the primary targeting motif of galectins for the export machinery Citation[42]. However, galectin-2 might also be deposited at the brush border following lumenal secretion from neighbouring goblet cells. Thus, in a recent study, galectin-2 was reported to be expressed primarily by goblet cells in the mouse small intestine Citation[5]. Although we failed to detect this lectin in goblet cells of pig small intestine, we cannot rule out that some of the galectin-2 in the enterocyte brush border has originated from goblet cells. Along this line, we have previously shown that another brush border lectin, intelectin (identical to the intestinal lactoferrin receptor Citation[43]), is synthesized both by enterocytes and goblet cells Citation[18], and in view of the brush border being a local β-galactoside hotspot (), much of the lectins secreted by goblet cells may well be deposited there.
In addition to the lectins already mentionned, galectins 3-and -6 have also been localized to the intestinal brush border Citation[5], so the question arises: Why are so many different lectins present at this body boundary? An answer may be illustrated by the surprising observation of the present work that galectin-2 was effectively released from the DRF by sucrose and, to a lesser extent, by maltose. Although it is difficult to imagine these dietary disaccharides being relevant physiological ligands for galectin-2, this finding exemplifies the broad diversity of possible ligand interactions displayed by different members of the galectin family. Interestingly, cholesterol 3-sulfate, a widespread membrane lipid, was recently reported to be an endogeneous ligand for galectin-4 Citation[44], supporting the notion that individual galectins may have noncanonical ligands. Furthermore, the diversity amongst galectins 1–3 with regard to recognition of sialylated glycans and blood group antigens was recently studied by a microarray approach that revealed mechanistic differences in the binding properties of individual galectins Citation[45]. Given the immense variety of the glycans present as part of glycolipids and glycoproteins at the brush border, the many lectins targeted to this site most likely act in concert, rather than in mutual competition, to ensure the optimal organization/stabilization of this membrane. This may be exemplified by our observation that galectin-2 preferentially binds to sucrase-isomaltase rather than to aminopeptidase N (which was previously shown to be a ligand of galectin-4 Citation[33]).
We have previously argued that lectins, together with locally synthesized lectin-like anti-glycosyl antibodies Citation[32], act as guardians of the lipid raft microdomains of the brush border Citation[20]. The present addition of galectin-2 to the battery of brush border lectins seems to strengthen this concept and we anticipate that future work will deepen our insight into the complex network of lectin-based protein-protein – and protein-lipid interactions formed at this important body boundary.
Acknowledgements
We thank Karina Rasmussen and Lise-Lotte Niels-Christiansen for excellent technical assistance. MKT was the recipient of a scholarship from the Danish Medical research Council (grant # FSS 271-08-0214), and the work was supported by grants from the Danish Medical Research Council (grant # FSS 22-04-0425) and the Novo-Nordic Foundation (grant # NN:5293). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta 2002; 1572: 263–273
- Demetter P, Nagy N, Martin B, Mathieu A, Dumont P, Decaestecker C, Salmon I. The galectin family and digestive disease. J Pathol 2008; 215: 1–12
- Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: Matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci 2007; 64: 1679–1700
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994; 76: 597–598
- Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J Histochem Cytochem 2009; 57: 41–50
- Nio J, Kon Y, Iwanaga T. Differential cellular expression of galectin family mRNAs in the epithelial cells of the mouse digestive tract. J Histochem Cytochem 2005; 53: 1323–1334
- Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol 2007; 17: 513–520
- Delacour D, Koch A, Ackermann W, Eude-Le PI, Elsasser HP, Poirier F, Jacob R. Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo. J Cell Sci 2008; 121: 458–465
- Oka T, Murakami S, Arata Y, Hirabayashi J, Kasai K, Wada Y, Futai M. Identification and cloning of rat galectin-2: Expression is predominantly in epithelial cells of the stomach. Arch Biochem Biophys 1999; 361: 195–201
- Paclik D, Lohse K, Wiedenmann B, Dignass AU, Sturm A. Galectin-2 and -4, but not Galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm Bowel Dis 2008; 14: 1366–1372
- Paclik D, Berndt U, Guzy C, Dankof A, Danese S, Holzloehner P, Rosewicz S, Wiedenmann B, Wittig BM, Dignass AU, Sturm A. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J Mol Med 2008; 86: 1395–1406
- Hokama A, Mizoguchi E, Mizoguchi A. Roles of galectins in inflammatory bowel disease. World J Gastroenterol 2008; 14: 5133–5137
- Trier, JS. 1968. Morphology of the epithelium of the small intestine. CF Code. Handbook of physiology – alimentary canal. 6, American Physiological Society, Washington. 1125–1176.
- Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol 2004; 4: 953–964
- Sansonetti PJ. War and peace at the intestinal epithelial surface: An integrated view of bacterial commensalism versus bacterial pathogenicity. J Pediatr Gastroenterol Nutr 2008; 46(Suppl. 1)E6–7
- Christiansen K, Carlsen J. Microvillus membrane vesicles from pig small intestine. Purity and lipid composition. Biochim Biophys Acta 1981; 647: 188–195
- Hauser H, Howell K, Dawson RM, Bowyer DE. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim Biophys Acta 1980; 602: 567–577
- Wrackmeyer U, Hansen GH, Seya T, Danielsen EM. Intelectin: A novel lipid raft-associated protein in the enterocyte brush border. Biochemistry 2006; 45: 9188–9197
- Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrom BT, Hansen GH, Danielsen EM. Microvillar membrane microdomains exist at physiological temperature – role of galectin-4 as lipid raft stabilizer revealed by ‘superrafts’. J Biologic Chem 2003; 278: 15679–15684
- Danielsen EM, Hansen GH. Lipid raft organization and function in brush borders of epithelial cells. Mol Membr Biol 2006; 23: 71–79
- Delacour D, Cramm-Behrens CI, Drobecq H, Le BA, Naim HY, Jacob R. Requirement for galectin-3 in apical protein sorting. Curr Biol 2006; 16: 408–414
- Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, Andre S, Le Bivic A, Gabius HJ, Manninen A, Simons K, Huet G. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol 2005; 169: 491–501
- Stechly L, Morelle W, Dessein AF, Andre S, Grard G, Trinel D, Dejonghe MJ, Leteurtre E, Drobecq H, Trugnan G, Gabius HJ, Huet G. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic 2009; 10: 438–450
- Danielsen EM, Hansen GH. Lipid rafts in epithelial brush borders: Atypical membrane microdomains with specialized functions. Biochim Biophys Acta 2003; 1617: 1–9
- Hansen GH, Dalskov SM, Rasmussen CR, Immerdal L, Niels-Christiansen LL, Danielsen EM. Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry 2005; 44: 873–882
- Duncan MJ, Shin JS, Abraham SN. Microbial entry through caveolae: Variations on a theme. Cell Microbiol 2002; 4: 783–791
- Manes S, del Real G, Martinez A. Pathogens: Raft hijackers. Nat Rev Immunol 2003; 3: 557–568
- Rosenberger CM, Brumell JH, Finlay BB. Microbial pathogenesis: Lipid rafts as pathogen portals. Curr Biol 2000; 10: R823–825
- Shin JS, Abraham SN. Caveolae as portals of entry for microbes. Microbes Infect 2001; 3: 755–761
- Brandtzaeg P, Johansen FE. Mucosal B cells: Phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev 2005; 206: 32–63
- Pazur JH, Dresher KL, Forsberg LS. Anti-glycosyl antibodies. Two sets of isoantibodies with specificity for different carbohydrate moieties of the same glycosyl antigen. J Biol Chem 1978; 253: 1832–1837
- Hansen GH, Pedersen ED, Immerdal L, Niels-Christiansen LL, Danielsen EM. Anti-glycosyl antibodies in lipid rafts of the enterocyte brush border: A possible host defense against pathogens. Am J Physiol Gastrointest Liver Physiol 2005; 289: G1100–1107
- Danielsen EM, Van Deurs B. Galectin-4 and small intestinal brush border enzymes form clusters. Mol Biol Cell 1997; 8: 2241–2251
- Hansen GH, Wetterberg LL, Sjostrom H, Noren O. Immunogold labelling is a quantitative method as demonstrated by studies on aminopeptidase N in microvillar membrane vesicles. Histochem J 1992; 24: 132–136
- Sjostrom H, Noren O, Christiansen L, Wacker H, Semenza G. A fully active, two-active-site, single-chain sucrase.isomaltase from pig small intestine. Implications for the biosynthesis of a mammalian integral stalked membrane protein. J Biol Chem 1980; 255: 11332–11338
- Booth AG, Kenny AJ. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J 1974; 142: 575–581
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- Lotan R, Skutelsky E, Danon D, Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem 1975; 250: 8518–8523
- Iskratsch T, Braun A, Paschinger K, Wilson IB. Specificity analysis of lectins and antibodies using remodeled glycoproteins. Anal Biochem. 2009; 386: 133–46
- Dudley MA, Hachey DL, Quaroni A, Hutchens TW, Nichols BL, Rosenberger J, Perkinson JS, Cook G, Reeds PJ. In vivo sucrase-isomaltase and lactase-phlorizin hydrolase turnover in the fed adult rat. J Biol Chem 1993; 268: 13609–13616
- Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function and therapeutic potential. Expert Rev Mol Med 2008; 10: e17
- Seelenmeyer C, Wegehingel S, Tews I, Kunzler M, Aebi M, Nickel W. Cell surface counter receptors are essential components of the unconventional export machinery of galectin-1. J Cell Biol 2005; 171: 373–381
- Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: Structure and function. Cell Mol Life Sci 2005; 62: 2560–2575
- Ideo H, Seko A, Yamashita K. Recognition mechanism of galectin-4 for cholesterol 3-sulfate. J Biol Chem 2007; 282: 21081–21089
- Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem 2008; 283: 10109–10123