Abstract
Mutations in the gene encoding the kidney anion exchanger 1 (kAE1) can lead to distal renal tubular acidosis (dRTA). dRTA mutations reported within the carboxyl (C)-terminal tail of kAE1 result in apical mis-targeting of the exchanger in polarized renal epithelial cells. As kAE1 physically interacts with the μ subunit of epithelial adaptor protein 1 B (AP-1B), we investigated the role of heterologously expressed μ1B subunit of the AP-1B complex for kAE1 retention to the basolateral membrane in polarized porcine LLC-PK1 renal epithelial cells that are devoid of endogenous AP-1B. We confirmed the interaction and close proximity between kAE1 and μ1B using immunoprecipitation and proximity ligation assay, respectively. Expressing the human μ1B subunit in these cells decreased significantly the amount of cell surface kAE1 at the steady state, but had no significant effect on kAE1 recycling and endocytosis. We show that (i) heterologous expression of μ1B displaces the physical interaction of endogenous GAPDH with kAE1 WT supporting that both AP-1B and GAPDH proteins bind to an overlapping site on kAE1 and (ii) phosphorylation of tyrosine 904 within the potential YDEV interaction motif does not alter the kAE1/AP-1B interaction. We conclude that μ1B subunit is not involved in recycling of kAE1.
Introduction
The kidneys play an essential role in regulating pH homeostasis. In the distal nephron, the kidney anion exchanger 1 (kAE1) is expressed at the basolateral membrane of the alpha intercalated cells where it mediates the exchange of chloride for bicarbonate (Kollert-Jons et al., Citation1993). Along with the apical H+-ATPase and cytosolic carbonic anhydrase II, kAE1 participates in urine acidification through the reabsorption of bicarbonate into the blood, while protons are secreted via the apical H+-ATPase into the urine.
Mutations in the SLC4A1 gene that encodes for AE1 including point mutation, deletion and frameshift mutations cause hereditary spherocytosis, Southeast Asian ovalocytosis or distal renal tubular acidosis (dRTA) or rarely a combination of red blood cell anomalies and renal defects (Fry et al., Citation2012; Karet et al., Citation1998; Shao et al., Citation2010; Zhang et al., Citation2012). dRTA is characterized by development of metabolic acidosis, hypokalemia, hyperchloremia, inability to acidify the urine, nephrocalcinosis and kidney stones if not treated (Alper, Citation2010).
The majority of SLC4A1 dominant dRTA mutations are reported to affect kAE1 protein trafficking to the surface of immortalized renal epithelial cells. For example, kAE1 R901X truncated mutant is mistargeted to the apical or to both apical and basolateral membranes when expressed in polarized Madin-Darby canine kidney (MDCK) cells depending on the degree of cell polarization (Cordat et al., Citation2006; Devonald et al., Citation2003; Toye et al., Citation2004), without affecting kAE1 protein function (Quilty et al., Citation2002). However, examination of the intercalated cells of a knockin mouse line carrying a dominant kAE1 dRTA mutation or of biopsies from dRTA patients showed a sharply reduced number of renal intercalated cells, but in the few remaining intercalated cells the mutant was either basolateral or cytosolic (Han et al., Citation2002; Mumtaz et al., Citation2017; Vichot et al., Citation2017). These data show that our understanding of the molecular root for dRTA and of kAE1 trafficking remains incomplete.
kAE1 protein is composed of a large N-terminal cytosolic domain, a 14 transmembrane domain, which is responsible for the anion exchange and a small cytosolic C-terminus (Arakawa et al., Citation2015). This last domain interacts with proteins that are important for the transporter’s trafficking and/or function. Carbonic anhydrase II interaction with kAE1 forms a functional metabolon that increases the ion exchanger’s activity (Vince & Reithmeier, Citation1998, Citation2000; Vince et al., Citation2000). The β subunit of Na+/K+-ATPase also interacts with kAE1 C-terminus, as knockdown of the former significantly reduced cell surface expression of the latter (Su et al., Citation2015). Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) (Su et al., Citation2011) and adaptor protein 1 (AP-1) (Almomani et al., Citation2012; Sawasdee et al., Citation2010) also play an essential role for kAE1 trafficking and residency at the plasma membrane. Importantly, the GAPDH interaction site on kAE1 (D902EYDEV) overlaps with that of AP-1 interaction site (Y904DEV907) (Almomani et al., Citation2012; Sawasdee et al., Citation2010; Su et al., Citation2011).
Our previous work showed that AP-1A is essential for proper plasma membrane trafficking of newly synthesized kAE1 protein (Almomani et al., Citation2012). We have reported that kAE1 not only co-immunoprecipitates with the ubiquitous AP-1A but also with the epithelial specific AP-1B. This adaptor complex sorts basolateral proteins such as the vesicular stomatitis virus G (VSV-G) protein in its biosynthetic route or the transferrin receptor (TfR) in both its biosynthetic and recycling routes in MDCK cells (Gravotta et al., Citation2007). Low-density lipoprotein receptor (LDLR) was mistargeted to the apical membrane when the interacting subunit μ1B was knocked down in MDCK cells. When expressed in Lilly Laboratories porcine kidney (LLC-PK1) cells, which lack μ1B, both LDLR and TfR were mistargeted to the apical membrane (Gan et al., Citation2002). Thus, AP-1B protein complex plays a specific role in sorting basolateral membrane proteins in their biosynthetic and recycling routes.
When the endogenous μ1A and to a less extent μ1B were knocked down in MDCK cells, kAE1 was rapidly degraded and did not reach the cell surface (Almomani et al., Citation2012). When either human μ1A or B was expressed in the knocked down cells, they stabilized and partially rescued kAE1 trafficking to the cell surface. Based on these previous findings, we aimed at identifying the physiological role of kAE1 and AP-1B interaction. We hypothesized that the interaction between kAE1 and μ1B is important for kAE1 surface expression and recycling in renal epithelial cells. AP-1B would help maintaining the amount of basolateral kAE1 necessary to efficiently reabsorb bicarbonate into the blood. Herein, we examined the effect of μ1B heterologous expression on kAE1 cell surface amount, endocytosis and recycling in LLC-PK1 cells that are devoid of μ1B.
Materials and methods
Plasmid constructs and antibodies
The pCDNA3 plasmid construct containing human kAE1-wild type cDNA with extracellular hemagglutinin (HA) or myc epitope in position 557 (numbering as per the erythroid isoform) was used to express kAE1 protein. The constructs encoding human μ1 A-HA and μ1B-HA were kindly provided by Dr. Heike Folsch (Northwestern University). The mouse monoclonal antibody against the HA epitope was purchased from Covance (Covance, Princeton, NJ). The mouse monoclonal and the rabbit polyclonal antibodies directed against Myc epitope were purchased from Cell signaling, and from Santa Cruz Biotechnology, respectively. Rabbit polyclonal antibody against the Na+/K+ ATPase was purchased from Cell signaling. Rat anti-HA antibody was purchased from Roche (Roche Diagnostics). Anti-AE1 antibodies were provided by Drs. Reinhart Reithmeier (University of Toronto) and Joe Casey (University of Alberta), Bric 155 antibody was purchased from the International Blood Group Laboratory, the anti-phosphotyrosine 904 antibody was a kind gift from Dr. Ashley Toye (Bristol University). The anti-GAPDH antibody was purchased from Millipore.
