Abstract
The sarcoplasmic reticulum (SR) is composed of two fractions, the heavy fraction that contains proteins involved in Ca2 + release, and the light fraction enriched in Ca2 + -ATPase (SERCA), an enzyme responsible for Ca2 + transport from the cytosol to the lumen of SR. It is known that in red muscle thyroid hormones regulate the expression of SERCA 1 and SERCA 2 isoforms. Here we show the effects of thyroid hormone on SERCA expression and distribution in light and heavy SR fractions from rabbit white and red muscles. In hyperthyroid red muscle there is an increase of SERCA 1 and a decrease of SERCA 2 expression. This is far more pronounced in the heavy than in the light SR fraction. As a result, the rates of Ca2 + - ATPase activity and Ca2 + -uptake by the heavy vesicles are increased. In hypothyroidism we observed a decrease in SERCA 1 and no changes in the amount of SERCA 2 expressed. This promoted a decrease of both Ca2 + -uptake and Ca2 + -ATPase activity. While the major differences in hyperthyroidism were found in the heavy SR fraction, the effects of hypothyroidism were restricted to light SR fraction. In white muscle we did not observe any significant changes in either hypo- or hyperthyroidism in both SR fractions. Thus, the regulation of SERCA isoforms by thyroid hormones is not only muscle specific but also varies depending on the subcellular compartment analyzed. These changes might correspond to the molecular basis of the altered contraction and relaxation rates detected in thyroid dysfunction.
Introduction
The sarcoplasmic reticulum (SR) is a membrane network found in skeletal muscle that functions as an intracellular calcium storage site. The SR structure is composed of two morphologically and functionally distinct fractions. The terminal cisternae or heavy SR fraction corresponds to an enlarged portion of SR, which in addition to the Ca2 + -ATPase contains proteins involved in calcium storage and release such as calsequestrin and the ryanodine Ca2 + channel. This part of the reticulum is connected to the muscle cell transverse tubule by the calcium channels and dihydropyridine receptors, a complex that plays a key role in the excitation-contraction coupling mechanism Citation[1], Citation[2]. The light SR fraction corresponds to the longitudinal SR membranes that are spread along the myofibrils. This fraction is enriched in Ca2 + -ATPase and is involved in muscle relaxation Citation[3–7].
The sarcoplasmic reticulum Ca2 + -ATPases (SERCA) are a family of membrane bound enzymes that drive the transport of calcium from the cytosol to the sarcoplasmic reticulum lumen using ATP hydrolysis as energy source Citation[8–12]. Different genes encode the sarco/endoplasmic reticulum Ca2 + -ATPase (SERCA) isoforms, but the physiological significance of this isoform diversity is not clear Citation[13–17]. SERCA 1 and SERCA 2a isoforms expression differ depending on skeletal muscle fiber types. While white muscle fibers (fast-twitch) express solely SERCA 1, red muscle fibers (slow-twitch) express both SERCA 1 and SERCA 2a. The SERCA 2b and SERCA 3 genes are expressed in non-muscular tissues such as blood platelets and lymphoid tissue Citation[13–17].
The thyroid hormone 3,5,3′-triiodo L-thyronine (T3) regulates the expression and function of SERCA proteins, and this effect varies depending on the fiber type Citation[18–25]. In white skeletal muscle, the SERCA 1 isoform content does not seem to be significantly changed by thyroid status. In the red muscle however, high T3 levels promote an over expression of SERCA 1 isoform, which is accompanied by an increase of both the SR rate of calcium transport and ATPase activity Citation[18], Citation[22]. In hypothyroidism, the expression of SERCA 2a is not altered but there is a decrease in SERCA 1 Citation[20], Citation[25].
Most of the studies measuring SERCA expression in either hypo- or hyperthyroid animals were performed in rats and the Western blot analyses were done using either muscle homogenates or immunohistochemical staining of muscle fibers. Using these methodological approaches it is not possible to ascertain whether the changes of SERCA proteins promoted by T3 differ in the longitudinal and terminal cisternae of the sarcoplasmic reticulum.
In this report we aimed to determine whether the action of T3 on muscles involves the subcellular redistribution of SERCA proteins. We isolated the light and heavy fractions of the SR and measured both the expression of the two SERCA isoforms and the Ca2 + -transport activity in hypo- and hyperthyroid rabbits.
