Abstract
The temperature-sensitive hemagglutinin (Tsh) is a representative of the growing subfamily of secreted bacterial virulence factors, known as serine protease autotransporters of the Enterobacteriaceae (SPATEs). Expressed by avian and human pathogenic strains of Escherichia coli Tsh acts as a serine protease and an adhesin to erythrocytes, hemoglobin, and extracellular matrix proteins. Mature Tsh is comprised of a 106-kDa secreted domain (Tshs) and a 33-kDa outer membrane β-domain (Tshβ). Based on the size of β-domains and functional properties of their passenger domains, all SPATEs are considered to be conventional autotransporters. However, it is unsettled if the conventional autotransporters exist as monomers, oligomers, or multimers (e.g., hexamers). To determine the quaternary structure of Tsh in vitro, we purified Tshβ from the outer membranes and showed that it is natively folded because it is heat modifiable and resistant to protease digestion. Blue-native polyacrylamide gel electrophoresis of Tshβ indicated that Tshβ exists as a monomer or a dimer. The cross-linking analysis demonstrated that purified Tshβ exists as a monomer. The size-exclusion chromatography and cross-linking analyses of purified Tshs also showed that the passenger domain of Tsh is a monomer. Overall, our data indicated that Tsh is a monomeric protein in vitro and support the concept that the SPATE autotransporters exist as monomers rather than as multimers. Implications of our findings on the mechanism of autotransporter secretion across the outer membrane are discussed.
Introduction
The presence of two membranes in the Gram-negative cell envelope complicates the process of protein secretion in these bacteria. Multiple distinct pathways have evolved in the Gram-negative bacteria for secretion of proteins from the bacterial cytoplasm to the extracellular space Citation[1]. The simplest pathway is the type V, also known as the autotransporter pathway. Autotransporters are large proteins with three distinct domains: a cleavable N-terminal signal sequence; an internal passenger domain, which carries out the functions specific to each autotransporter; and a C-terminal transmembrane domain Citation[1–4]. The process of autotransporter secretion consists of several steps. First, the signal sequence directs the protein to the Sec-translocase complex Citation[2]. After the Sec-mediated translocation across the inner membrane, a signal peptidase removes the signal sequence. Next, the C-terminal domain inserts itself into the outer membrane and mediates transport of the passenger domain to the extracellular environment. Once translocation is complete, the protein has two fates. It either remains attached to the bacterial surface or is released into the extracellular space Citation[1], Citation[3], Citation[5], Citation[6].
Several hundred autotransporters have been identified to date in Gram-negative bacteria Citation[7]. Autotransporters could be broadly subdivided into conventional and trimeric autotransporters. Trimeric autotransporters possess a short transmembrane domain that is not enough to form a β-barrel pore to transport its passenger domain. It has been proposed that in this case three autotransporters come together in a TolC-like fashion to form a single β-barrel Citation[6]. Conventional autotransporters contain a large transmembrane domain capable of forming a complete pore for its passenger domain. Among conventional autotransporters, the serine protease autotransporters of the Enterobacteriaceae (SPATEs) form a distinct group of virulence factors unified by the presence of a conserved serine protease motif, GDSGS, at the N-termini of their passenger domains Citation[8]. Some representative examples of the SPATEs are: the hemoglobin protease and heme adhesin Hbp from human pathogenic Escherichia coli Citation[9], the protease and cytotoxin Pet from enteroaggregative E. coli Citation[8], Citation[10], Citation[11], and the protease and adhesin Tsh from avian and human pathogenic strains of E. coli Citation[12–14].
The temperature-sensitive hemagglutinin (Tsh) was the first SPATE identified and since then has served as a model to study this autotransporter subfamily Citation[14]. After translocation across the inner membrane and cleavage of the 52-amino acid signal sequence, mature Tsh is comprised of a 106-kDa passenger domain (Tshs) and a 33-kDa β-domain (Tshβ). Tshs contains separate domains responsible for the adhesive and proteolytic properties of the protein Citation[12].
Despite the accumulated data on the functional activities of conventional autotransporters like the SPATEs, their structural details are sparse. Currently it is unclear whether conventional autotransporters are monomeric or multimeric. The crystal structure of NalP translocator domain indicates that conventional autotransporters may exist as monomers Citation[15]. On the other hand, in vitro studies performed with another conventional autotransporter, the IgA1 protease of Neisseria gonorrhoeae, suggest that six or more C-terminal subunits may form a 2 nm-wide central pore in the outer membrane Citation[16]. In this study we examine in vitro the quaternary structure of the SPATE group of conventional autotransporters using Tsh as a model and compare our results to the data obtained from the purified β-domains of other autotransporter proteins.