Cell culture
MDCK (CCL-34), LLC-PK1 (CL-101) and HEK 293 T (CRL-11268) cells were purchased from the American Type Culture Collection (ATCC). MDCK or LLC-PK1 cells stably expressing kAE1 WT were prepared according to the following procedure: HEK 293 T cells were transfected with p-VPack-GP, p-VPack-VSV-G and pFB-Neo-kAE1 WT plasmids using XtremeGENE9 (Roche Applied Science). Cell culture supernatants containing infectious viral particles were added to dividing LLC-PK1 cells supplemented with 8 μg/ml of polybrene (Sigma-Aldrich). After 24-hour incubation, a heterogenous population of LLC-PK1 or MDCK cells expressing kAE1 was selected with 3 mg/ml geneticin (Sigma-Aldrich). The cells were further maintained in DMEM:F12 containing 10% FBS, 3 mg/ml geneticin and 1 mg/ml penicillin/streptomycin. For immunofluorescence experiments, 50% confluent LLC-PK1 cells were transfected with 1 μg cDNA and 4 μl XtremeGENE 9.
For LLC-PK1 cells stably expressing μ1B HA, 50% confluent HEK 293 T cells were transfected with PLVX-IRES plasmid (Clontech) containing μ1B HA cDNA and the lentivirus packaging system (Clontech) using XtremeGENE9. Cell culture supernatants containing infectious viral particles were collected after 48 h and added to dividing LLC-PK1 cells complemented with 8 μg/ml of polybrene (Sigma-Aldrich). After 24-hour incubation, a heterogeneous population of LLC-PK1 cells stably expressing μ1B HA was selected with 1 mg/ml hygromycin (Invitrogen).
LLC-PK1 or MDCK cells expressing both kAE1 WT (myc or HA) and μ1B-HA were either prepared by transient transfection using XtremeGENE 9 (according to the manufacturer’s instructions) or the NEON transfection system (see below), or by transfecting HEK 293T cells with p-VPack-GP, p-VPack-VSV-G, and pFB-Neo-kAE1 WT plasmid using XtremeGENE9. HEK 293 cell culture supernatants containing infectious viral particles were added to cells stably expressing μ1B-HA protein. After 24-hour incubation, cells expressing both kAE1 and μ1B-HA were selected and maintained by complementing the growth medium with both 1 mg/ml geneticin and 1 mg/ml hygromycin.
Immunoprecipitation and immunoblotting
Confluent LLC-PK1 cells transiently expressing μ1B HA and pcDNA3 empty vector or μ1B HA and kAE1-myc were lysed in PBS containing 1% Triton X-100 and protease inhibitors (1 μg/ml aprotinin, 2 μg/ml leupeptin, 1 μg/ml pepstatin A and 100 μg/ml PMSF). Protein concentration was measured using BCA assay (Pierce). A fraction of the cell lysate (15 μg) was saved (Total lysate) and the remaining lysates were incubated with 4 μl rabbit anti-AE1 N-terminus antibody (provided by Dr. Reinhart Reithmeier, University of Toronto) at 4 °C for 2 hours prior to incubation with 40 μl protein G-Sepharose beads (Thermo Scientific, Rockford, IL) for 1 hour at 4 °C. The bound proteins were eluted from the beads with 40 μl Laemmli buffer and detected by immunoblotting with mouse anti-myc and mouse anti-HA antibodies. The blots were further incubated with anti-mouse secondary antibody coupled to horseradish peroxidase and developed by enhanced chemiluminescence (ECL western blotting substrate from Thermo Scientific, or ECL prime western blotting detection reagent from GE Healthcare). Relative band intensities were determined using the freeware ImageJ.
Proximity ligation assay
Semi-confluent LLC-PK1 cells were seeded on coverslips and transfected with cDNA encoding either empty vector and μ1B as a negative control, kAE1 myc and CAII as a positive control, or with kAE1 myc and μ1B HA. The following day, the cells were treated as per the Duolink detection reagent kit’s (Olink Bioscience) instructions: they were fixed with 4% paraformaldehyde, then quenched with 50 mM NH4Cl and permeabilized with 0.2% Triton X-100. The slides were then blocked in 5% donkey serum (Jackson Immunoscience, Jackson Immuno Research Europe Ltd, Suffolk, UK), 2 mg/ml salmon sperm (Sigma), 5 mg/ml bovine serum albumin (Sigma) and 2 mM cysteine (Sigma) in TBS-Tween (TBST) with 5 mM EDTA (Sigma) for 30 min in humidifying chamber at 37 °C. The samples were then incubated with the appropriate combination of primary antibodies (mouse anti-HA and either rabbit anti-myc or rabbit anti-CAII) diluted 1:50 in blocking solution for 1 hour at 37 °C. After washing, the slides were incubated with Duolink PLA Rabbit MINUS and PLA Mouse PLUS proximity probes (Olink Bioscience, Uppsala, Sweden) and proximity ligation was performed using the Duolink detection reagent kit (Olink Bioscience) according to the manufacturer’s protocol. High resolution images were acquired using the 63× oil immersion objective on an Angstrom Illumination system (Quorum Technologies Inc.) equipped with OptiGrid structured illumination (Qioptiq), excitation and emission filter wheels (Ludl Electronic Products) and the Flash 4.0 camera (Hamamatsu). The Angstrom and associated hardware are mounted to the 100% sideport on a DMI6000 (Leica Microsystems), fully motorized inverted microscope. All hardware was controlled with the Metamorph software (Molecular Devices).
Phosphorylation experiment
MDCK cells were either transfected with 2.5 μg kAE1-myc and 2.5 μg μ1 A HA cDNA or 2.5 μg kAE1-myc and 2.5 μg μ1B HA cDNA using the NEON electroporation system (Invitrogen) (1400-V pulse voltage, 20-ms pulse width, and 3 pulses). Twenty-four hours after transfection, cells were either treated with 200 μM pervanadate in warm DMEM:F12 medium containing 10% FBS for 30 min at 37 °C or kept as control (warm medium only). The cells were lysed with 500 μl phosphatase inhibitor lysis buffer (0.05% Triton X-100, 10 mM HEPES, 100 mM NaCl, 14 mM beta-mercaptoethanol, 0.5 mM EGTA, MgCl2, PhosSTOP Phosphatase Inhibitor Cocktail Tablets (Roche), 100 nM Calyculin A (Sigma)) and protein concentrations were measured using a BCA assay. A 20 μg aliquot of the total cell lysate was kept as the total fraction. The remaining lysate (1 mg) was divided into two halves: one half was immunoprecipitated with 3 μl mouse anti-myc antibody and the other with 3 μl mouse anti Bric 155 antibody for 2 hours at 4 °C, followed by 40 μl protein G-Sepharose. The bound proteins were eluted with 50 μl Laemmli buffer and phosphorylated kAE1 was immunoblotted with an antibody raised against phosphorylated tyrosine 904. Total kAE1 was detected by a rabbit anti-AE1 N-terminus antibody (provided by Dr. Joe Casey, University of Alberta), μ1 A or μ1B HA proteins were detected by rat anti-HA antibody.
Competition between μ1 and GAPDH binding to kAE1 protein
MDCK cells were transfected with 2 μg of kAE1-myc and 5 μg pCDNA3 empty vector cDNA or with 2 μg of kAE1-myc and 5 μg μ1 A HA or μ1B HA cDNA using the NEON electroporation system. Twenty-four hours after transfection, the cells were lysed and fractions containing 15 μg of proteins were kept as total cell lysate. Proteins in the remaining lysate (approximately 300 μg) were immunoprecipitated with 3 μl rabbit anti-kAE1-myc antibody, followed by 40 μl protein G-Sepharose. Eluted proteins were immunoblotted using rat anti-HA antibody to detect μ1 A HA or μ1B HA. Mouse anti-GAPDH antibody was used to detect the endogenous GAPDH protein. Relative band intensities were determined using the freeware ImageJ.