Materials and methods
Light and heavy SR vesicles derived from rabbit skeletal muscle
Hind limb red and white muscles were dissected from white male rabbits based on their macroscopic aspect. This is a procedure adopted in previous reports (22,41). The rabbits were treated in accordance with the published regulations for animal laboratorial use. Vesicles derived from light and heavy sarcoplasmic reticulum were prepared as previous reports with small modifications Citation[3], Citation[5]. Briefly, white (40 g) and red (10 g) muscles were homogenized in 10 mM MOPS/Tris pH 7.0, 10% Sucrose 0.1 mM EDTA (buffer 1) in a blender. Homogenization and all subsequent steps were performed at 4°C. The homogenates were centrifuged for 10 min at 1,600×g. The supernatants (S1) were filtered through four layers of cheesecloth. The pellet was resuspended in buffer 1 and centrifuged for 10 min at 1,600×g. The supernatant of the second centrifugation (S2) was mixed with S1 and centrifuged for 10 min at 5,200×g. The pellet was discarded and the supernatant was re-centrifuged for 30 min at 15,000×g. The supernatant of this centrifugation was collected (S3). The pellet was homogenized in 10 mM MOPS/Tris pH 7.0 and 0.6 M KCl (buffer 2) and centrifuged for 30 min at 20,000×g. The supernatant was discarded ann the pellet was collected (P1). S3 was centrifuged for 40 min at 30,000×g, the supernatant was discarded and the pellet was homogenized with buffer 2. This homogenate was centrifuged for 20 min at 20,000×g, the supernatant was collected (S4) and the pellet was mixed with the P1 and homogenized in a small volume of 50 mM MOPS/Tris pH 7.0 with 0.6 M sucrose and stored at –70°C. These vesicles correspond to the heavy SR fraction. The S4 was centrifuged for 40 min at 30,000×g, the supernatant was discarded and the pellet was homogenized in a small volume of B3 and stored at −70°C. These vesicles correspond to light SR vesicles. Prior to use, vesicles were diluted in a medium containing 50 mM MOPS/Tris buffer, 100 mM KCl, 10 mM Pi and 10 µM CaCl2.
Hypo- and hyperthyroid animals
Hypothyroid state was induced by oral administration of propylthiouracil (0.08%), in drinking water for 21 days Citation[26]. Hyperthyroidism was induced in rabbits by injection of T4 (100 µg/kg body weight), subcutaneously, for 10 days Citation[16], Citation[18], Citation[22]. After the period of treatment the animals were sacrificed and blood was collected for T4 measurement. Hypo- and hyperthyroidism were confirmed by measurements of body weight and serum T4 (). Serum total T4 was measured by specific radioimmunoassay kits using 125I as tracer (T4: DLS −3200 Active, sensitivity of 0.4 µg/dl, inter- and intra assay coefficients of variation varied from 7.1 to 7.4% and 2.9 to 5.1%, respectively, TX, EUA), based on the presence of specific antibodies adhered to the internal surface of propylene tubes. Rabbit hormone free serum was used in the standard curves for total T4. All the procedures were carried out following the recommendations of the kit.
Table I. Body weight and serum total T4 in control, hyper and hypothyroid rabbits
Gel electrophoresis and Western blot
Proteins were separated on a 7.5% polyacrylamide (SDS-PAGE) gel according to Laemmli Citation[27]. Electro transfer of protein from the gel to polyvinylidene difluoride (PVDF) membranes was performed for 20 min at 250 mA per gel in 25 mM Tris, 192 mM glycine and 20% methanol using a Mini Trans-Blot cell from Bio-Rad. Membranes were blocked with 3% non-fat dry milk in Tris-buffered saline with 0.1% Tween-20 for 1 h at room temperature. Membranes were then washed and incubated for 1 h with anti-SERCA 1, anti-SERCA 2 monoclonal antibodies at room temperature. The membranes were washed and blots were revealed using an ECL detection kit from Amersham-Pharmacia Biotech, UK. Monoclonal antibodies for SERCA 1 (clone IIH11) and SERCA 2 (clone IID8) were obtained from Affinity BioReagents, Inc. (Brazil). Densitometric analyses were performed using Scion Image 4.02 for windows, downloaded from web site:scioncorp.com.france.fr (NIH). Total homogenates were obtained by muscle centrifugation at 10,000×g and the supernatant containing practically all the cell components were used, except the actomiosin filaments.
