Abstract
UapA, a member of the NAT/NCS2 family, is a high affinity, high capacity, uric acid-xanthine/H+ symporter in Aspergillus nidulans. Determinants critical for substrate binding and transport lie in a highly conserved signature motif downstream from TMS8 and within TMS12. Here we examine the role of TMS1 in UapA biogenesis and function. First, using a mutational analysis, we studied the role of a short motif (Q85H86), conserved in all NATs. Q85 mutants were cryosensitive, decreasing (Q85L, Q85N, Q85E) or abolishing (Q85T) the capacity for purine transport, without affecting physiological substrate binding or expression in the plasma membrane. All H86 mutants showed nearly normal substrate binding affinities but most (H86A, H86K, H86D) were cryosensitive, a phenotype associated with partial ER retention and/or targeting of UapA in small vacuoles. Only mutant H86N showed nearly wild-type function, suggesting that His or Asn residues might act as H donors in interactions affecting UapA topology. Thus, residues Q85 and H86 seem to affect the flexibility of UapA, in a way that affects either transport catalysis per se (Q85), or expression in the plasma membrane (H86). We then examined the role of a transmembrane Leu Repeat (LR) motif present in TMS1 of UapA, but not in other NATs. Mutations replacing Leu with Ala residues altered differentially the binding affinities of xanthine and uric acid, in a temperature-sensitive manner. This result strongly suggested that the presence of L77, L84 and L91 affects the flexibility of UapA substrate binding site, in a way that is necessary for high affinity uric acid transport. A possible role of the LR motif in intramolecular interactions or in UapA dimerization is discussed.
Introduction
UapA is a high affinity (7–10 µM), high capacity, uric acid-xanthine/H+ symporter of the filamentous ascomycete Aspergillus nidulans (Diallinas & Scazzocchio [Citation1989], Gorfinkiel et al. [Citation1993], Argyrou & Blanchard [Citation2001]). UapA expression is transcriptionally activated during germination and is further induced in the presence of purines (Gorfinkiel et al. [Citation1993], Amillis et al. [Citation2004]). While transcription in germinating conidiospores is independent of the presence of purines or the presence of N and C sources, in mycelia, uapA transcription is under strict nitrogen catabolite repression and purine-inducible (Gorfinkiel et al. [Citation1993], Amillis et al. [Citation2004]). The UapA protein is predicted to consist of 12 α-helical transmembrane segments (TMS) and cytoplasmic N- and C-termini (Koukaki et al. [Citation2005]). A. nidulans also possesses a paralogue of UapA, called UapC (Diallinas et al. [Citation1995]). UapC is also specific for uric acid and xanthine, but it has distinct kinetics, substrate profile and physiology from UapA (Diallinas et al. [Citation1995], [Citation1998], H. Tsilivi & G. Diallinas, unpublished). A third purine transporter in A. nidulans is encoded in the azgA gene (Cecchetto et al. [Citation2004]). The AzgA protein is an adenine-guanine-hypoxanthine/H+ symporter defining a distinct transporter family (Cecchetto et al. [Citation2004]).
UapA and UapC are the best-studied members of the Nucleobase-Ascorbate Transporter family (NAT), also known as the Nucleobase-Cation Symporter family (NCS2) (Diallinas et al. [Citation1995], De Koning & Diallinas [Citation2000]). The NAT family consists of more than 500 currently sequenced proteins derived from bacteria, archaea, diatoms, fungi, plants and animals (http://www.tcdb.org/). These proteins, which consist of 450–650 amino acid residues, are predicted to have very similar hydrophobicity profiles, corresponding, in most cases, to 12 α-helical TMS. A dozen functionally characterized members of the NAT family are microbial or plant, proton-driven, symporters specific for xanthine and/or uric acid or uracil (De Koning & Diallinas [Citation2000], Argyrou & Blanchard [Citation2001], Karatza & Frillingos [Citation2005]; Goudela et al. [Citation2005]). The only characterized metazoan NAT members are the products of the mammalian svct1 and svct2 cDNAs. These proteins, rather than being nucleobase/H+ symporters, are highly specific L-ascorbate/Na+ symporters (Tsukaguchi et al. [Citation1999], Liang et al. [Citation2001]). The NAT family is only distantly related to the two other major nucleobase transporter families, which are restricted to microorganisms and plants, the NCS1 family (http://www.tcdb.org/) and the AzgA-like family (Cecchetto et al. [Citation2004]).
Using a plethora of purine analogues, we have recently proposed speculative models on how UapA and homologous transporters from Escherichia coli and Candida albicans contact the purine ring (Goudela et al. [Citation2005]). These models proposed that UapA and its homologues bind xanthine or uric acid via H-bonds, principally with N1-H,=O6, N9 (xanthine) or = O8 (uric acid), and to a lesser extent with = O2. By analysing the function, kinetics and specificity of UapA-UapC chimeric transporters and a series of UapA mutations, we have presented evidence showing that several residues critical for UapA function map within the so called NAT signature motif, [Q/E/P]408-N409-X-G411-X-X-X-X-T416-[R/K/G]417 (numbering corresponds to UapA) (Diallinas et al. [Citation1998], Meintanis et al. [Citation2000], Koukaki et al. [Citation2005]). This sequence is located downstream from TMS8, within a loop of amphipathic character. A second-site suppressor of one of the mutations in the signature motif (Q408E) has suggested that residue F528, in TMS12, phenomenologically acts as a ‘molecular filter’, excluding purines, other than uric acid or xanthine, from being substrates of UapA (Amillis et al. [Citation2001]). Recently, the role of conserved residues Q408, N409, G411, T416, R417, in the NAT signature motif (Koukaki et al. [Citation2005]), as well as, of F528 in TMS12 (Vlanti et al. [Citation2006]), has been systematically studied by analysing the kinetics and specificity of several UapA mutants, towards different nucleobase analogues as potential substrates. This analysis has shown that, amino acids Q408-N409-N-G411 might be involved in interactions with the imidazol ring of purines, and supported the notion that these residues define part of the UapA purine translocation pathway (Koukaki et al. [Citation2005]). The analysis of F528 mutations has in turn suggested that the presence of an aromatic amino acid residue at this position is involved in a ‘gating’-like mechanism, which in conjunction with the purine-binding site controls UapA-mediated substrate translocation (Vlanti et al. [Citation2006]).