Cell surface biotinylation
Confluent LLC-PK1 cells expressing kAE1 were transfected with pCDNA3 empty vector or μ1B HA. Twenty-four hours after transfection, the cells were incubated twice with EZ-Link Sulfo-NHS-SS-Biotin reagent (1 mg/ml) (Pierce) at 4 °C for 15 min in borate buffer (10 mM Boric acid, 145 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, pH 9). The excess of biotin was quenched 4 times with 100 mM glycine in PBS. The cells were lysed in 300 μl TNT lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.2% SDS, 1 μg/ml aprotinin, 2 μg/ml leupeptin, 1 μg/ml pepstatin A and 100 μg/ml PMSF). 10% of the lysate was kept as total lysate and the remaining was incubated with 100 μl streptavidin agarose resin (Thermo Scientific) for 1 hour at 4 °C. Cell surface proteins were eluted with 50 μl Laemmli buffer that contained 15% beta-mercaptoethanol. Samples were immunoblotted with a mouse anti-HA antibody to detect both kAE1 and μ1B proteins. The membranes were stripped (7 M Guanidine HCl, 50 mM Glycine, 100 mM KCl, 5 μM EDTA, 19.6 mM β-Mercaptoethanol) and reincubated with rabbit anti-Na+/K+-ATPase antibody. Relative band intensities were determined using the freeware Image J. To quantify the ratios of surface kAE1, we considered that amounts loaded in “Total kAE1” fraction only represented 1/9 of the amount incubated with avidin beads.
Immunofluorescence
Image acquisition was done using an Olympus IX81 microscope equipped with a Nipkow spinning disk optimized by Quorum Technologies (Guelph, ON, Canada) and a 63× oil objective.
• Surface kAE1 at the steady state
To detect kAE1 protein at the cell surface at the steady state, confluent LL-CPK1 cells stably expressing kAE1 myc or both kAE1 myc and μ1B HA were grown on semi-permeable filters for 7 days to polarization. Growth medium was changed every day. The cells were next fixed with 1% PFA for 10 min on ice to avoid permeabilization and surface kAE1 was detected by adding mouse anti-myc antibody for 30 min followed by anti-mouse Cy3 antibody (red). The cells were then permeabilized with 0.2% Triton-X 100 for 15 min, blocked with 1% bovine serum albumin (BSA) before incubation with rat anti-HA antibody followed by anti-rat Alexa 488 antibody (shown in blue) to detect μ1B HA. Mouse anti-myc antibody followed by anti-mouse Dylight 649 antibody detected total kAE1 (shown in green).
• Endocytosis experiment
LL-CPK1 cells stably expressing kAE1 myc or both kAE1 myc and μ1B HA were grown on semi-permeable filters for 7 days to polarization. Growth medium was changed every day. Mouse anti-myc antibody was added to the basolateral side of LLC-PK1 cells in warm medium at 37 °C for 1 h to allow antibody binding to the extracellular epitope and endocytosis. After rinsing the cells with ice cold PBS to stop endocytosis, the non-endocytosed antibodies were acid-stripped with ice cold Acetate buffer (500 mM NaCl, 200 mM acetic acid in PBS, pH 3) for 10 min (Gan et al., Citation2002), then the extracellular pH was neutralized by washing with cold PBS. To detect endocytosed kAE1 myc, LLC-PK1 cells were next fixed with 1% PFA, permeabilized with 0.2% Triton-X 100 and blocked with 1% bovine serum albumin (BSA) for 20 min before incubation with the Cy3 coupled anti-mouse antibody. Rat anti-HA antibody was added for 30 minutes followed by Alexa 488 coupled anti-rat antibody (Invitrogen, shown in blue) to detect μ1B HA. Mouse anti-myc antibody was added again to the cells followed by anti-mouse Dylight 649 (shown in green) secondary antibody to detect total kAE1.
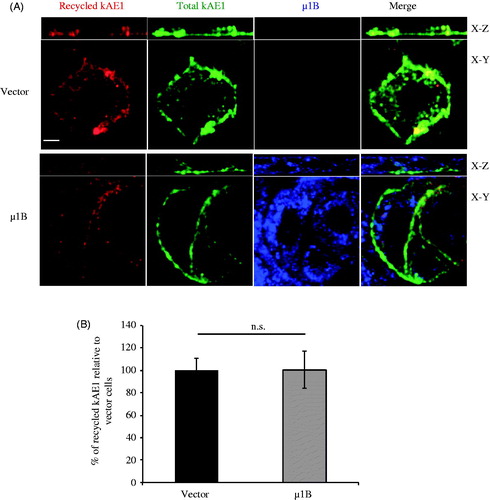
• Recycling experiment
LL-CPK1 cells stably expressing kAE1 myc or both kAE1 myc and μ1B HA were grown on semi-permeable filters for 7 days to polarization. Growth medium was changed every day. Mouse anti-myc antibody was added to the basolateral side of LLC-PK1 cells in warm medium at 37 °C for 1 h to allow antibody binding to the extracellular epitope and endocytosis. After rinsing the cells with ice cold PBS to stop endocytosis, the non-endocytosed antibodies were acid-stripped with ice cold Acetate buffer (500 mM NaCl, 200 mM acetic acid in PBS, pH 3) for 10 min (Gan et al., Citation2002), then the extracellular pH was neutralized by washing with cold PBS. Protein recycling was induced by incubating cells at 37° C for 90 min in warm medium. The recycled proteins were then detected by fixing the cells with 1% PFA for 10 minutes on ice followed by Cy3 coupled anti-mouse antibody. To detect μ1B-HA, the cells were permeabilized with 0.2% Triton-X 100, blocked with 1% BSA before incubation with rat anti-HA antibody followed by anti-rat Alexa488 antibody (Invitrogen, shown in blue). Mouse anti-myc and anti-mouse Dylight 649 antibodies detected total kAE1 (shown in green).
Immunofluorescence and statistical analysis
Quantification of surface, endocytosed or recycled kAE1 was calculated as following: For each cell ± μ1B, the average background corrected Cy3 fluorescence (reflecting the amount of surface kAE1) was divided by the average background corrected Cy5 fluorescence (shown in green, reflecting the amount of total kAE1 protein). The ratios for vector-transfected cells were then set at 100% and compared with the ratios of cells expressing μ1B. Imaging settings (exposure times and laser power) were kept the same for each channel for cells ± mu1B within each experiment. Intensities from a minimum of 63 cells from three independent experiments were measured to calculate the percentage of surface, endocytosed and recycled kAE1 protein relative to vector transfected cells. Results are expressed as mean values ± standard error of the mean (SE). Statistical comparisons were made using Student’s t-test to compare between two groups of cells. All the experiments were repeated at least three independent times. p < 0.05 was considered significant.