Ca2 + uptake
Trace amounts of 45Ca were included in the assay medium. The reaction was arrested by filtering samples of the assay medium through Millipore filters Citation[28]. After filtration, the filters were washed five times with 5 ml of 3 mM La(NO3)3 and the radioactivity remaining on the filters was counted using a liquid scintillation counter.
ATPase activity
Enzyme activity was assayed by measuring the release of 32Pi from [γ-32P]ATP. The reaction was arrested with trichloroacetic acid (final concentration 5% w/v). The [γ-32P]ATP not hydrolyzed during the reaction was extracted with activated charcoal as previously described Citation[29]. Two different ATPase activities can be distinguished in sarcoplasmic reticulum vesicles Citation[8–11], Citation[30]. The Mg2 + -dependent activity requires only Mg2 + for its activation and is measured in the presence of 2 mM EGTA to remove contaminant Ca2 + from the medium. The Ca2 + -dependent ATPase activity, which is correlated with Ca2 + transport, is determined by subtracting the Mg2 + -dependent activity from the activity measured in the presence of both Mg2 + and Ca2 + . In previous reports, skeletal muscle calcium dependent ATPase has been shown to be fully inhibited by sub micromolar concentrations of thapsigargin Citation[31].
Experimental procedure
All the experiments were performed at 35°C. In a typical experiment the assay media was divided into three samples, which were used for the simultaneous measurement of Ca2 + uptake and ATP hydrolysis. These measurements were started simultaneously with vesicles to a final concentration of 5 µg protein/ml. NaN3 (5 mM), an inhibitor of mitochondrial ATP synthase, was added to the assay medium The free Ca2 + concentration in the medium was calculated as previously described Citation[32], Citation[33].
Statistical analyses
All enzymatic data were analysed by unpaired t-test. Initial and final rabbit body weights were analysed by paired t-test. Serum T4 levels were analysed by one-way analysis of variance followed by Newman–Keuls multiple comparison tests.
Results and discussion
Characterization of light and heavy SR fractions derived from white and red skeletal muscle
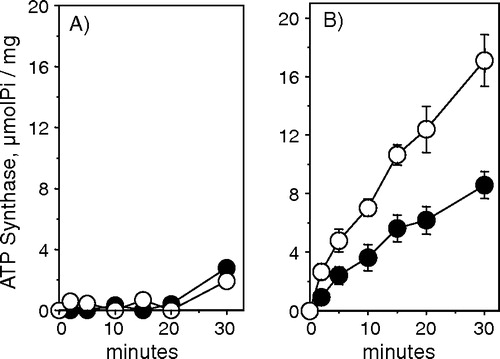
Electrophoretic analyses of muscle vesicles revealed a band of 110 kDa, characteristic of SERCA proteins (A). In both red and white muscle from control rabbits, the amount of Ca2 + -ATPases was more conspicuous in the light than in the heavy fraction (B). When compared with white muscle, both the light and heavy fractions of red muscle showed more protein bands. Red muscle contains a larger quantity of mitochondria than white muscle, and during the process of preparation of the SR vesicles mitochondria lysis can lead to the formation of sub-mitochondrial particles that cannot be separated from the SR vesicles fraction. Therefore, the sub-mitochondrial particles are one of the possible contaminants prevailing in red muscle. To explore this possibility we measured the ATPase activity of the mitochondrial Fo F1 ATP synthase () in the two vesicle fractions. For white muscle, a tissue with low mitochondria content, there was little or no mitochondrial ATPase activity in either the light or the heavy SR fraction (A), but in red muscle there was a significant sodium azide sensitive mitochondrial ATPase activity, which was more pronounced in the heavy than in the light SR fraction (B).
Figure 1. Electrophoresis of vesicles obtained from red (RM) and white (WM) skeletal muscle. LF refers to light fraction and HF to heavy fraction. A) The amount of protein used in SDS-PAGE gel electrophoresis was 20 µg for red muscle and 15 µg for white muscle. The gel was stained with Coomassie Brilliant Blue. B) Immunodetection was obtained with SERCA 1 and SERCA 2 specific monoclonal antibodies. 7 µg protein of light and heavy SR vesicles from red muscle and 0.5 µg protein of light and heavy SR vesicles from white muscle were used to load the gels.