Similar genetic approaches have been employed in structure-function studies concerning a handful of other A. nidulans transporters, such as those specific for proline (PrnB; Tavoularis et al. [Citation2003]), nitrate (CrnA; Unkles et al. [Citation2005], Kinghorn et al. [Citation2005]) or ammonium (MrpA; Monahan et al. [Citation2002]).
In this work, we present approaches to investigate the role of TMS1 in UapA biogenesis and function. We first investigated the role of the highly conserved Q85H86 motif, present within TMS1 in all NAT members (A), by constructing appropriate mutations and analysing their effects on UapA targeting and function. Then, using a similar approach, we examined the role of a UapA-specific, Leu Repeat (LR) motif, also present in TMS1 (B). Our results showed that TMS1 includes flexible structural determinants critical for UapA stable expression in the plasma membrane and for transport catalysis.
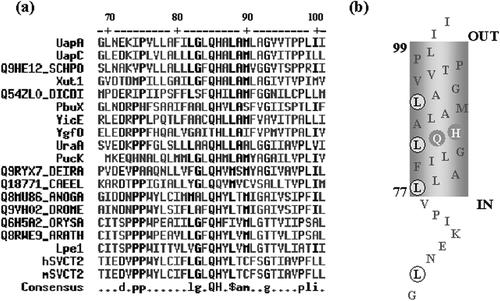
Figure 1. (A) A highly conserved QH motif in TMS1 of NAT homologues (http://prodes.toulouse.inra.fr/multalin/multalin.html). UapA and UapC are purine transporters of A. nidulans referred in the text. YicE and Ygfo are xanthine transporters in E. coli. PbuX and PucK are xanthine and uric acid transporters, respectively, in Bacillus subtilis. Xut1 is a xanthine/uric acid transporter in C. albicans. Lpe1 is a maize xanthine/uric acid transporter. hSVCT2 and mSVCT2 are ascorbic acid/Na+ symporters in human and mouse, respectively. UraA is a uracil transporter in E. coli. The rest of the NAT homologues shown are of unknown function and come from Schizosaccharomyces pombe (SCHPO), Dictyosteliun discoideum (DICDI), Deinococcus radiodurans (DEIRA), mosquito (ANOGA), Drosophila melanogaster (DROME) and Caenorhabditis elegans (CAEEL), rice (ORYSA) and Arabidopsis thaliana (ARATH) (B) Speculative topology of TMS1 based on http://www.ch.embnet.org/software/TMPRED_form.html and http://sosui.proteome.bio.tuat.ac.jp/sosuiframe0.html. The QH and LR motifs are highlighted.
Materials and methods
Strains, growth conditions and transformations
Standard media for A. nidulans (Cove [Citation1966]) and E. coli (Sambrook et al. [Citation1989]) were used. The E. coli strain used was DH5α. Two strains were used for the analysis of mutations. The first was ACZF9 (uapA24 uapC401 azgA4 furR argB2 yA2), an A. nidulans strain previously described and used to analyse several uapA mutations (Amillis et al. [Citation2001]). It is a strain, carrying total loss-of-function mutations, known as uapA24, uapC401 and azgA4, in the genomic loci encoding the three major A. nidulans purine transporters, a genetically unidentified fluorouracil resistance mutation (furR) leading to lack of uracil uptake and an arginine auxotrophy (argB2). yA2 is a conidiospore colour marker. The second strain became available in the course of this study (ΔuapA ΔuapC ΔazgA argB2 pabaA1). It carries knockout deletions of all three purine transporter genes (ΔuapA, ΔuapC, ΔazgA). It also carries the same arginine auxotrophy as ACZF9 (argB2) and an auxotrophic mutation (pabaA1) for p-amino benzoic acid (A. Pantazopoulou, C. Drevet, G. Cecchetto, C. Scazzocchio and G. Diallinas, unpublished). In all cases, mutants and control strains were isogenic, except for the specific uapA mutations constructed. UapA positive and negative controls were ACZF9 or ΔuapA ΔuapC ΔazgA strains transformed with pAN510 or pAN510-GFP (uapA+, positive) or pAN5 (uapA−, negative) plasmids. The controls carry single-copy intact copies of these plasmids. Growth tests of mutants and control strains were performed at both 25 and 37°C, at different pH values (5.0, 6.8 and 8.0) and at different purine concentrations (0.25, 0.5, 1.0 and 2.0 mM). 0.5 mM is the standard purine concentration used in all previous studies concerning purine catabolism in A. nidulans. Transformation of DH5α E. coli was with standard chemical protocols. Transformation of A. nidulans was carried out according to Koukaki et al. [Citation2003].
Construction of mutants
Specific uapA mutations were constructed in plasmid pAN510 (30 ng) by oligonucleotide-directed mutagenesis, using complementary oligonucleotides (135 ng) carrying the desired substitution, in a PCR reaction (30 sec 95°C, followed by 18 cycles of 30 sec 95°C, 1 min at the oligonucleotide Tm, 1 min/Kb 68°C, and finally 68°C 10 min) with Pfu Turbo (Stratagene). The oligonucleotides used are listed in . The parental strands were eliminated with DpnI (NEB) digest and the mutated strands were used to transform E. coli. Plasmids were isolated (NucleoSpin plasmid Macherey-Nagel) from several transformants and mutations were confirmed at the sequencing facilities of Microchemistry Lab, IMBB, Crete. The different uapA alleles were introduced into ACZF9 or ΔuapA ΔuapC ΔazgA strains as described previously (Koukaki et al. [Citation2003], [Citation2005]), based on complementation of an arginine auxotrophy. Several transformants were analysed by Southern blot analysis and transformants, arising from single-copy or multiple-copy integration of pAN510 were selected for further work. The same tools and methodology were used to re-construct selected mutations on a plasmid carrying a chimeric uapA-gfp gene (pAN510-GFP) (Vlanti et al. [Citation2006]).