Materials and methods
Bacterial strains, plasmids, and growth conditions
E. coli strain XL1-Blue (recA1 lac endA1 gyrA96 thi hsdR17 supE44 relA1 (F+ proAB lacIq lacZD M15 Tn10 [Tetr]) Citation[18] was acquired from Promega (Madison, WI). E. coli strain AW741 (▵ompF, ▵ompA, OmpC) was a generous gift from Dr A. Delcour (University of Houston, Houston, TX). It was constructed from AW739 Citation[19] by selecting for a mutant resistant to phage K3, which utilizes OmpA as its receptor. The XL1 Blue strain expresses both OmpF and OmpA, whereas the AW741 strain is a deletion mutant for OmpA and OmpF but expresses a functional OmpC. Plasmid pTsh-ΔN2, a derivative of pYA3418 Citation[13], expresses a Tsh protein missing the Asn57-Val1099 sequence, which corresponds to the entire Tshs passenger domain.
All strains were grown overnight at 37°C in LB medium (10 g/l tryptone, 5 g/l yeast extract, 10 g/l NaCl) in the presence of 0.1 mg/ml ampicillin (Sigma-Aldrich, St Louis, MO). One milliliter of overnight culture was used to inoculate 1 l of fresh LB medium containing 0.1 mg/ml ampicillin. The culture was grown at 37°C until OD600 of 0.6. Expression of Tshβ was induced by addition of 0.8 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 3 h. Cells were harvested by a centrifugation at 5,000 rpm for 30 min at 4°C (Beckman J 2-21 M centrifuge, JA-10 rotor).
Outer membrane isolation
Harvested cell pellets were resuspended in 20 mM Tris-HCl buffer (pH 7.5), 1 mM EDTA, 0.1 mM PMSF. The cells were lysed by passing through a French Pressure Cell Press (SLM Aminco, SLM Instruments, Urbana, IL) at 16,000 psi. Unbroken cells were separated by centrifugation at 5,000 rpm (Beckman J2-21M centrifuge, Piramoon F 10B rotor) for 30 min at 4°C and then discarded. Cell lysate was centrifuged at 100,000 g for 1 h at 4°C (Beckman L7-55 ultracentrifuge) to pellet the total membranes. The collected total membranes were washed in 20 mM Tris-HCl (pH 7.5). Inner membranes were solubilized by incubation on ice for 5 min in the presence of 0.5% sarkosyl NL (Sigma-Aldrich, St Louis, MO). Outer membranes were harvested by ultracentrifugation at 100,000 g for 1 h at 4°C. Isolated outer membranes were resuspended in 20 mM Tris-HCl buffer (pH 7.5). Protein concentration was determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA).
Protein purification
Tshs was purified as described earlier Citation[12]. Tshβ and OmpC were purified using the procedure described by Kramer and colleagues Citation[20] with some modifications. Briefly, Tshβ and OmpC were extracted from the outer membranes of AW741/pTsh-ΔN2 after incubation with 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 5 mM EDTA for 1 h at 4°C. Detergent Triton X-100 contributes to the disruption of the lipid bilayer releasing integral membrane proteins. Then it forms detergent micelles around these proteins stabilizing them and preventing their unfolding. According to our studies, both OmpC and Tshβ get solubilized by Triton X-100. Membrane lipids were pelleted by centrifugation at 10,000 rpm for 30 min at 4°C (Eppendorf 5804 R centrifuge, F34-6-38 rotor). Five milliliters containing 0.2 mg of the resulting protein extract were loaded onto a 5 ml HiTrap DEAE FF anion-exchange column (Amersham Biosciences, Corp., Piscataway, NJ) equilibrated with 20 mM Tris-HCl (pH 7.5), 10 mM SB3-12 [3-(Dodecyldimethylammonio)-propanesulfonate] (Sigma-Aldrich, St Louis, MO). The proteins were eluted with a linear ionic strength gradient ranging from 0–0.5 M NaCl. Their presence was monitored at the 280 nm wavelength. The eluted proteins were collected in 2 ml fractions. Two hundred microliters of each fraction were acetone precipitated overnight. The precipitated proteins were visualized using Coomassie-blue staining following separation on a 12% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) to assess the molecular mass and the purity of the obtained proteins. Fractions containing Tshβ and OmpC were dialysed against 20 mM Tris-HCl (pH 7.5), 10 mM SB3-12 using 10 kDa cut-off dialysis cassette (Pierce, Rockford, IL) and concentrated using 10 kDa cut-off Amicon Ultra Centrifugal Filter Devices (Millipore, Billerica, MA). The protein concentration was determined by the BCA assay (Pierce, Rockford, IL).