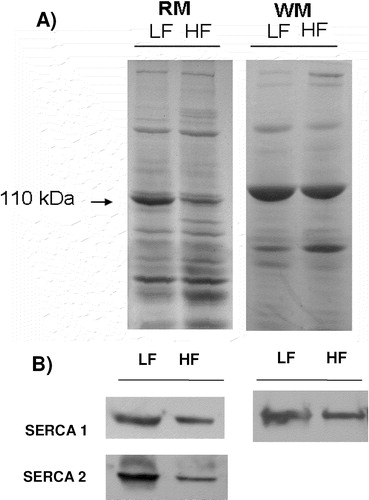
Figure 2. ATP Synthase activity of vesicles obtained from white (A) and red (B) skeletal muscle. This activity was calculated by subtracting the ATPase activity without sodium azide from the ATPase activity in the presence of sodium azide. The assay medium composition was 50 mM Tris/HCl buffer (pH 8.0), 1 mM ATP, 2 mM MgCl2, 5 mM EGTA, 100 mM KCl and 5 mM NaN3. The reaction was performed at 35°C and was started by the addition of vesicles (5 µg protein/ml). The vesicles used in the experiment were derived from light (•) and heavy (○) SR fractions. The figure shows the average±SE of 4 experiments.
In agreement with previous reports Citation[34], Citation[35], we found that the rate of Ca2 + uptake and the amount of Ca2 + accumulated at steady state by the heavy SR are smaller than that measured with the light fractions (compare B and D with A and C). In the literature it is well established that caffeine impairs the Ca2 + accumulation of the heavy fraction by increasing the Ca2 + release from the vesicles, but has no effect in the light vesicles fraction Citation[7], Citation[36–38]. Accordingly, and show that caffeine impairs Ca2 + accumulation from SR of both white and red muscle heavy fractions but has little or no effect on the light fraction of the two muscles.
Figure 3. Effect of caffeine in light (A and C) and heavy SR (B and D) fraction obtained from red and white skeletal muscles. The assay medium composition was 50 mM Mops/Tris buffer (pH 7.0), 1 mM ATP, 2 mM MgCl2, 0.2 mM CaCl2, 0.2 mM EGTA, 10 mM Pi, 100 mM KCl, 10 mM caffeine, 5 mM NaN3 and trace amounts of 45Ca2 + . The reaction was performed at 35°C and was started by the addition of vesicles (5 µg protein/ml). The calculated free Ca2 + concentration in the medium was 5 µM. Upper panel – red muscle and lower panel – white muscle. Control (○), 10 mM caffeine (•). The figure shows a typical experiment.
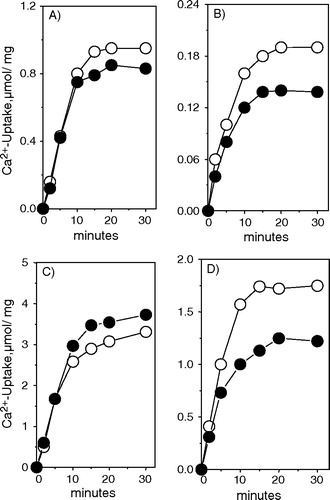
Table II. Effect of caffeine on Ca2 + -uptake in white and red skeletal muscle.
Hyperthyroidism
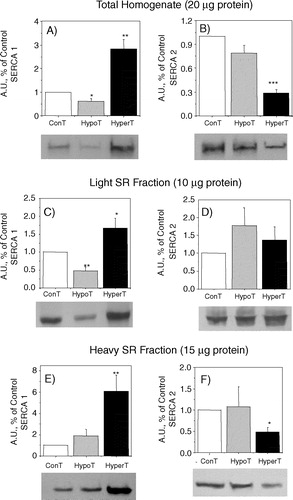
There is general agreement that in both rats Citation[18–21], Citation[24] and rabbits Citation[22], Citation[23], hyperthyroidism promotes an up-regulation of SERCA 1 and a down-regulation of SERCA 2 in red muscle. An increase of Ca2 + -ATPase content in hyperthyroid vastus lateralis muscle has also been detected in humans Citation[39]. These measurements were performed using total muscle homogenates that do not allow the distinction between the two regions of the SR. We now show that the effect of hyperthyroidism varies depending on the SR fraction evaluated. With total homogenate we obtained the same results as those previously described (A and B). However, in red muscle, the effect of hyperthyroidism in the two SERCA isoforms content was not the same in the light and heavy SR fractions. In the light fraction there was a ∼50% increase of SERCA 1 and no significant change of the SERCA 2 level ( C and D). Thus, the variation of SERCA content in the light fraction does not match the pattern observed with the total homogenate. In the heavy SR fraction however, there was a 6 to 7 fold increase of SERCA 1 and a significant decrease of SERCA 2 ( E and F). These data suggest that in hyperthyroidism the change of SERCA content in red muscle is observed mainly in the terminal cisternae and only a small modification is detected in the longitudinal part of the reticulum. In fact, the pattern observed in the heavy fraction is practically the same as that detected in the total homogenate (compare A and B with 4E and F).