Table I. ‘Forward’ primers used in the PCR mutagenesis experiments. The nucleobase substitutions of the wild-type uapA codon are shown in bold. The ‘reverse’ primers were complementary to the forward primers.
Hybridizations and standard nucleic acid manipulations
Southern analysis and other techniques involving DNA manipulations were as described in Sambrook et al. [Citation1989]. [32P]-dCTP labelled DNA gene-specific probes were prepared using a random hexanucleotide-primer kit following the supplier's instructions (Promega). Total genomic DNA isolation from A. nidulans strains has been described in Lockington et al. [Citation1985].
Radiolabelled purine uptake measurements
[3H]-xanthine uptake was assayed in conidiospores at 37 or 25°C as previously described (Koukaki et al. [Citation2005], Vlanti et al. [Citation2006]). All experiments were carried out in triplicate. Initial velocities were corrected by subtracting background uptake values measured in the negative control strains (azgA− uapA− uapC− or ΔazgA ΔuapA ΔuapC). Km and IC50 values for compounds inhibiting the uptake of radiolabelled permeants were determined from full dose-response curves with a minimum of eight points spread over the relevant range. In all cases, the Hill coefficients were close to − 1, consistent with competitive inhibition. Hence, Ki values were calculated from the Cheng and Prusoff ([Citation1973]) equation Ki=IC50/[1+(L/Km)], in which L is the permeant concentration. Based on initial competition experiments, performed at the wild-type UapA Ki, some inhibitors were found to bind to several mutants with affinities nearly similar to wild-type (±10%). In those cases, only approximate Ki values were estimated.
Fluorescence microscopy
Samples were prepared as described previously (Koukaki et al. [Citation2005], Vlanti et al. [Citation2006]). Vacuole staining was with CMAC (4-Chloro-methyl-coumarin), according to the supplier (Molecular Probes, Inc., USA) and as described in Vlanti et al. [Citation2006]. Samples were observed and photographed with an AXIOPLAN ZEISS phase-contrast epifluorescent microscope and appropriate filters.
Results
Mutational analysis of the QH motif
Mutations substituting the nearly absolutely conserved residues Q85 and H86 in TMS1 of UapA were designed based on the following criteria: Q85 was substituted by (i) Leu (L85), a hydrophobic residue, (ii) Asn (N85), an isofunctional residue, (iii) Glu (E85), an isostructural residue, and (iv) Thr (T85), another polar amino acid conserved in the same position of a few members of the NAT family. H86 was substituted by (i) Ala (A86), a hydrophobic residue, (ii) Asn (N86), a polar amino acid conserved in some members of the NAT family, (iii) Asp (D86), a residue with the opposite charge, (iv) Lys (K86), a different residue with the same charge. All mutations were designed taking into account the A. nidulans codon usage, and constructed by an oligonucleotide-directed mutagenesis protocol using plasmid pAN510 (see Materials and methods). Mutant alleles carried on pAN510 were introduced initially into strain uapA24 uapC401 azgA4 furR, and eventually into strain ΔuapA ΔuapC ΔazgA. The use of the second strain eliminates some residual purine uptake present in the originally used uapA24 uapC401 azgA4 furR strain (for details see Materials and methods). Both strains allow direct assessment of the function of any uapA allele introduced by transformation. Several transformants were selected for each mutation, based on complementation of arginine auxotrophy (argB−), carried by the recipient strain. At least six transformants for each mutation were purified from conidiospores and tested for stable growth on CM or MM, supplemented with urea as sole nitrogen source. Total DNA was isolated from 48 selected transformants and used in PCR and Southern blot analysis with uapA- and argB-specific probes. This established the profile of the recombination events that took place in each case (not shown). Among all isolates analysed, we selected at least one single-copy transformant and one multi-copy transformant (3–8 plasmid copies in tandem), arising from integration events in the argB locus or from heterologous integration.
Selected transformants were tested by growth tests on different nitrogen sources, including urea, uric acid, xanthine, hypoxanthine and adenine. Growth tests were performed at both 25 and 37°C, at different pH values (5.0, 6.8 and 8.0) and at three different purine concentrations (0.5, 1.0 and 2.0 mM). The recipient strain (uapA24 uapC401 azgA4 furR or ΔuapA ΔuapC ΔazgA), complemented with ‘wild-type’ pAN510 (uapA+), or with plasmid pAN5, carrying only the selection marker argB, was used as positive or negative control for UapA activity, respectively.
A shows that all transformants carrying substitutions of Q85, including the highly conserved substitution Q85N, presented reduced or no growth on uric acid. The corresponding multi-copy transformants showed significantly improved growth on uric acid for Q85E, very slight increase for Q85N and Q85L, and no difference for Q85T. A similar picture was also obtained for growth on xanthine (not shown). In fact, Q85T grew exactly as isogenic ΔuapA strains and was thus considered as a total loss-of-function mutant. None of the mutants showed differential growth on uric acid or xanthine at different pH values, or at different purine concentrations (results not shown). Furthermore, none of the mutants showed a capacity for growth on other purines, such as hypoxanthine or adenine (not shown), with the probable exception of Q85N, which showed a little better growth, compared to uapA+ or uapA- strains, on 2 mM hypoxanthine, that is 4-fold higher concentration than the standard used in growth tests (not shown).