Heat modifiability of Tshβ
The assay was performed essentially as described by Thanassi and colleagues Citation[21]. In short, purified Tshβ and the outer membranes from XL1 Blue cells were mixed with SDS loading buffer so that the final concentration of SDS was 2% w/v and β-mercaptoethanol was 5% v/v. The samples were heated at either 42°C or 100°C for 5 min, cooled down for 5 min on ice, and resolved on a 12% SDS-polyacrylamide gel. Protein bands were visualized with Coomassie.
Protease accessibility assay for Tshβ
XL1-Blue outer membranes and purified Tshβ were incubated with trypsin (Sigma-Aldrich, St Louis, MO) under native and denaturing conditions. To denature proteins, the samples were mixed with SDS-loading buffer (SDS, β-mercaptoethanol) and boiled for 10 min, followed by immediate cooling on ice for 5 min. Digestion conditions were: 2.8 µg of the purified protein or 0.1 mg of outer membranes were treated with 30 µg of trypsin (Sigma-Aldrich, St Louis, MO) for 1 h at 37°C in a final volume of 1 ml of 20 mM Tris-HCl buffer (pH 7.5). To terminate digestion, the reaction mixtures were boiled for 5 min and combined with 10 mM PMSF (Sigma-Aldrich, St Louis, MO). The reactions containing the purified proteins were precipitated with acetone (Sigma-Aldrich, St Louis, MO) overnight. 9 µl of outer membrane digestions and resuspended precipitates were resolved on a 12% SDS-polyacrylamide. The protein bands were visualized using silver staining.
Blue Native PAGE (BN-PAGE) for Tshβ
Totals of 15–25% gradient gels were prepared as previously described Citation[22]. The protein samples were prepared as outlined by Thanassi and colleagues Citation[21], except that OmpC and Tshβ were in 10 mM SB3-12. Anode and cathode buffers were the same as described by Thanassi and colleagues Citation[21]. The protein markers were from Sigma-Aldrich (St Louis, MO). Protein complexes were resolved at 4°C for 5 h at 100 V. The protein bands were visualized as described before Citation[23].
Cross-linking analysis
Purified Tshβ and OmpC were dialysed twice against 20 mM HEPES buffer (pH 7.5), 150 mM NaCl, 10 mM SB3-12 and concentrated using 10 kDa cut-off Amicon Ultra Centrifugal Filter Devices (Millipore, Billerica, MA). Tshs was treated in the same manner, but without detergent in the dialysis buffer. The resulting protein concentration was measured using BCA assay. DSS (Disuccinimidyl suberate) (Sigma-Aldrich, St Louis, MO) was dissolved in DMSO (Fisher Scientific, Pittsburgh, PA). Two millimolar of DSS was mixed with 4.5 µg of protein and incubated at RT for 1 h in a final volume of 1 ml of 20 mM HEPES buffer (pH 7.5) Citation[24]. Reactions were terminated with 20 mM Tris-Cl buffer (pH 7.5) Citation[24]. Membrane proteins were precipitated with acetone overnight. All proteins were resolved on 10% or 12% SDS-PAGE and visualized using Coomassie-blue staining.
Size-exclusion chromatography for Tshs
Gel filtration column Superdex 200 26/60 (Amersham Biosciences, Corp., Piscataway, N.J.) was calibrated with 20 mM Tris buffer, 300 mM NaCl and then by two subsequent runs using first the gel filtration MW standards thyroglobulin (MW 669 kDa), ferritin (MW 440 kDa), catalase (MW 232 kDa), aldolase (MW 158 kDa), albumin (MW 67 kDa) and then the molecular weight standards ferritin (MW 440 kDa), catalase (MW 232 kDa), albumin (MW 67 kDa), ovalbumin (MW 43 kDa) (Amersham Biosciences, Corp., Piscataway, NJ), prepared in 20 mM Tris buffer, 300 mM NaCl according to the manufacturer's instructions. The void volume was calculated by the elution of blue dextran 2000 (Amersham Biosciences, Corp., Piscataway, NJ), which was added each time in the protein sample. The flow rate was 1 ml/min and 6 ml fractions were collected. Elution of the standards was monitored at the 280 nm wavelength. Five milliliters of the purified Tshs sample containing 6.42 mg of protein were loaded on the Superdex column and ran under the same conditions. Presence of Tshs in the eluted peak was confirmed by SDS-polyacrylamide gel electrophoresis and calculation of its molecular weight was carried out using the standard curve created from the calibration runs with the standards. Native folding of the protein was confirmed by testing its adhesive properties as previously described Citation[12] (data not shown).