Figure 4. Western blots showing SERCA isoform contents of red muscle from total homogenate, light SR fraction and heavy SR fraction. Immunodetection was obtained with SERCA 1 and SERCA 2 specific monoclonal antibodies. 20 µg of total homogenate (A and B), 10 µg of light SR vesicles (C and D) and 15 µg of heavy SR vesicles (E and F) were used to load the gels. The tissue homogenate were centrifuged at 10,000 g for 20 min and the supernatants were used. Hypo- and hyper- refers to samples obtained from hypo- or hyperthyroid animals. The densitometric values represent arbitrary units (A.U.) and the values represent the average ± SE. The number of Western blots performed varied between 3 and 8. The differences between control and either hyper- or hypothyroid were statistically significant (t-test) *p < 0.025, **p < 0.005 and ***p < 0.0005.
There was a reasonably good correlation between the variations of SERCA noted in Western blots and the Ca2 + transport activity of the two red muscle fractions. In the light fraction, there was only a ∼50% increase of SERCA 1 and no variation in the level of SERCA 2. Accordingly, there was a ∼50% increase of both the rates of Ca2 + uptake and Ca2 + -dependent ATPase activity (A and B, ). For the heavy fraction, the calcium transport rate depends on the balance between the two SERCA isoforms. In early reports it has been shown that vesicles containing SERCA 1 accumulate more Ca2 + and at a faster rate than vesicles containing SERCA 2 Citation[40], Citation[41]. The Western blot analysis indicates a 6 fold increase of SERCA 1 and a 50% decrease of SERCA 2. As a result of these two divergent variations there was a 3-fold increase of both Ca2 + transport and ATPase activity (C and D, ).
Figure 5. Ca2 + -uptake and Ca2 + -ATPase activity of light and heavy vesicles derived from red muscle. A and C refer to 45Ca2 + -uptake from light vesicles and heavy vesicles respectively. B and D refer to thapsigargin sensitive Ca2 + -ATPase activity from light and heavy vesicles. The assay medium composition was described in and for Ca2 + -ATPase activity we added trace amounts of 32ATP. The vesicles used in the experiment were derived from Control (○), hypothyroid (Δ) and hyperthyroid (•) animals. The figure shows a typical experiment.
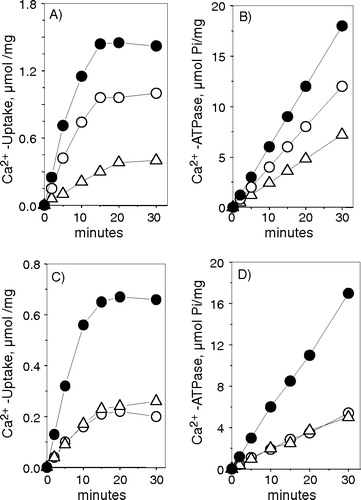
Table III. Ca2 + -uptake and Ca2 + -ATPase activity from red skeletal muscle.
Rabbit white muscle expresses only SERCA 1 and the level of this protein did not vary significantly in either the total homogenate or in the two SR fractions (). Similar to the Western blot, hyperthyroidism did not promote a significant change in the Ca2 + uptake rate of the two SR fractions (A and C). However, in both fractions, there was an increase of Ca2 + -ATPase activity, which was not accompanied by a decrease of Ca2 + transport, indicating that hyperthyroidism promotes an uncoupling of SERCA 1 (B and D, ). In a previous report Citation[22] we did already observe that hyperthyroidism produces an increase in the SERCA 1 uncoupled ATPase activity in the light SR vesicle, but in our early work, the enhancement of ATPase activity promoted by hyperthyroidism was accompanied by a decrease of Ca2 + -uptake, which was not detected in and A. We do not know at present the reason for this discrepancy. One possibility is variability among the different groups of animals used.