Figure 2. Selected growth tests of transformants carrying single-copy plasmids with the uapA mutations. All Q85 mutations are in a uapA24 uapC401 azgA4 genetic background (yellow conidiospores; for detailed description of genotypes see Materials and methods). All H86 and LR mutations are in a ΔuapA ΔuapC ΔazgA genetic background (green conidiospores). Isogenic positive (uapA+), and negative (uapA−) control strains in both genetic backgrounds are also shown (for more details see Materials and methods). Mutants are shown by the corresponding amino acid substitution, as described in the text. dLA stands for L77A/L84A, trLA for L77A/l84A/L91A. hc refers to high copy transformants. UA is uric acid. Growth tests shown are after 2 days at 37°C, or after 4 days at 25°C. Growth on xanthine is similar to growth on uric acid (not shown). This Figure is reproduced in colour in Molecular Membrane Biology online
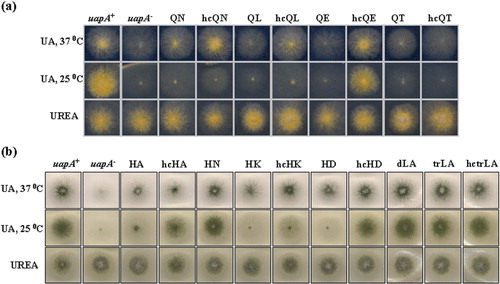
B shows that none of the H86 substitutions led to total loss-of-function at 37°C. All mutants were still able to grow, albeit at reduced rates, on uric acid or xanthine (not shown). Transformants carrying more than a single-copy plasmid grew with significantly improved rates, compared to transformants with single-copy plasmids. This suggested that H86 mutations affected the transport capacity of UapA, either by reducing the amount of protein expressed in the plasma membrane or by affecting transport catalysis per se. Mutants H86A, H86K and H86D showed dramatically reduced growth rates on uric acid (or xanthine) at 25°C. None of the H86 mutations seemed to affect the specificity of UapA for other purines (no growth on hypoxanthine, adenine, guanine) or the pH-dependence of UapA-mediated uric acid transport (results not shown). The observation that none of the Q86 or the H86 mutations affected growth on uric acid or xanthine on different pHs, was in agreement with transport assays showing that in all mutants UapA-mediated transport showed a wild-type dependence on the H+ gradient across the plasma membrane (see later).
Taken together, our results strongly suggested that mutations in residues Q85 and H86 affected the transport capacity of UapA. The cryosensitive nature of most substitutions suggested that these mutations might affect proper folding of the UapA protein or targeting to the plasma membrane.
UapA-GFP topology in Q85 and H86 mutants
We used the Green Fluorescent Protein (GFP) methodology, as previously described (Koukaki et al. [Citation2005], Vlanti et al. [Citation2006]), to study UapA topogenesis in Q85 or H86 mutants. We studied mutations Q85L and Q85T, which had the most dramatic effect on UapA activity, and all four mutations in H86. Mutations were reconstructed into vector pAN510-GFP, which carries a chimeric uapA-gfp gene. The UapA-GFP protein has identical kinetic and specificity properties with the native UapA transporter. pAN510-GFP versions of mutations Q85L, Q85T, H86A, H86N, H86K and H86D were introduced into strain ΔuapA ΔuapC ΔazgA and several transformants were selected as described previously. On the basis of a PCR analysis, which confirmed the presence of intact uapA-gfp copies, and on a Southern analysis, which allowed us to identify single-copy integration events (analyses not shown), selected transformants were chosen for epifluorescence microscopy. Specimens for microscopy were prepared from very young mycelium, germinated in MM supplemented with urea as sole nitrogen source, at 25°C for 14–16 h. At this temperature, the mutants analysed, except H86N, should have very low or no UapA transport activities, as judged from growth tests on uric acid or xanthine (see ). This was also confirmed later on by direct uptake measurements.
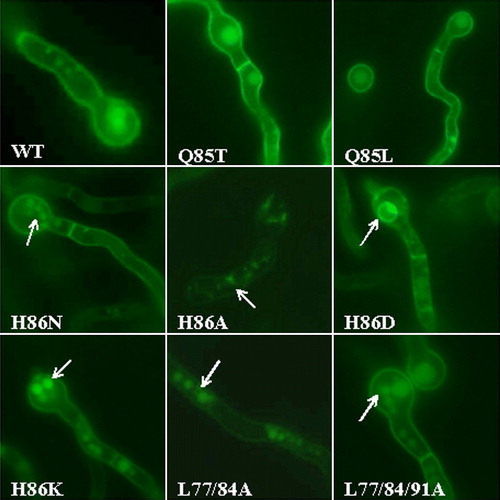
shows selected photographs from the epifluorescence microscopic analysis. Both Q85L and Q85T mutants showed UapA cellular topology identical to the wild-type UapA-GFP control strain, that is, localization mostly at the plasma membrane. Some vacuolar localization, as this was confirmed by double staining with the vacuolar dye CMAC (not shown), was also observed. This is the normal picture of the wild-type UapA-GFP (Vlanti et al. [Citation2006], A. Pantazopoulou and G. Diallinas, unpublished). Mutant H86N, which grew normally on uric acid or xanthine, had a nearly wild-type UapA-GFP topology. Compared to a UapA-GFP strain, a number of UapA-H86N-GFP germlings showed an increased number of small, vacuole-type bodies. In all other H86 mutants, that showed lack of growth on uric acid or xanthine at 25°C (H86A, H86K, H86D), UapA-GFP expression in the plasma membrane was significantly reduced. In these mutants, fluorescence could still be seen associated with the plasma membrane, but its intensity was much reduced compared to wild-type UapA-GFP. Moreover, fluorescence was mostly associated with punctuate granular bodies (H86A, H86K) or typical ER membranous rings (H86D) (Erpapazoglou et al. [Citation2006], Vlanti et al. [Citation2006]). Judging from their compact appearance and positive CMAC staining, the granular bodies in H86A and H86K should be small vacuoles or prevacuolar (PVC) bodies (Penalva [Citation2005]). In line with partial ER-retention, mutant H86D showed evidence of an unfolded protein response (Zhang & Kaufman, [Citation2004]). This was shown by the fact that the mRNA of the A. nidulans homologue of the ER chaperone BiP/Kar2p (Gething [Citation1999]) was 4-fold induced, compared to the wild-type or other mutants with physiological trafficking (not shown). These observations suggested that several H86 substitutions partially and differentially affect UapA folding and targeting to the plasma membrane.