Results
kAE1 interacts with μ1B in LLC-PK1 cells
In our previous work, we observed that kAE1 co-immunoprecipitates with both μ1A and μ1B in MDCK cells (Almomani et al., Citation2012). To confirm that kAE1 and μ1B interact, we transiently transfected LLC-PK1 cells [that neither express endogenous μ1B (Gan et al., Citation2002) nor kAE1 (Devonald et al., Citation2003)] with μ1B-HA and kAE1 myc or with μ1B-HA only as a negative control. Heterologously expressed μ1B shows a perinuclear localization (Fölsch, Citation2015; Nakatsu & Ohno, Citation2003) and restores a functional AP-1B complex in these cells (Duffield et al., Citation2004; Folsch et al., Citation1999, Citation2003). As seen on , kAE1 protein migrates as two main bands in LLC-PK1 cells: the top band corresponds to proteins carrying complex oligosaccharide (open circle) and the bottom band corresponds to kAE1 carrying high mannose oligosaccharide (closed circle) (Almomani et al., Citation2016; Cordat et al., Citation2006; Toye et al., Citation2004). We observed that μ1B co-immunoprecipitates with kAE1 in LLC-PK1 cells transfected with both kAE1 and μ1B (lane 4), as seen by the appearance of the expected 50 kDa band (corresponding to μ1B) that is absent from cells expressing kAE1 only ( lane 7). These results, which are in agreement with our previous findings in MDCK cells (Almomani et al., Citation2012), thus show that μ1B interacts with kAE1 in LLC-PK1 cells ().
Figure 1. µ1B interacts and colocalizes in the perinuclear region with kAE1 in LLC-PK1 cells. (A), In LLC-PK1 (left panels) or MDCK (right panels) cell lysates containing either kAE1-myc only, µ1B HA only or kAE1-myc and µ1B-HA proteins, kAE1 was immunoprecipitated with rabbit anti-myc (kAE1) antibody before immunoblotting with anti-myc antibody to detect kAE1 and with anti-HA to detect μ1B protein. IP means immunoprecipitation. Open circle corresponds to kAE1 carrying complex oligosaccharides, and filled circle indicates kAE1 carrying high mannose oligosaccharides. µ1B migrates as a 50 kDa band. (B), For the proximity ligation assay, LLC-PK1 cells were transiently transfected with empty vector and μ1B as a negative control, kAE1 and CAII as a positive control, and with kAE1 and µ1B. Red dots appear when the two proteins are within 30–40 nm from each other. Nuclei were stained with DAPI (blue). Bar = 10 μm. (C), Immunofluorescence experiment showing colocalization between kAE1-myc and µ1B HA. Polarized LLC-PK1 cells expressing kAE1 and µ1B were fixed, permeabilized, blocked before incubation with an anti-myc antibody (kAE1) followed by anti-mouse antibody coupled to Alexa 488 (green) and anti-HA antibody (µ1B) followed by Cy5 coupled antibody (shown in red). Bar = 5 μm. The inset shows a region of the cell with yellow staining indicating colocalization.
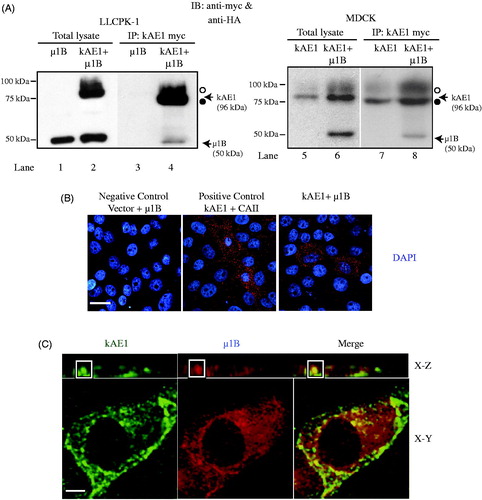
To confirm this result, we performed a proximity ligation assay, which provides a positive signal if the two proteins of interest are within 30 to 40 nm distance from each other. Using LLC-PK1 cells that were either transiently transfected with empty vector and μ1B HA cDNA as a negative control, or with kAE1 myc and μ1B HA cDNA, we assessed the proximity between kAE1 and carbonic anhydrase II (as a positive control) or with μ1B HA. As seen on , red fluorescent dots were detected in the positive control and in cells expressing kAE1 and mutants and μ1B but not in the negative control sample. These results confirm that kAE1 is in close proximity to μ1B proteins and support our immunoprecipitation results.
We further determined whether kAE1 and μ1B colocalize in the same organelle in cells using immunofluorescence with an anti-myc antibody to detect kAE1 (, green) and with an anti-HA antibody to detect μ1B (red). The predominant perinuclear staining observed for μ1B in transfected LLC-PK1 cells support that it is most likely incorporated into functional AP-1B complexes as previously shown (Gan et al., Citation2002) (). Our results show that μ1B and a minor sub-set of kAE1 colocalize in the perinuclear region as shown by the yellow signal within the inset, confirming that kAE1 and μ1B are at the same confocal plane in renal epithelial cells. Together, our immunoprecipitation, proximity ligation assay and immunofluorescence results support that kAE1 is in close proximity to and interacts with μ1B subunit of the AP-1B adaptor complex.
Phosphorylation of the C-terminal tyrosine 904 does not affect kAE1/μ1B interaction
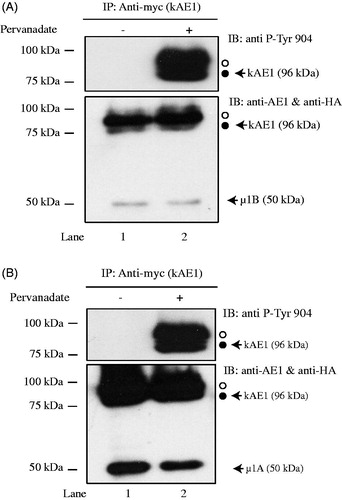
Tyrosine 904 in the carboxyl-terminal domain of kAE1 is phosphorylated in MDCK cells (Williamson et al., Citation2008). This tyrosine is part of the canonical YXXΦ binding site for μ1B. As our results support the interaction between μ1B and kAE1 C-terminus, we next assessed the effect of kAE1 phosphorylation on kAE1/μ1B binding. MDCK cells stably expressing kAE1 myc were transiently transfected with μ1B HA and either kept in control conditions or treated for 30 minutes with pervanadate (Williamson et al., Citation2008) before kAE1 immunoprecipitation and immunoblotting with either anti-myc antibody, an anti-phosphorylated tyrosine 904 antibody, or anti-HA antibody to detect co-immunoprecipitated μ1B HA. shows that in conditions where tyrosine 904 was phosphorylated (, lane 2), μ1B HA co-immunoprecipitates with kAE1, supporting that the phosphorylation status of this tyrosine does not dramatically impair the interaction between kAE1 and μ1B subunit. Of note, tyrosine 904 phosphorylation did not affect the interaction between kAE1 and μ1 A either (, lane 2). As μ1 A and μ1B binding sites on Y904DEV907 amino acids overlap with the GAPDH interaction site (D902EYDEV) (Su et al., Citation2011), we next tested whether μ1B and GAPDH are competing for the same or an overlapping interaction site on kAE1.
Figure 2. Phosphorylation of tyrosine 904 does not impair µ1B HA interaction with kAE1. MDCK cells expressing kAE1-myc and µ1B HA were either kept in control conditions (lane 1) or treated with pervanadate for 30 minutes (lane 2) prior to cell lysis and imunoprecipitation of kAE1 proteins. Eluted proteins were resolved by immunoblot and identified using an anti-HA antibody to detect µ1B, anti-kAE1 antibody to detect kAE1 and anti-phospho-tyrosine 904 antibody to detect phosphorylated kAE1. Open circle corresponds to kAE1 carrying complex oligosaccharides, and filled circle indicates kAE1 carrying high mannose oligosaccharides. This is representative of 3 independent experiments.