Figure 6. Western blots showing SERCA isoform contents of white muscle from total homogenate, Light SR Fraction and Heavy SR fraction. Immunodetection was obtained with SERCA 1 specific monoclonal antibodies. 8 µg of total homogenate (A), 0.5 µg of light SR vesicles (B) and 1 µg of heavy SR vesicles (C) were used to load the gel. The tissue homogenate were centrifuged at 10,000 g for 20 min and the supernatants were used. HypoT and HyperT refer to samples obtained from hypo- or hyperthyroid animals. Densitometric analysis showing arbitrary units (A.U.) relative to control. The figure shows the average±SE of 3 and 4 different experiments.
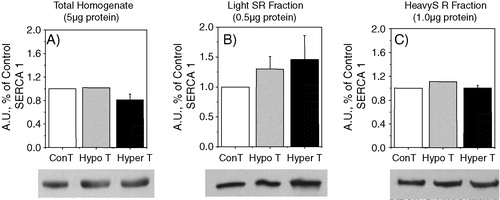
Figure 7. Ca2 + -uptake and ATPase activity of light and heavy vesicles derived from white muscle. A and C refer to 45Ca2 + -uptake from light vesicles and heavy vesicles respectively. B and D refer to thapsigargin sensitive Ca2 + -ATPase activity from light and heavy vesicles. The assay medium composition was described in and for Ca2 + -ATPase activity we added trace amounts of 32ATP. The vesicles used in the experiment were derived from Control (○), hypothyroid (Δ) and hyperthyroid (•) animals. The figure shows a typical experiment.
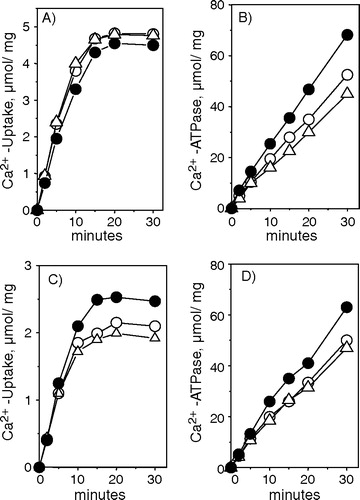
Table IV. Ca2 + -uptake and Ca2 + -ATPase activity from white skeletal muscle.
Hypothyroidism
The information available on hypothyroidism is scant and, as far as we know, the studies available on the effect of thyroid status on SERCA were performed only in rats. Hypothyroidism was shown to reduce Ca2 + -ATPase in rat soleus muscle Citation[42], and this decrement is mainly due to the decreased SERCA 1 expression Citation[20]. We confirm these findings in rabbit red muscle ( A and B) and in addition observed that the decrease of SERCA 1 is confined to the light fraction (C and D). The levels of both SERCA 1 and SERCA 2 in the heavy fraction of hypothyroid rabbit red muscle were the same as those detected in control animals ( E and F). Accordingly, A and B and show that hypothyroidism promoted a decrease of both the rate of Ca2 + uptake and Ca2 + -dependent ATPase activity of the light SR vesicles but had no effect on Ca2 + transport of the heavy SR fraction. In white muscle, hypothyroidism did not promote any measurable changes in SERCA 1 expression (), Ca2 + uptake or Ca2 + -ATPase activity (, ).
Final remarks
In humans, thyroid disease promotes abnormalities in skeletal muscle function. The contraction and relaxation rates are increased in hyperthyroidism and decreased in hypothyroidism. The muscles more affected by thyroid hormone are those used for maintenance of posture, which are predominantly red muscles Citation[42], Citation[43]. A typical symptom is the shortening of the Achilles-tendon reflex time in hyperthyroidism and its prolongation in hypothyroidism Citation[42]. These alterations correlate well with the changes of the SERCA noted in this study. We observed a higher SERCA 1 content in hyperthyroid red muscle, specifically in the heavy SR fraction, which is a portion of the reticulum involved in the muscle excitation-contraction coupling. Hudecova et al. Citation[44] observed that thyroid hormone status promote alterations in the mRNA level of other proteins found in the heavy SR fraction. These include the ryanodine channel, the Na+/Ca2 + exchanger and the IP3 channel. In conclusion, in this report we show that regulation of SERCA isoforms by thyroid hormones are not only muscle specific but also vary depending on the sub cellular compartment of the reticulum analyzed. These changes might correspond to one of the molecular basis of the altered contraction-relaxation rates detected in thyroid dysfunction.