Figure 3. Epifluorescence microscopy of strains expressing wild-type or mutant chimeric UapA-GFP molecules. All strains are isogenic except the uapA allele. Arrows highlight ER membranous rings and vacuolar bodies, also evidenced by appropriate staining (see text, not shown). This figure is reproduced in colour in Molecular Membrane Biology on line.
Q85 and H86 mutations affect substrate transport rather than substrate or H+ binding
We directly assessed the effect of the different mutations on UapA transport activity by a comparative analysis of initial uptake rates of xanthine, in single-copy transformants. Radiolabelled [3H]-xanthine transport assays were performed at 37°C, and for the Q85 mutants also at 25°C, as described in Materials and methods. The results () showed that, with the exception of Q85T, all mutants conserved measurable, and in some cases significant, UapA activities at 37°C. This was further confirmed by uptake studies using multi-copy transformants (see insert in ).
Figure 4. Relative [3H]-xanthine transport rates of single-copy (major figure) and selected multicopy (insert) mutants expressed as % of initial uptake rates (V) compared to the wild-type rate, taken as 100%. Measurements were carried out in the presence of 0.5 µ? [3H]-xanthine, as described in Materials and methods. Results shown are means from four to five independent experiments. Standard deviations in all experiments were always < 20% of the mean values shown. Mutant description as in legend of .
![Figure 4. Relative [3H]-xanthine transport rates of single-copy (major figure) and selected multicopy (insert) mutants expressed as % of initial uptake rates (V) compared to the wild-type rate, taken as 100%. Measurements were carried out in the presence of 0.5 µ? [3H]-xanthine, as described in Materials and methods. Results shown are means from four to five independent experiments. Standard deviations in all experiments were always < 20% of the mean values shown. Mutant description as in legend of Figure 2.](/cms/asset/050065e8-5c9a-4dc0-bec5-59feefcd2915/imbc_a_173786_f0004_b.gif)
All functional mutants were studied kinetically in respect to their capacity to bind and/or transport the two physiological substrates of UapA, xanthine and uric acid. For mutants showing very low transport rates when expressed from single-copies, we also used the respective multicopy transformants in order to confirm Km or Ki values. Km values for xanthine and Ki values for uric acid were calculated as described in Materials and methods, and are shown on . Our results showed that none of the mutations studied affected significantly the affinity of UapA for its physiological substrates. UapA-mediated transport activities in all mutants were also found to be fully dependent on the plasma membrane proton gradient, similarly to a wild-type (uapA+) control. In particular, the presence of H+-gradient uncouplers, such as DCCD or CCCP, abolished transport, while the pH range of UapA-mediated transport in mutants and wild-type was identical (not shown).
Table II. Specificity profile of QH mutants. Km/i values were calculated as described in Materials and methods. Km values are shown only for xanthine. In all other cases Ki values are estimated. As shown previously, using radiolabelled xanthine or uric acid, Km and Ki values are identical. Results are averages of at least two independent experiments, each experiment carried out in triplicate. Standard deviations were always < 30%. All strains are isogenic except for uapA alleles.
We further investigated the possibility that the mutations analysed might affect the architecture of the purine binding site by calculating binding affinities of 25 substrate analogues. Purine analogues were assayed for their ability to inhibit UapA-mediated [3H]-xanthine transport in functional mutants. Inhibitor compounds were tested for their ability to compete with 0.5 µM [3H]-xanthine uptake. shows Ki values from this analysis.
Certain mutants (Q85N, Q85L) had generally increased binding affinities (4–10 fold) for some xanthine analogues with bulky substitutions (1-methylxanthine, 2-thioxanthine, 6-thioxanthine, 8-methylxanthine). Q85N also had low, but measurable, affinity for hypoxanthine, while Q85E has generally 2–5 fold reduced affinity for uric acid, 1-methylxanthine and 9-methylxanthine. The overall picture suggests that the mutations analysed do not affect substrate binding significantly. Moreover, altered affinities could not be rationalized according to a hierarchy, based upon substitutions at particular positions of the purine ring. The importance of this is discussed later.
All H86 mutations caused a minor 2–4 fold negative effect on uric acid binding. H86A had also modified affinity for 8-methylxanthine (5-fold reduction). Similarly to Q85, these modifications do not seem to directly affect specific interactions of UapA with the purine ring.
Thus, growth tests, kinetics and GFP analysis, confirm that the apparent lack of UapA-mediated transport in several mutants is caused from either a defect in substrate translocation, rather than substrate-binding (substitutions of Q85), or a defect in UapA folding and topogenesis (substitutions of H86).
Mutational analysis of the LR motif
Mutations replacing simultaneously L77/ L84 or L77/ L84/L91, with Ala residues, were constructed as previously described, on plasmid pAN510. Appropriate transformants of strain ΔuapA ΔuapC ΔazgA, expressing these mutations from a single-copy plasmid integration event, were isolated as described before (Materials and methods). B shows that none of the mutations affected significantly UapA-mediated growth on purines, at 25 or 37°C.
GFP epifluorescence microscopic analysis of the same substitutions constructed in pAN510-GFP has shown that, in both mutants, UapA associates with the plasma membrane and can be clearly seen in septa. This picture is characteristic of a wild-type UapA topology. An increased number of small vacuoles were evident in both mutants, indicating that substitutions of Leu residues might have increased UapA turnover (). This assumption was not however supported by a kinetic analysis of mutants L77A/ L84A and L77A/ L84A/L91A, which showed that these mutations either increased (180% in L77A/ L84A) or not affected significantly (80% in L77A/ L84A/L91A) UapA transport capacity for xanthine, at both 25°C and 37°C (). A similar result was obtained when the pAN510-GFP versions of these mutations were analysed kinetically (not shown).