Results
Purification of Tshβ
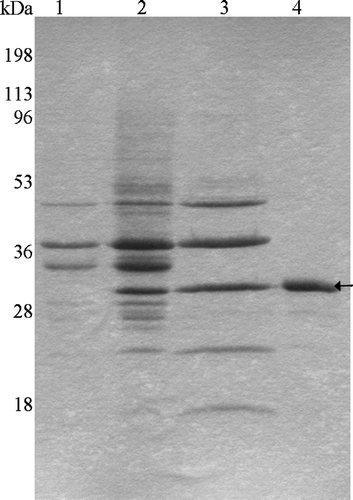
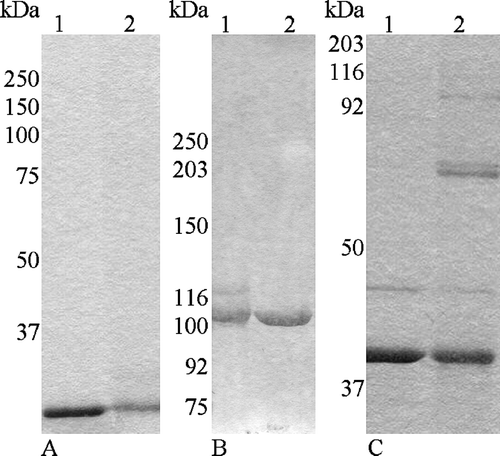
A truncated tsh gene containing only its signal sequence and Tshβ domain was engineered into a low copy vector (pWSK30) under the control of the lac promoter. Expression and outer membrane localization of Tshβ was determined by cell fractionation (, lane 2). Proteins, including Tshβ, were extracted from the outer membrane with Triton X-100 detergent (, lane 3). Tshβ was purified from the other proteins in the extract using ion-exchange chromatography (, lane 4). Excess detergent and salt were removed from the protein sample by dialysis against 20 mM Tris-HCl (pH 7.5), 10 mM SB3-12 prior to loading on the gel. Lane 1 in corresponds to the negative control which consisted of outer membranes isolated from XL1 Blue cells carrying the vector plasmid without the insert.
Figure 1. SDS-PAGE profile of Tshβ purification. The outer membranes were collected following the lysis of bacterial cells. Proteins were extracted from the outer membranes. Tshβ was purified from the extract. Fractions containing Tshβ were combined, dialysed, and concentrated. Protein samples were resolved on 12% SDS-PAGE followed by Coomassie-blue staining. Lanes: 1, outer membranes of XL1-Blue/pSWK30; 2, outer membranes of XL1-Blue/pTsh-ΔN2 (Tshβ); 3, protein extract from AW741/pTsh-ΔN2 (Tshβ) membranes, acetone precipitated; 4, purified Tshβ. Arrow indicates the position of Tshβ.