μ1B and GAPDH share a common binding site on kAE1
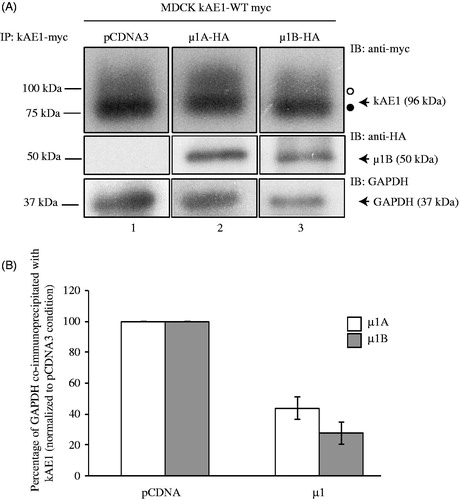
The kAE1 protein physically interacts with GAPDH via the carboxyl-terminal D902EYDE motif, which encompasses the binding site for μ1 A that was reported by Sawasdee et al. (Citation2010). We thus asked whether heterologously expressing μ1B subunit impedes endogenous GAPDH binding to kAE1. kAE1 myc stably expressing-MDCK cells were transiently transfected with vector cDNA or cDNA encoding for μ1B-HA and kAE1 was then immunoprecipitated prior to detection with anti-myc antibody (to detect kAE1), anti-HA antibody (to detect μ1B) and anti-GAPDH antibody to detect endogenous GAPDH (). Expression of μ1B reduced GAPDH interaction with kAE1 as seen by the decreased band intensity of GAPDH in (compare lane 3 to lane 1). Similar observations were made with the μ1 A isoform (lane 2, ). Quantification of the relative band intensities from a minimum of 4 independent experiments confirmed that only 44 ± 7% (n = 5, ± SEM) and 27 ± 7% (n = 4, ± SEM) of GAPDH co-immunoprecipitated with kAE1 protein after expression of μ1 A and μ1B subunits, respectively (). These experiments support that GAPDH and adaptor protein complexes AP-1A and B compete for the same binding site on kAE1 carboxyl-terminus.
Figure 3. Expression of µ1B subunit displaces GAPDH interaction with kAE1 carboxyl-terminus. (A), MDCK cells expressing kAE1-myc and either vector cDNA (lane 1), cDNA-encoding µ1A-HA (lane 2) or µ1B-HA (lane 3) were lysed and kAE1 was immunoprecipitated with a rabbit anti-myc antibody. Proteins were then immunoblotted using mouse anti-myc antibody (kAE1), anti-HA antibody (µ1A or B) and anti-GAPDH antibody. (B), GAPDH and kAE1 band intensities were measured using ImageJ software and the ratio of GAPDH band intensity after expression of either µ1A or µ1B normalized to GAPDH band intensity in absence of µ1A or B was measured. Error bars correspond to SEM, from a minimum of 3 independent experiments. Star indicates significant difference versus corresponding vector-transfected cells.
Expression of μ1B decreases cell surface amount of kAE1 in polarized LLC-PK1 cells
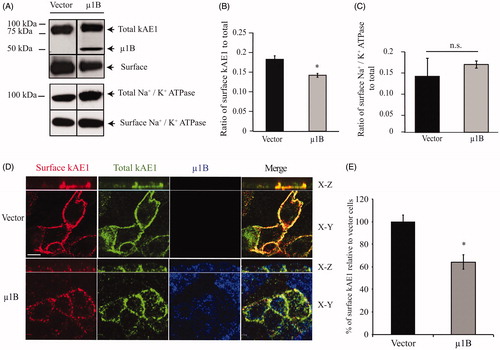
AP-1B is located in common recycling endosomes and is necessary for recycling of basolateral membrane proteins (Ang et al., Citation2004). We therefore determined whether expression of μ1B affects the amount of kAE1 or Na+/K+-ATPase as a control at the plasma membrane (). We observed that expression of μ1B decreased plasma membrane abundance of kAE1 but had no significant effect on the ATPase, in agreement with previous reports (Farr et al., Citation2009; Folsch et al., Citation1999). To confirm the effect on kAE1, we next performed immunofluorescence experiments. Polarized LLC-PK1 cells stably expressing kAE1 with an extracellular epitope and μ1B were stained for cell surface kAE1 (red), total kAE1 (green) and μ1B expression (blue) (). Comparison of the fluorescence intensity of surface kAE1 ± μ1B using Volocity (see materials and methods) showed that μ1B expression resulted in a significant decrease of the amount of surface kAE1 (64 ± 4% of the vector-transfected cells) and a more “patchy” distribution of the protein. Thus, this experiment showed a significant decrease in cell surface kAE1 after μ1B expression.
Figure 4. In polarized LLC-PK1 cells, kAE1 surface abundance decreases upon of µ1B expression. (A) Confluent LLC-PK1 cells expressing kAE1-HA only or kAE1 and µ1B-HA were incubated with membrane impermeant EZ-Link Sulfo-NHS-SS-Biotin reagent followed by streptavidin resin. Surface proteins were immunoblotted with mouse anti-HA antibody to detect kAE1 and µ1B. The immunoblot membranes were stripped and reincubated with rabbit anti-Na+/K+-ATPase to detect surface and total endogenous protein. (B), Histogram showing the ratio of surface to total kAE1 in cells expressing kAE1 only or with µ1B. Error bars correspond to SEM, from a minimum of 3 independent experiments. (C), Histogram showing the ratio of surface to total endogenous Na+/K+-ATPase in cells expressing kAE1 only or with µ1B. *p < 0.05 compared to vector-transfected cells. Error bars correspond to SEM from a minimum of 3 independent experiments. (D), Immunofluorescence experiment of polarized LLC-PK1 cells showing surface kAE1 in the presence and absence of µ1B. LLC-PK1 cells expressing kAE1 and µ1B-HA were grown on filters to polarization, then fixed and incubated with anti-myc antibody to detect surface kAE1 followed by a Cy3-coupled antibody (red). The cells were then permeabilized and incubated with anti-HA antibody to detect µ1B followed by an Alexa 488-coupled antibody (shown in blue). Total kAE1 was detected with an anti-myc antibody followed by a Dylight 649-coupled antibody (shown in green to facilitate observation). Bar =5 μm. (E), Histogram representing the percentage of surface kAE1 in the presence of µ1B relative to vector-transfected cells (see materials and methods). Mean fluorescence intensities for at least 50 cells for each condition from 3 independent experiments were measured using Volocity Image analysis software. Bars correspond to SEM. *p < 0.05 versus vector transfected cells.
The decrease in surface kAE1 upon μ1B expression could have three origins: expression of μ1B could (i) reduce the amount of newly synthesized kAE1 protein that reaches the cell surface, (ii) accelerate the rate of endocytosis of kAE1; or (iii) reduce the amount of recycled kAE1 to the cell surface. Thus we next examined the effect of μ1B expression on kAE1 endocytosis.
The expression of μ1B does not affect kAE1 endocytosis in polarized cells
We next assessed whether µ1B subunit expression altered kAE1 internalization. After labeling surface kAE1 proteins, we first verified the efficiency of the acid stripping in removing bound antibodies on polarized LLC-PK1 cells stably expressing kAE1-myc or both kAE1-myc and µ1B HA. As shown in , the acid wash removed all the non-endocytosed antibodies as indicated from the absence of red labeling that reflects surface kAE1.