Interestingly, the L77A/L84A and L77A/L84A/L91A mutations altered differentially the binding of xanthine and uric acid, and this effect was temperature-dependent. shows Km or Ki values for xanthine or uric acid, calculated at 25 and 37°C. At 25°C, in wild-type (UapA), Km/i values for xanthine and uric acid were 2 and 65 µM respectively. That is, lower temperature had a positive effect (3–4-fold) for xanthine and a negative effect (9-fold) for uric acid. At 37°C, UapA-L77A/L84A had 5-fold reduced affinities for both xanthine and uric acid (32–36 µM), while in UapA-L77A/L84A/L91A, compared to wild-type, xanthine binding increased 4-fold (2 µM) and diminished 16-fold (110 µ?) for uric acid. At 25°C the effect of both mutants was suppressed, as their Km/i values for xanthine and uric acid were very similar to the wild-type. In other words, replacing the two internal Leu of the LR motif had a temperature-sensitive negative effect on substrate binding in general, while replacing one extra Leu of the LR, affected differentially uric acid and xanthine, still in a temperature-sensitive manner. The end result was that at 37°C, UapA- L77A/L84A/L91A binds xanthine 55-fold more efficiently than uric acid. Interestingly, the transformant with the GFP version of UapA-L77A/L84A/L91A was thermosensitive for growth on uric acid or xanthine (not shown). It seems that the presence of the GFP tag in association with the three Leu substitutions further enhanced a negative effect of the mutations.
Table III. Effect of Leu substitution on UapA affinity for physiological substrates. Km/i values were calculated as described in Materials and methods. Results are averages of at least two independent experiments, each experiment carried out in triplicate. Standard deviations were always < 30%. All strains are isogenic except for uapA alleles. Uptakes were performed at 25 or 37°C.
We then investigated, as described previously for the QH motif mutations, whether L77A/L84A and L77A/L84A/L91A mutations alter the specificity of UapA towards various purines and analogues. Competition assays were performed at 37°C, where these mutants present altered kinetics in respect to their physiological substrates. shows that none of the two mutations altered significantly Ki values for all possible substrates tested, except for a reduction in 1-methylxanthine (2.5-fold) and 9-methylxanthine (6-fold) binding by UapA-L77A/L84A.
Table IV. Specificity profile of LR mutants. Km/i values were calculated as described in Materials and methods. Results are averages of at least two independent experiments, each experiment carried out in triplicate. Standard deviations were always < 30%. All strains are isogenic except for uapA alleles.
The kinetic phenotypes of L77A/L84A and L77A/L84A/L91A mutants strongly suggest that repeated Leu residues in TMS1 are not necessary for expression in the plasma membrane but should rather determine the local flexibility of UapA, in a way that affects uric acid binding much more prominently than xanthine binding.
Discussion
We have analysed several elements associated with TMS1 of UapA. In particular, we examined the role of rationally designed substitutions on two motifs, a nearly absolutely conserved QH element conserved in all NAT members, and a LR consisting of four Leu residues (L70 -X6- L77 -X6- L84 -X6- L91), the last three of which were predicted to lie in one side of TMS1. This motif is not present, at least in canonical form, in other NAT members.
The QH motif
The fact that the QH is conserved in the mammalian ascorbate/Na+ symporters, the only non-nucleobase/H+ carriers of the NAT family known, allowed the prediction that its function, if any, would not be connected with substrate or ion specificity. On the other hand, its absolute conservation in homologues with diverse substrate specificity suggested that it might be involved in critical structural intra-molecular interactions. The possibility that the QH motif might rather be involved in inter-molecular interactions with chaperones or other factors assisting its trafficking, could also be dismissed given its conservation in prokaryotes and eukaryotes, and generally in organisms with divergent topogenetic mechanisms for membrane proteins. Of course, considering the conservation of the basic translocase (Sec61/SecY) channel and of the mechanism of co-translational integration of transporters, we could not formally dismiss that this motif is playing a family-specific role in interactions with the translocase machinery. Despite the fact that we have noticed that several transporters families have exceptionally well conserved short motifs in their TMS1 (unpublished observations), no function has ever been assigned to those. Our results were in perfect agreement with the first prediction, that the QH motif has nothing to do with substrate or ion binding. We presented evidence that these polar/charged residues, at mid-point of TMS1, stabilize the UapA polypeptide in a way that is critical for either its trafficking (H86) or for substrate transport catalysis (Q85). Even conservative substitutions dramatically affect UapA function at 25°C, the physiological temperature of A. nidulans in nature. Despite their importance in UapA function, these neighbouring residues seem to affect differently the structure of the carrier. The fact the mutation Q85L has a severe phenotype is indicative of the involvement of Gln in a polar intramolecular interaction. This interaction should involve the amino group of the Gln side chain, possibly as a H donor, given that the Q85E mutation also compromised UapA-mediated transport. This is further supported by the fact that, despite the severe negative effect of Q85E on transport catalysis, this isostructural substitution had a minor effect on general folding, as judged from its very mild cryosensitivity, in a multicopy transformant (see A). Furthermore, the nearly absolute evolutionary conservation of Q85 and the fact that neither Asn nor Thr could replace its role, suggested the Gln side chain should be involved in a very specific and dynamic interaction, necessary for transport catalysis per se. Second-site suppressors of Q85 mutations are currently screened for identifying interacting residues. Interestingly, in YgfO, an E. coli UapA homologue recognizing xanthine but not uric acid, Thr, a residue not tolerated for UapA function, “replaces” Q85.
Substitutions of H86 were in general more tolerated than those in Q85 and, except H86N, most affected UapA trafficking. Despite not being able to quantify accurately the amount of different H86 mutant versions that reach the plasma membrane, it was evident that such mutants were functional for purine transport. Neither substrate and ion binding, nor rate of transport was affected. H86 is most likely critical for folding of TMS1 and UapA, in a way that does not affect the general structure of the protein, but rather a specific topogenetic domain. Defective folding was further supported by the behaviour of different H86 mutants and of the wild-type UapA to diethyl pyrocarbonate (DEPC), a reagent reacting specifically with aqueous-accessible His residues. In uptake experiments not shown herein, functional UapA molecules (wild-type and H86N respectively) had different accessibility to DEPC compared to mutants with severely affected function (H86A). H86 mutations affecting function do not affect the hydrophobicity of TMS1 significantly. Asp reduces moderately α-helicity, while Ala increases it (http://biophysics.biol.uoa.gr/cgi-bin/SecStr/SecStr). H86 might be involved in specific interactions through its imidazol ring. Sequence-specific interactions between α-helical TMSs support folding and assembly of many integral membrane proteins (Popot & Engelman [Citation2000], Shai [Citation2001]; DeGrado et al. [Citation2003]). These interactions depend on mutual recognition of complementary surfaces of the helices. The fact that H86 could be replaced by Asn, a residue found in a few other NAT members, but not by Asp, suggests that the imidazol ring might act, analogously to the amino group of Asn, as a H donor. Such an interaction is most likely specifically critical for ER-exit, trafficking and UapA stability, but does not affect the process of transport. Suppressors of H86 mutations should be revealing of this independent topological element in UapA, and NAT homologues in general, and might also include extragenic suppressors eventually leading to specific chaperone and/or trafficking factors.