Assessment of folding of purified Tshβ
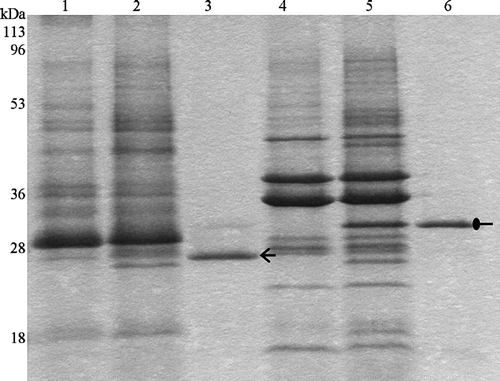
To verify the correct folding of the purified Tshβ, we relied on two properties of natively folded outer membrane proteins: their heat modifiability and their resistance to proteases Citation[25]. One of the hallmarks of the outer membrane proteins is that they form extremely stable β-barrels. Moderate heat (42°C) and the presence of SDS-loading buffer are not enough to completely denature outer membrane proteins. As a result, the non-denatured outer membrane proteins run faster than their predicted molecular weight. Only when these proteins are completely denatured by boiling at 100°C, they migrate according to their molecular masses. When boiled with SDS-loading buffer, Tshβ ran at its calculated molecular mass, as a 33-kDa protein (, lane 6). When heated to 42°C in the presence of SDS detergent, Tshβ ran as a ∼26-kDa protein (, lane 3). A faint band at 33 kDa that corresponds to the denatured Tshβ was detected (, lane 3). However, the amount of unfolded protein is negligible compared to the amount of the folded protein. Lanes 1 and 4 in correspond to negative controls which consisted of outer membranes isolated from XL1 Blue cells without insert-containing plasmid heated to 42°C and 100°C respectively. Lanes 2 and 5 in correspond to positive controls which consisted of outer membranes isolated from XL1 Blue cells with insert-containing plasmid heated to 42°C and 100°C respectively.
Figure 2. Heat modifiability of purified Tshβ. Purified Tshβ and outer membranes were heated for 5 min at either 100°C or 42°C in the presence of SDS loading buffer and then cooled down for 5 min on ice. Samples were loaded immediately and resolved on 12% SDS-PAGE. Protein bands were visualized by overnight Coomassie staining. Lanes: 1, outer membranes of XL1-Blue/pWSK30 heated to 42°C; 2, outer membranes of XL1-Blue/pTsh-ΔN2 heated to 42°C; 3, purified Tshβ heated to 42°C; 4, outer membranes of XL1-Blue/pWSK30 heated to 100°C; 5, outer membranes of XL1-Blue/pTsh-ΔN2 heated to 100°C; 6, purified Tshβ heated to 100°C. Oval arrow indicates the unfolded Tshβ and open arrow indicates the folded Tshβ.
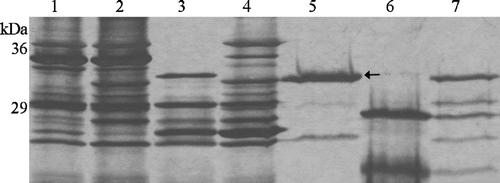
Another characteristic of natively-folded outer membrane proteins is that they form β-barrels resistant to proteases. To demonstrate the presence of trypsin cleavage sites in Tshβ sequence, Tshβ protein, denatured by heat and SDS, was subjected to protease treatment. The denatured Tshβ was equally susceptible to trypsin degradation in both the outer membranes (, lane 3) and in the purified fraction (, lane 6). On the other hand, the non-denatured Tshβ remained mostly intact even after 1 h of incubation with trypsin in the outer membranes (, lane 4) and in the purified fraction (, lane 7). Lane 1 in corresponds to the negative control which consisted of outer membrane isolated from XL1 Blue cells carrying the vector plasmid without the insert. Lane 2 in corresponds to the positive control which consisted of outer membrane isolated from XL1 Blue cells carrying the vector plasmid with the insert. The results presented in and confirm that, in the outer membranes, Tshβ adapts a β-barrel conformation resistant to heat and protease treatment and indicate that this conformation is retained even after the protein purification.
Figure 3. Trypsin digestion of Tshβ. Native and denatured outer membrane proteins and purified Tshβ were incubated with 30 µg/ml trypsin for 1 h at 37°C. Reactions were terminated by boiling for 10 min and addition of 10 mM PMSF. Fractions in lanes 5–7 were acetone precipitated overnight. All fractions were mixed with loading buffer, boiled for 5 min and resolved on 12% SDS-PAGE. Protein bands were visualized following the silver staining. Lanes: 1, outer membranes of XL1-Blue/pWSK30; 2, outer membranes of XL1-Blue/pTsh-ΔN2; 3, denatured outer membranes of XL1-Blue/pTsh-ΔN2 with 30 µg/ml trypsin; 4, outer membranes of XL1-Blue/pTsh-ΔN2 with 30 µg/ml trypsin; 5, Tshβ, the band at 25 kDa is unrelated to Tshβ or trypsin; 6, denatured Tshβ with 30 µg/ml trypsin; 7, folded Tshβ with 30 µg/ml trypsin. Arrow indicates position of Tshβ.