This work was supported by grants from PRONEX-Financiadora de Estudos e Projetos (FINEP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). APA and GMO are recipient of the CNPq fellowship. The authors are grateful to Prof. Paulo Arruda for helpful discussion and Mr V. A. Suzano and A.C. Miranda for technical assistance.
References
- Protasi F, Franzini-Armstrong C, Allen PD. Role of ryanodine receptor in the assembly of calcium release units in skeletal muscle. J Cell Biol 1998; 140: 831–842
- Franzini-Armstrong C. The sarcoplasmic reticulum and the control of muscle contraction. FASEB J 1999; 13: S266–S270
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta 1975; 389: 51–68
- Chu A, Volpe P, Costello B, Fleischer S. Functional characterization of junctional terminal cisternae from mammalian fast skeletal muscle sarcoplasmic reticulum. Biochemistry 1986; 25: 8315–8324
- Saito A, Seiler SD, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol 1984; 99: 875–885
- Murray BE, Ohlendieck K. Complex formation between calsequestrin and the ryanodine receptor in fast- and slow-twitch rabbit skeletal muscle. FEBS Lett 1998; 429: 317–322
- Marie V, Silva JE. Calcium pool size modulates the sensitivity of the ryanodine receptor channel and calcium-dependent ATPase of heavy sarcoplasmic reticulum to extravesicular free calcium concentration. J Cell Physiol 1998; 175: 283–294
- Hasselbach W, Makinose M. On the mechanism of calcium transport across the membrane of the sarcoplasmic reticulum. Biochem Z 1963; 339: 94–111
- de Meis L, Vianna AL. Energy interconversion by the Ca2 + -transport ATPase of sarcoplasmic reticulum. Annu Rev Biochem 1979; 48: 275–292
- de Meis L. The sarcoplasmic reticulum: Transport and energy transduction, vol.2 (Bittar E, editor). John Wiley & Sons, New York 1981
- Inesi G. Mechanism of Ca2 + transport. Annu Rev Physiol 1985; 47: 573–601
- de Meis L. Ca2 + -ATPases (SERCA): Energy transduction and heat production in transport ATPases. J Membr Biol 2002; 188: 1–9
- Lytton J, MacLennan DH. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of cardiac Ca2 + -ATPase gene. J Biol Chem 1988; 263: 15024–15031
- Lytton J, Westin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic reticulum family of calcium pumps. J Biol Chem 1992; 267: 14483–14489
- MacLennan DH, Brandl CJ, Korczak B, Green NM. Amino-acid sequence of Ca2 + , Mg2 + -dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature 1985; 316: 696–700
- Arai M, Otsu K, MacLennan DH, Alpert NR, Periasamy M. Effect of thyroid-hormone on the expression of messenger RNA encoding sarcoplasmic-reticulum proteins. Circ Res 1991; 69: 266–276
- Wuytack F, Papp B, Verboomen H, Raeymaekers L, Dode L, Bobe R, Enouf J, Bokkala S, Authi KS, Casteels R. A sarco/endoplasmic reticulum Ca2 + -ATPase 3-type Ca2 + pump is expressed in platelets, in lymphoid cells, and in mast cells. J Biol Chem 1994; 269: 1410–1416
- Nunes MT, Bianco AC, Migala A, Agostin B, Hasselbach W. Tyroxine induced transformation in sarcoplasmic reticulum of rabbit soleus and psoas muscles. Z Naturforsch 1985; 40: 726–734
- Muller A, van der Linden GC, Zuidwijk MJ, Simonides WS, van der Laarse WJ, van Hardeveld C. Differential effects of thyroid hormone on the expression of sarcoplasmic reticulum Ca2 + -ATPase isoforms in rat skeletal muscle fibers. Biochem Biophys Res Commun 1994; 203: 1035–1042
- Van der Linden CG, Simonides WS, Muller A, Van der Laarse WJ, Vermeulen JL, Zuidwijk MJ, Moorman AF, Van Harveld C. Fiber-specific regulation of Ca(2 + )-ATPase isoform expression by thyroid hormone in rat skeletal muscle. Am J Physiol 1996; 271: C1908–C1919
- Simonides WS, Thelen MHM, van der Linden CG, Muller A, Van Hardeveld C. Mechanism of thyroid-hormone regulated expression of SERCA genes in skeletal muscle: implications for thermogenesis. Biosci Rep 2001; 21: 139–154