The LR element
The LR motif is unique for UapA. Thus, if it has any role, this is not probably part of the basic elements involved in protein translocation, or in substrate binding and transport. We rather favoured a specific role in the fine regulation of expression of UapA. We obtained evidence that the transmembrane Leu residues of the LR are critical for the fine regulation of UapA kinetics. This was highlighted by the observation that the UapA version with the triple substitution L77A/L84A/L91A binds xanthine 55-fold more efficiently than uric acid at 37°C. This contrasts sharply with the wild-type UapA, which binds both purines with equal affinity. Our results also suggest that xanthine and uric acid, the two physiological substrates of UapA, probably use different molecular interactions to be accommodated in the binding pocket of UapA. This is in line with previously proposed kinetic models describing UapA-substrate interactions (Goudela et al. [Citation2005]).
Unlike other UapA mutations, mapping in the NAT signature motif downstream TMS8 which defined part of the substrate binding (Koukaki et al. [Citation2005]), no evidence was obtained for a direct involvement of L77, L84 and L91 in substrate binding. Replacement of Leu with Ala reduces significantly overall hydrophobicity of TMS1. It seems that the Leu repeat creates a specific hydrophobic interface that interacts with, either another part of the UapA polypeptide, possibly a helix lining the substrate-binding site and thus indirectly affects purine binding, or another UapA molecule promoting UapA dimerization.
Transmembrane LR motifs have gained considerable attention recently. It was shown that transmembrane helix-helix interfaces of a set of crystallized membrane proteins might be structurally equivalent to soluble leucine zipper interaction domains (Langosch et al. [Citation1996], Gurezka et al. [Citation1999], Gurezka & Langosch [Citation2001]). Degenerate versions of leucine motifs were found within transmembrane segments of a variety of functionally different proteins. For several of these natural transmembrane segments, self-interaction was experimentally verified (Gurezka et al. [Citation1999]). Moreover, oligo-leucine sequences were shown to function as an artificial transmembrane segment that efficiently self-assembles in membranes (Gurezka & Langosch [Citation2001]). These results support the notion that transmembrane LR motifs are implicated in the oligomeric assembly of the corresponding proteins. In line with that, Torres et al. ([Citation2003]) showed that expressing the first two transmembrane domains of the dopamine transporter (DAT) inhibited wild-type transporter function, but not when an LR motif, present in TMS2, was mutated. Recently however, Sen et al. (2005) presented evidence suggesting that it is unlikely that the LR motif plays a role, as a zipper, in symmetrical TMS2 dimerization. Sen et al. ([Citation2005]) also proposed, based on the high-resolution structure of LeuT (Yamashita et al. [Citation2005]), a sodium-dependent leucine transporter that is sufficiently homologous to DAT, that TMS2 does not contribute directly to the substrate-binding site or the transport pathway, suggesting that the Leu mutations in this region affect cocaine binding indirectly (Sen at al. 2005).
In summary, we showed that TMS1 has at least a dual role in UapA functioning. Some elements, revealed by mutations in Q85 and in the LR motif, regulate transport, while other elements, highlighted by mutations in H86 are critical for topology. These two elements seem to be independent of each other. The involvement of TMS1 elements as determinants of UapA kinetics predicts an association with two other domains shown previously to be critical for substrate binding and transport, the NAT signature motif downstream from TMS8, and a selectivity filter in TMS12 (Amillis et al. [Citation2001], Koukaki et al. [Citation2005], Vlanti et al. [Citation2006]). Alternatively, UapA dimerization through the TMS1 LR motif might finely regulate binding and transport of specific substrates such as uric acid. Experiments are in progress to evaluate the role of the TMS1 LR motif in UapA dimerization or in intramolecular interactions. Finally, mutations studied in this work also provide ideal tools for isolating new genes whose function assist UapA trafficking.
This paper was first published online on prEview on 18 May 2006.
We are grateful to C. Scazzocchio, C. Drevet and G. Cecchetto for the original ΔuapA ΔuapC ΔazgA strain. A.P. was supported by the Archimedes 2002 prize of the EU and a grant from the Ministry of Development (PENED 2001). Work in the laboratory of G.D. was supported by the European Social Fund and National resources –(EPEAEKII) PYTHAGORAS and the University of Athens (ELKE).