Figure 5. µ1B expression has no effect on kAE1 endocytosis rate. (A), Immunofluorescence experiment controlling for the efficacy of the acid wash. Polarized LLC-PK1 cells stably expressing kAE1-myc or both kAE1-myc and µ1B-HA were incubated with anti-myc antibody for 1 h at 4 °C, washed with acetate buffer (pH 3) for 10 min, then fixed and incubated with Cy3-coupled antibody (red) to detect surface antibodies after the acid wash. The cells were permeabilized, incubated with anti-HA antibody followed by Alexa488-coupled antibody to detect µ1B (shown in blue), and anti-myc antibody followed by Dylight649-coupled antibody to detect the total kAE1 (shown in green). Bar = 5 μm. (B), polarized LLC-PK1 cells stably expressing kAE1-myc or both kAE1-myc and µ1B HA were incubated with anti-myc antibody for 1 h at 37 °C cell incubator, then washed with acetate buffer (pH 3) prior to fixation, permeabilized and incubated with Cy3-coupled antibody (red). µ1B-HA was detected with anti-HA antibody followed by Alexa488-coupled antibody (shown in blue) and total kAE1 with an anti-myc antibody coupled to Dylight 649 (green). Bar = 5 μm. (C), Histogram representing percentage of endocytosed kAE1 in the presence of µ1B relative to vector-transfected cells. Mean fluorescence intensities were measured using Volocity image analysis program for at least 50 cells for each condition from 3 independent experiments. Bars correspond to means ± SEM. There was no significant difference between the 2 sets of values (n.s.).
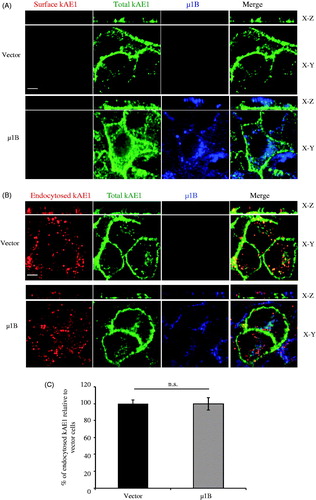
To test µ1B effect on kAE1 endocytosis, polarized LLC-PK1 cells either expressing kAE1 only or both kAE1 and µ1B were incubated with an anti-myc antibody for 1 h at 37 °C, acid stripped, fixed and permeabilized before incubation with a secondary Cy3 coupled antibody (red). μ1B HA (blue) and total kAE1 (green) were also detected. Not all the red spots colocalized with the green spots, most likely due to the incomplete ability of the Alexa488 conjugated antibodies to interact with the primary antibodies attached to endocytosed kAE1 since they are already bound to the first secondary Cy3-coupled antibody. Our results shown on show no significant difference in the amount of endocytosed kAE1 upon µ1B expression compared with vector-transfected cells (100 ± 7% of the vector-transfected cells). We conclude that, as expected for a complex subunit that is not known to alter endocytosis, expression of μ1B did not alter kAE1 endocytosis.
Expression of µ1B in polarized LLC-PK1 cells has no significant effect on the recycling of kAE1
As AP-1B complex is involved in recycling of basolateral membrane proteins, we next wondered whether expression of µ1B affects the recycling rate of kAE1. Polarized LLC-PK1 cells expressing either kAE1 or both kAE1 and µ1B were submitted to the endocytosis protocol described above, however, after the acid strip, they were re-incubated in warm media at 37° C for 90 min to induce kAE1 recycling to the cell surface (). After fixation, the recycled proteins were detected by adding the secondary Cy3-coupled antibody (red). Total kAE1 and μ1B were stained in green and blue, respectively. Quantification of fluorescence intensity () showed no significant difference in the amount of recycled kAE1 upon or in absence of μ1B expression (100 ± 16% of the vector-transfected cells).
Figure 6. µ1B expression has no effect on kAE1 recycling rate. (A), Polarized LLC-PK1 cells stably expressing kAE1-myc or both kAE1-myc and µ1B-HA were incubated with anti-myc antibody for 1 h at 37 °C, then washed with acetate buffer (pH 3) prior to reincubation again at 37 °C for 90 min to induce protein recycling. The cells were fixed, incubated with Cy3-coupled antibody (red), permeablized before incubation with anti HA antibody followed by Alexa488-coupled antibody (shown in blue). Anti-myc antibody was added again followed by Dylight 649-coupled antibody to detect the total kAE1 (shown in green). Bar = 5 μm. (B), Histogram representing percentage of recycled kAE1 in the presence of µ1B relative to vector-transfected cells. Mean fluorescence intensities for at least 50 cells for each condition from 3 independent experiments were measured using Volocity image analysis program. Bars correspond to means ± SEM. There was no significant difference between the 2 sets of values (n.s.).
Discussion
AP-1A and epithelial AP-1B complexes share the small and two large subunits that form the tetrameric AP-1 complex. These two complexes only differ by their medium subunit, µ1A for AP-1A and µ1B for AP-1B. The respective location of the complexes appears to be dependent on the nature of phosphatidyl-inositides that are enriched in the membrane (Fields et al., Citation2010). Their function is a matter of debate in the current literature. Although both AP-1A and AP-1B were originally thought to regulate basolateral trafficking of cargo proteins (Gravotta et al., Citation2007), AP-1B is now reported to regulate trafficking from the recycling endosomes while AP-1 A directs trafficking from the trans-Golgi network and early endosomes (Folsch et al., Citation2001, Citation2003; Gravotta et al., Citation2012). However, the absence of one AP-1 complex could be compensated by the presence of the other in mouse embryonic fibroblasts and in polarized MDCK cells (Eskelinen et al., Citation2002; Gravotta et al., Citation2012). More recent data support that AP-1B sorts basolateral cargo proteins that are not efficiently recognized by AP-1 A (Guo et al., Citation2013). In mice, µ1B is required for protein sorting and polarization of cargoes in intestinal cells, as the lack of this subunit in these cells causes cell proliferation, hyperplasia and mistargeting of E-cadherin/beta-catenin complex (Hase et al., Citation2013). Given that newly synthesized kAE1 does not seem to traffic through recycling endosomes (Almomani et al., Citation2012), we assessed the role of its interaction with μ1B. As kAE1 is expressed in polarized epithelial cells, we wondered whether AP-1B is involved in its recycling.
In our study, we examined the trafficking of kAE1 in polarized porcine epithelial LLC-PK1 cells that are naturally devoid of endogenous AP-1B complex (Duffield et al., Citation2004; Folsch et al., Citation2003). To specifically examine the effect of µ1B on kAE1 trafficking, we heterologously expressed human µ1B, a maneuver known to produce functional AP-1B complexes (Gan et al., Citation2002). We have previously expressed kAE1 in these cells and shown that it behaves similarly to when expressed in MDCK and intercalated cells (Hase et al., Citation2013), although LLC-PK1 cells do not polarize to the same extent as MDCK cells.