- Arruda AP, da-Silva WS, Carvalho DP, de Meis L. Hyperthyroidism increases the uncoupled ATPase activity and heat production by the sarcoplasmic reticulum Ca2 + -ATPase. Biochem J 2003; 375: 753–760
- Jiang M, Xu A, Jones DL, Narayanan N. Coordinate downregulation of CaM kinase II and phospholamban accompanies contractile phenotype transition in the hyperthyroid rabbit soleus. Am J Physiol Cell Physiol 2004; 287: C622–632
- Yamada T, Inashima S, Matsunaga S, Nara I, Kajihara H, Wada M. Different time course of changes in sarcoplasmic reticulum and myosin isoforms in rat soleus muscle at early stage of hyperthyroidism. Acta Physiol Scand 2004; 180: 79–87
- Simonides WS, van Hardeveld C. The effect of hypothyroidism on sarcoplasmic reticulum in fast-twitch muscle of the rat. Biochim Biophys Acta 1985; 844: 129–141
- Boerth SR, Artman M. Thyroid hormone regulates Na(+)-Ca2 + exchanger expression during postnatal maturation and in adult rabbit ventricular myocardium. Cardiovasc Res 1996; 31: E145–52
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- Chiesi M, Inesi G. The use of quench reagents for resolution of single transport cycles in sarcoplasmic reticulum. J Biol Chem 1979; 254: 10370–10377
- Grubmeyer C, Penefsky HS. The presence of two hydrolytic sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem 1981; 256: 3718–3727
- Hasselbach W. Relaxing factor and relaxation of muscle. Prog Biophys Biophys Chem 1964; 14: 167–222
- Sagara Y, Fernandez-Belda F, de Meis L, Inesi G. Characterization of the inhibition of intracellular Ca2 + transport ATPases by thapsigargin. J Biol Chem 1992; 267: 12606–12613
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol 1972; 75: 463–505
- Scwartzenbach G, Senn H, Anderegg G. Komplexone XXIX Eingrosser chelateffekt besonderer. Helv Chim Acta 1957; 40: 1886–1900
- Louis CF, Nash-Adler PA, Fudyma G, Shigekawa M, Akowitz A, Katz AM. A comparison of vesicles derived from terminal cisternae and longitudinal tubules of sarcoplasmic reticulum isolated from rabbit skeletal muscle. Eur J Biochem 1980; 111: 1–9
- Watras J. Effects of Mg2 + on calcium accumulation by two fractions of sarcoplasmic reticulum from rabbit skeletal muscle. Biochim Biophys Acta 1985; 812: 333–344
- Kirino Y, Shimizu H. Ca2 + -induced Ca2 + release from fragmented sarcoplasmic reticulum: a comparison with skinned muscle fiber studies. J Biochem 1982; 92: 1287–1296
- Hasselbach W, Migala A. Modulation by monovalent anions of calcium and caffeine induced calcium release from heavy sarcoplasmic reticulum vesicles. Z Naturforsch 1992; 47: 440–448
- Hasselbach W, Migala A. Modulation by ryanodine of active calcium loading and caffeine induced calcium release of heavy sarcoplasmic reticulum vesicles. Z Naturforsch 1992; 47: 429–439
- Riis AL, Jorgensen JO, Moller N, Weeke J, Clausen T. Hyperthyroidism and cation pumps in human skeletal muscle. Am J Physiol Endocrinol Metab 2005; 288: E1265–9126
- Sumbilla C, Cavagna M, Zhong L, Ma H, Lewis D, Farrance I, Inesi G. Comparison of SERCA1 and SERCA2a expressed in COS-1 cells and cardiac myocytes. Am J Physiol 1999; 277: H2381–2391
- Reis M, Farage M, de Meis L. Thermogenesis and energy expenditure: control of heat production by the Ca (2 + )-ATPase of fast and slow muscle. Mol Membr Biol 2002; 19: 301–310
- Everts ME. Effects of thyroid hormones on contractility and cation transport in skeletal muscle. Acta Physiol Scand 1996; 156: 325–333
- Wiles CM, Young A, Jones DA, Edwards RH. Muscle relaxation rate, fibre-type composition and energy turnover in hyper-and hypo-thyroid patients. Clin Sci 1979; 57: 375–384
- Hudecova S, Vadaszova A, Soukup T, Krizanova O. Effect of thyroid hormones on the gene expression of calcium transport systems in rat muscles. Life Sci 2004; 75: 923–931