References
- Amillis S, Koukaki M, Diallinas G. Substitution F569S converts UapA, a specific uric acid-xanthine transporter, into a broad specificity transporter for purine-related solutes. J Mol Biol 2001; 313: 765–774
- Amillis S, Cecchetto G, Sophianopoulou V, Koukaki M, Scazzocchio C, Diallinas G. Transcription of purine transporter genes is activated during the isotropic growth phase of Aspergillus nidulans conidia. Mol Microbiol 2004; 52: 205–216
- Argyrou A, Blanchard JS. Mycobacterium tuberculosis lipoamide dehydrogenase is encoded by Rv0462 and not by the lpdA or lpdB genes. Biochemistry 2001; 40: 11353–11363
- Cecchetto G, Amillis S, Diallinas G, Scazzocchio C, Drevet C. The AzgA purine transporter of Aspergillus nidulans. Characterization of a protein belonging to a new phylogenetic cluster. J Biol Chem 2004; 279: 3132–3141
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973; 22: 3099–3108
- Cove DJ. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta 1966; 113: 51–56
- DeGrado WF, Gratkowski H, Lear JD. How do helix-helix interactions help determine the folds of membrane proteins? Perspectives from the study of homo-oligomeric helical bundles. Protein Sci 2003; 12: 647–665
- De Koning HP, Diallinas G. Nucleobase transporters. Mol Membr Biol 2000; 17: 75–94
- Diallinas G, Scazzocchio C. A gene coding for the uric acid-xanthine permease of Aspergillus nidulans: inactivational cloning, characterization and sequence of a cis-acting mutation. Genetics 1989; 122: 341–350
- Diallinas G, Gorfinkiel L, Arst HN, Jr, Cecchetto G, Scazzocchio C. 1995. Genetic and molecular characterization of a gene encoding a wide specificity purine permease of Aspergillus nidulans reveals a novel family of transporters conserved in prokaryotes and eukaryotes. J Biol Chem 270:8610–8622.
- Diallinas G, Valdez J, Sophianopoulou V, Rosa A, Scazzocchio C. Chimeric purine transporters of Aspergillus nidulans define a domain critical for function and specificity conserved in bacterial, plant and metazoand homologues. EMBO J 1998; 17: 3827–3837
- Erpapazoglou Z, Kafasla P, Sophianopoulou V. 2006. The SHR3 homologue of Aspergillus nidulans has restricted range of amino acid transporter targets. Fungal Genet Biol.43:222–223.
- Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol 1999; 10: 465–472
- Gorfinkiel L, Diallinas G, Scazzocchio C. Sequence and regulation of the uapA gene encoding a uric acid-xanthine permease in the fungus Aspergillus nidulans. J Biol Chem. 1993; 268: 23376–23381
- Goudela S, Karatza P, Koukaki M, Frillingos S, Diallinas G. Comparative substrate recognition by bacterial and fungal purine transporters of the NAT/NCS2 family. Mol Membr Biol 2005; 22: 263–275
- Gurezka R, Laage R, Brosig B, Langosch D. A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J Biol Chem 1999; 274: 9265–9270
- Gurezka R, Langosch D. In vitro selection of membrane-spanning leucine zipper protein-protein interaction motifs using POSSYCCAT. J Biol Chem 2001; 276: 45580–45587
- Karatza P, Frillingos S. Cloning and functional characterization of two bacterial members of the NAT/NCS2 family in Escherichia coli. Mol Membr Biol 2005; 22: 251–261
- Kinghorn JR, Sloan J, Kana'n GJ, Dasilva ER, Rouch DA, Unkles SE. Missense mutations that inactivate the Aspergillus nidulans nrtA gene encoding a high-affinity nitrate transporter. Genetics 2005; 169: 1369–1377
- Koukaki M, Giannoutsou E, Karagouni A, Diallinas G. A novel improved method for Aspergillus nidulans transformation. J Microbiol Methods 2003; 55: 687–695
- Koukaki M, Vlanti A, Goudela S, Pantazopoulou A, Gioule H, Tournaviti S, Diallinas G. The nucleobase-ascorbate transporter (NAT) signature motif in UapA defines the function of the purine translocation pathway. J Mol Biol 2005; 350: 499–513
- Langosch D, Brosig B, Kolmar H, Fritz HJ. Dimerisation of the glycophorin A transmembrane segment in membranes probed with the ToxR transcription activator. J Mol Biol 1996; 263: 525–530
- Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol Membr Biol 2001; 18: 87–95
- Lockington RA, Sealy-Lewis HM, Scazzocchio C, Davies RW. Cloning and characterization of the ethanol utilization regulon in Aspergillus nidulans. Gene 1985; 33: 137–149
- Meintanis C, Karagouni AD, Diallinas G. Amino acid residues N450 and Q449 are critical for the uptake capacity and specificity of UapA, a prototype of a nucleobase-ascorbate transporter family. Mol Membr Biol 2000; 17: 47–57
- Monahan BJ, Unkles SE, Tsing IT, Kinghorn JR, Hynes MJ, Davis M.A. Mutation and functional analysis of the Aspergillus nidulans ammonium permease MeaA and evidence for interaction with itself and MepA. Fungal Genet Biol 2002; 36: 35–46
- Peňalva AM. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fung Genet Biol 2005; 42: 963–975
- Popot JL, Engelman DM. Helical membrane protein folding, stability and evolution. Ann Rev Biochem 2000; 69: 881–922
- Sambrook J, Fritisch EF, Maniatis T. 1989. Molecular cloning: A laboratory manual. USA: Cold Spring harbour Laboratory.
- Sen N, Shi L, Beuming T, Weinstein H, Javitch JA. A pincer-like configuration of TM2 in the human dopamine transporter is responsible for indirect effects on cocaine binding. Neuropharmacology 2005; 49: 780–790
- Shai Y. Molecular recognition within the membrane milieu: Implications for the structure and function of membrane proteins. J Membr Biol 2001; 182: 91–104
- Tavoularis SN, Tazebay UH, Diallinas G, Sideridou M, Rosa A, Scazzocchio C, Sophianopoulou V. Mutational analysis of the major proline transporter (PrnB) of Aspergillus nidulans. Mol Membr Biol 2003; 20: 285–297
- Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem 2003; 278: 2731–2739
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker R F, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999; 399: 70–75
- Unkles SE, Rouch DA, Wang Y, Siddiqi MY, Okamoto M, Stephenson RM, Kinghorn JR, Glass AD. Determination of the essentiality of the eight cysteine residues of the NrtA protein for high-affinity nitrate transport and the generation of a functional cysteine-less transporter. Biochemistry 2005; 44: 5471–5477
- Vlanti A, Amillis S, Koukaki M, Diallinas G. 2006. A novel-type substrate-selectivity filter and ER-exit determinants in the Uapa purine transporter. J Mol Biol, in press.357:808–819.
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 2005; 437: 215–223
- Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 2004; 279: 25935–25938