We showed that µ1B immunoprecipitated and colocalized with kAE1 in polarized LLC-PK1 cells (), confirming our previous finding that both µ1A and µ1B interact with kAE1. As µ1A binds to kAE1 via the carboxyl-terminal Y904DEV motif (Sawasdee et al., Citation2010) and µ1B binds to canonical tyrosine motifs YXXΦ (where Y is a tyrosine, X any amino acid and Φ an hydrophobic bulky amino acid) (Carvajal-Gonzalez et al., Citation2012), we assumed that µ1B binding site on kAE1 encompasses at minimum the same Y904DEV motif. Phosphorylation of tyrosine 904, within the Y904DEV binding motif, induces internalization of surface kAE1 (Williamson et al., Citation2008), and the DEY904 motif within kAE1 C-terminus also interacts with GAPDH (Su et al., Citation2011). Thus, we next determined whether phosphorylation of tyrosine 904 could alter kAE1/µ1B interaction. Upon pervanadate treatment to induce Y904 phosphorylation, we observed a binding of kAE1 to µ1B similar to control conditions, suggesting that phosphorylation of tyrosine 904 does not affect kAE1/AP-1B interaction. Of note, this phosphorylation does not affect kAE1/AP-1 A interaction either (). While this result was unexpected as phosphorylation of the tyrosine residue within the YXXΦ motif impairs binding to μ subunits (Nesterov et al., Citation1995; Ohno et al., Citation1996), it is possible that kAE1 interacts with AP-1B through various subunits. Interestingly, recent results support that phosphorylation of serine residues within acidic clusters (similar to the acidic amino acid patch surrounding Y904) does not alter µ1B binding, and rather enhances it (Navarro Negredo et al., Citation2017). However, our experimental strategy did not allow us to measure binding affinities. Our results support that AP-1B and GAPDH are competing for the same or an overlapping interaction site on kAE1 (), as expressing µ1B or µ1A decreased the binding of kAE1 to endogenous GAPDH. Su and colleagues previously reported that in polarized MDCK cells that endogenously express µ1B and µ1A, knockdown of GAPDH reduced cell surface abundance of kAE1 (Su et al., Citation2011), indicating that both GAPDH and AP1 complexes are necessary for proper targeting of kAE1. Our finding supports that µ1B binds to kAE1 via the C-terminal DEY904 and most probably the Y904DEV motif. Interestingly, in contrast with our results, Junking and colleagues reported that in non-polarized MDCK cells µ1B does not directly interact with kAE1 protein using split-GFP and µ1B immunoprecipitations (Junking et al., Citation2014). As in our hands kAE1 co-immunoprecipitated with µ1B, it is possible that the interaction occurs via AP-1B subunits other than µ1B, such as the β1 subunit or a combination of γ and σ subunits within the AP-1B complex (Mattera et al., Citation2011; Rapoport et al. Citation1998) or that the cell type or polarization state of the cells affected the interaction, since our experiments were performed in polarized cells.
Using immunofluorescence and cell surface biotinylation, we found that upon expression of µ1B, kAE1 cell surface ratio decreased significantly in LLC-PK1 cells compared to cells devoid of µ1B (). As we previously reported that µ1B partially rescued kAE1 surface trafficking in µ1A knockdown MDCK cells, we expected that expressing µ1B may increase plasma membrane kAE1. However, the opposite was observed. We hypothesize that if the endogenously expressed µ1A is the preferred adaptor for kAE1 cell surface trafficking, introducing µ1B may have jammed the normal trafficking process and resulted in the unexpected decrease in plasma membrane kAE1. This result again contrasts with that of Junking et al. (Citation2014) who observed no effect of µ1B depletion on kAE1 cell surface abundance in MDCK cells. This discrepancy may be due to the polarization status of the cells, or to the different cell type used (LLC-PK1 versus MDCK cells).
We next assessed whether the decrease in kAE1 surface abundance upon μ1B expression was due to its increased endocytosis rate. As µ1B is not involved in membrane protein endocytosis, a role rather performed by AP-2 complex, we did not anticipate detecting an effect of µ1B on kAE1 endocytosis. Accordingly, we found that the expression of µ1B in LLC-PK1 cells has no significant effect on the amount of endocytosed kAE1 (). We and others have previously characterized human kAE1 WT endocytosis pathway (Almomani et al., Citation2016; Junking et al., Citation2014; Toye et al., Citation2004; Williamson et al., Citation2008). kAE1 is constitutively endocytosed in a dynamin-dependent pathway and colocalizes with clathrin-endocytosed transferrin receptor, which accumulates in recycling endosomes. In agreement with these findings, in erythroleukemia K562 cells and in human embryonic kidney 293 cells, murine erythroid Ae1 is also endocytosed in a clathrin dependent pathway and colocalizes with transferrin receptor after 20 minutes (Wang et al., Citation2012).
Finally, as μ1B is involved in membrane protein recycling, we asked whether its expression in LLC-PK1 cells would affect kAE1 recycling. Unexpectedly however, the amount of recycled kAE1 did not significantly change upon expression of µ1B in polarized LLC-PK1 cells (). This result could either originate from the inability of μ1B to form functional complexes when transfected in LLC-PK1 cells, however the contrary was previously shown (Duffield et al., Citation2004; Folsch et al., Citation1999, Citation2003), and our immunostaining experiments show reasonable amounts of perinuclear μ1B staining to support that the heterologous protein is most likely incorporated into functional AP-1B complexes. Alternatively, this result supports that kAE1 recycling is not dependent on μ1B. kAE1 is localized at the basolateral membrane of polarized LLC-PK1 cells (Devonald et al., Citation2003), indicating that the protein does not require µ1B for basolateral localization in this cell line. The fact that kAE1 efficiently recycled back to the plasma membrane in polarized LLC-PK1 cells further supports that these cells may have a recycling mechanism independent from µ1B, possibly via AP-4 (Junking et al., Citation2014) although this complex does not seem to interact with kAE1 (Almomani et al., Citation2012). Further experiments examining the effect of µ1B on kAE1 trafficking and recycling in LLC-PK1 knocked down for µ1A or in MDCK cells exclusively knocked down for µ1B would be useful, although we and others have so far failed to specifically target this subunit without altering μ1 A. Thus, further work will be needed to determine how kAE1 recycles efficiently in LLC-PK1 cells.
Our previous results showed that µ1B improves trafficking efficiency of newly synthesized proteins from the Golgi in MDCK cells (Almomani et al., Citation2012). In our current study using LLC-PK1 cells that only endogenously express μ1 A (and no µ1B), our experiments not only confirmed the role of µ1B in Golgi-to-plasma membrane kAE1 trafficking, but also complement these previous data by showing that µ1B affects neither kAE1 endocytosis nor recycling. This work together with previous publications (Almomani et al., Citation2012; Devonald et al., Citation2003; Junking et al., Citation2014) confirms that µ1A rather than µ1B may play an important role for basolateral targeting of kAE1 protein in polarized epithelial cells.
Acknowledgements
We thank Drs. Joe Casey and Reinhart Reithmeier for providing anti-AE1 antibodies and human kAE1 WT cDNA in the pCDNA3 vector. Dr. Ashley Toye kindly provided the antibody against phosphorylated tyrosine 904. We finally thank Dr. Heike Folsch who provided the µ1A HA and µ1B HA constructs.
Disclosure statement
The authors have no financial interest or benefit from the direct applications of their research to declare.
Additional information
Funding
References
- Almomani E, Lashhab R, Alexander RT, Cordat E. 2016. The carboxyl-terminally truncated kidney anion exchanger 1 R901X dRTA mutant is unstable at the plasma membrane. Am J Physiol Cell Physiol 310:C764–C772.
- Almomani EY, King JC, Netsawang J, Yenchitsomanus PT, Malasit P, Limjindaporn T, et al. 2012. Adaptor protein 1 complexes regulate intracellular trafficking of the kidney anion exchanger 1 in epithelial cells. Am J Physiol Cell Physiol 303:C554–C566.
- Alper SL. 2010. Familial renal tubular acidosis. J Nephrol 23(Suppl 1):S57–S76.
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, et al. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 167:531–543.
- Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, et al. 2015. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350:680–684.
- Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, et al. 2012. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXX{Phi} motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci USA 109:3820–3825.
- Cordat E, Kittanakom S, Yenchitsomanus P-T, Li J, Du K, Lukacs GL, Reithmeier RAF. 2006. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger induce distinct trafficking defects in MDCK cells. Traffic 7:117–128.
- Devonald MA, Smith AN, Poon JP, Ihrke G, Karet FE. 2003. Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat Genet 33:125–127.
- Duffield A, Folsch H, Mellman I, Caplan MJ. 2004. Sorting of H,K-ATPase beta-subunit in MDCK and LLC-PK cells is independent of mu 1B adaptin expression. Traffic 5:449–461.
- Eskelinen EL, Meyer C, Ohno H, von Figura K, Schu P. 2002. The polarized epithelia-specific mu 1B-adaptin complements mu 1A-deficiency in fibroblasts. EMBO Rep 3:471–477.
- Farr GA, Hull M, Mellman I, Caplan MJ. 2009. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol 186:269–282.
- Fields IC, King SM, Shteyn E, Kang RS, Folsch H. 2010. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol Biol Cell 21:95–105.
- Fölsch H. 2015. Role of the epithelial cell-specific clathrin adaptor complex AP-1B in cell polarity. Cell Logist 5:e1074331.
- Folsch H, Ohno H, Bonifacino JS, Mellman I. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99:189–198.
- Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol 163:351–362.
- Folsch H, Pypaert M, Schu P, Mellman I. 2001. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol 152:595–606.
- Fry AC, Su Y, Yiu V, Cuthbert AW, Trachtman H, Frankl FE. 2012. Mutation conferring apical-targeting motif on AE1 exchanger causes autosomal dominant distal RTA. J Am Soc Nephrol 23:1238–1249.
- Gan Y, McGraw TE, Rodriguez-Boulan E. 2002. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol 4:605–609.
- Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E. 2012. The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell 22:811–823.
- Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, et al. 2007. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA 104:1564–1569.
- Guo X, Mattera R, Ren X, Chen Y, Retamal C, González A, Bonifacino JS. 2013. The adaptor Protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev Cell 27:353–366.
- Han JS, Kim G-H, Kim J, Jeon US, Joo KW, Na KY, et al. 2002. Secretory-defect distal renal tubular acidosis is associated with transporter defect in H(+)-ATPase and anion exchanger-1. J Am Soc Nephrol 13:1425–1432.
- Hase K, Nakatsu F, Ohmae M, Sugihara K, Shioda N, Takahashi D, et al. 2013. AP-1B-mediated protein sorting regulates polarity and proliferation of intestinal epithelial cells in mice. Gastroenterology 145:625–635.
- Junking M, Sawasdee N, Duangtum N, Cheunsuchon B, Limjindaporn T, Yenchitsomanus PT. 2014. Role of adaptor proteins and clathrin in the trafficking of human kidney anion exchanger 1 (kAE1) to the cell surface. Traffic 15:788–802.
- Karet FE, Gainza FJ, Gyory AZ, Unwin RJ, Wrong O, Tanner MJ, et al. 1998. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA 95:6337–6342.
- Kollert-Jons A, Wagner S, Hubner S, Appelhans H, Drenckhahn D. 1993. Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol 265:F813–F821.
- Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS. 2011. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem 286:2022–2030.
- Mumtaz R, Trepiccione F, Hennings JC, Huebner AK, Serbin B, Picard N, et al. 2017. Intercalated cell depletion and vacuolar H+-ATPase mistargeting in an Ae1 R607H knockin model. J Am Soc Nephrol 28:1507–1520.
- Nakatsu F, Ohno H. 2003. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct 28:419–429.
- Navarro Negredo P, Edgar JR, Wrobel AG, Zaccai NR, Antrobus R, Owen DJ, Robinson MS. 2017. Contribution of the clathrin adaptor AP-1 subunit µ1 to acidic cluster protein sorting. J Cell Biol 216:2927–2943.
- Nesterov A, Kurten RC, Gill GN. 1995. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism. J Biol Chem 270:6320–6327.
- Ohno H, Fournier MC, Poy G, Bonifacino JS. 1996. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem 271:29009–29015.
- Quilty JA, Cordat E, Reithmeier RA. 2002. Impaired trafficking of human kidney anion exchanger (kAE1) caused by hetero-oligomer formation with a truncated mutant associated with distal renal tubular acidosis. Biochem J 368:895–903.
- Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. 1998. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. Embo J 17:2148–2155.
- Sawasdee N, Junking M, Ngaojanlar P, Sukomon N, Ungsupravate D, Limjindaporn T, et al. 2010. Human kidney anion exchanger 1 interacts with adaptor-related protein complex 1 mu1A (AP-1 mu1A). Biochem Biophys Res Commun 401:85–91.
- Shao L, Xu Y, Dong Q, Lang Y, Yue S, Miao Z. 2010. A novel SLC4A1 variant in an autosomal dominant distal renal tubular acidosis family with a severe phenotype. Endocrine 37:473–478.
- Su Y, Al-Lamki RS, Blake-Palmer KG, Best A, Golder ZJ, Zhou A, Karet Frankl FE. 2015. Physical and functional links between Anion Exchanger-1 and Sodium Pump. J Am Soc Nephrol 26:400–409.
- Su Y, Blake-Palmer KG, Fry AC, Best A, Brown AC, Hiemstra TF, et al. 2011. Glyceraldehyde 3-phosphate dehydrogenase is required for band 3 (anion exchanger 1) membrane residency in the mammalian kidney. Am J Physiol Ren Physiol 300:F157–F166.
- Toye AM, Banting G, Tanner MJ. 2004. Regions of human kidney anion exchanger 1 (kAE1) required for basolateral targeting of kAE1 in polarised kidney cells: mis-targeting explains dominant renal tubular acidosis (dRTA). J Cell Sci 117:1399–1410.
- Vichot AA, Zsengellér ZK, Shmukler BE, Adams ND, Dahl NK, Alper SL. 2017. Loss of kAE1 expression in collecting ducts of end-stage kidneys from a family with SLC4A1 G609R-associated distal renal tubular acidosis. Clin Kidney J 10:135–140.
- Vince JW, Carlsson U, Reithmeier RA. 2000. Localization of the Cl-/HCO3- anion exchanger binding site to the amino-terminal region of carbonic anhydrase II. Biochemistry 39:13344–13349.
- Vince JW, Reithmeier RA. 1998. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J Biol Chem 273:28430–28437.
- Vince JW, Reithmeier RA. 2000. Identification of the carbonic anhydrase II binding site in the Cl(-)/HCO(3)(-) anion exchanger AE1. Biochemistry 39:5527–5533.
- Wang CC, Sato K, Otsuka Y, Otsu W, Inaba M. 2012. Clathrin-mediated endocytosis of mammalian erythroid AE1 anion exchanger facilitated by a YXXPhi or a noncanonical YXXXPhi motif in the N-terminal stretch. J Vet Med Sci 74:17–25.
- Williamson RC, Brown AC, Mawby WJ, Toye AM. 2008. Human kidney anion exchanger 1 localisation in MDCK cells is controlled by the phosphorylation status of two critical tyrosines. J Cell Sci 121:3422–3432.
- Zhang Z, Liu KX, He JW, Fu WZ, Yue H, Zhang H, et al. 2012. Identification of two novel mutations in the SLC4A1 gene in two unrelated Chinese families with distal renal tubular acidosis. Arch Med Res 43:298–304.