Quaternary structure of the purified Tshβ
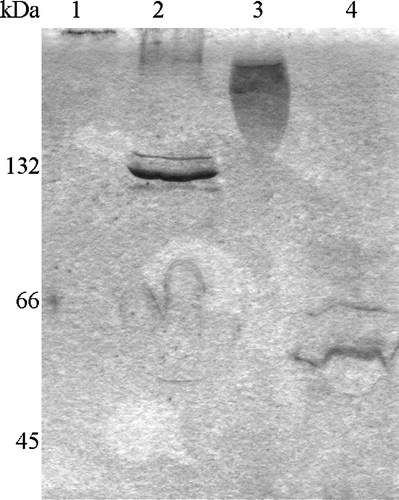
To assess the quaternary structure of Tshβ, we performed Blue-Native PAGE (BN-PAGE) Citation[22]. This is a procedure that allows the resolution of the native membrane protein complexes in the absence of any cross-linkers. The Coomassie-blue G dye coats the protein complexes without disrupting them or causing aggregation. We used purified OmpC, a well-studied outer membrane protein, as a control Citation[26]. OmpC monomer is 38.3 kDa. In the outer membrane it exists as a 115-kDa-trimer. OmpC associates with LPS and detergent molecules, even after the purification process, similarly to Tshβ.
We found that when OmpC and Tshβ were dialysed against a buffer containing no detergent, they formed high-molecular-weight aggregates (, lanes 1 and 3). The OmpC aggregate barely entered the resolving gel (, lane 1) and the Tshβ aggregate ran as a smear (, lane 3) well above 132 kDa marker. When the proteins were in the buffer containing 10 mM detergent, OmpC ran as 130-kDa complex (, lane 2) and Tshβ as 60-kDa complex (, lane 4). A minor fraction of Tshβ gets unfolded as seen on , lane 3. In that lane, one can see a faint band above the band corresponding to the folded Tshβ and matches the band of the unfolded Tshβ in lane 6. The actual molecular mass of OmpC is approximately 115 kDa and that of Tshβ is about 33 kDa. The proteins extracted from the membranes are still associated with LPS molecules, as could be detected on silver stained gels (data not shown). The presence of LPS in OmpC and Tshβ is one of possible explanations why these proteins run slightly slower than their predicted sizes. The proteins resolved on the BN-PAGE were a part of 18.5 kDa SB3-12 detergent micelle which contributes to a higher apparent molecular weight of protein. As a result the 115-kDa OmpC trimer with detergent is about 124 kDa and 33-kDa Tshβ is 52 kDa. An alternative explanation is that Tshβ runs as a dimer on the BN-PAGE. Since the molecular weight of the folded monomer Tshβ is 26 kDa (, lane 3), the folded Tshβ dimer would have the molecular weight of 52 kDa. LPS, Coomassie-blue G, and detergent account for the remaining 8 kDa (13% of apparent molecular weight of Tshβ). This further agrees with the observation that the apparent molecular weight of the OmpC trimer (130 kDa) is 12% greater than its predicted molecular weight (115 kDa). The molecular weight of Tshβ is too low to be resolved accurately on the BN-PAGE, due to high interference from detergent, LPS, and Coomassie, to state unambiguously whether Tshβ is a monomer or a dimer. However, our results do exclude the formation of hexamers by the Tsh autotransporter.
Figure 4. Profile of Tshβ and OmpC on BN-PAGE. Protein samples were incubated with loading buffer for 5 min on ice and resolved on 15–25% gradient gel. Lanes: 1, OmpC without detergent; 2, OmpC with 10 mM SB3-12, 3, Tshβ without detergent; 4, Tshβ with 10 mM SB3-12.
Results of cross-linking of Tshβ with DSS support the monomeric state of this protein in vitro (A). There was no protein band corresponding to a hexameric complex (A, Lane 2). The only observed band corresponded to the 33-kDa Tshβ monomer. OmpC under the same conditions had three protein bands. The upper one corresponded to a trimer, the next one to a dimer, and the lowest one to a monomer (C). Three bands for OmpC were observed previously after a cross-linker treatment Citation[27]. The results of our experiments with the membrane domain of Tsh support the monomeric nature of this domain.
Figure 5. Cross-linking of Tshβ, Tshs, and OmpC. After purified proteins were cross-linked with DSS, reactions were terminated with 20 mM Tris-Cl (pH 7.5). Tshβ and OmpC were precipitated overnight with acetone. Protein samples were mixed with loading buffer, and after the boiling for 5 min, were resolved on SDS-PAGE (6% for Tshs and 10% for Tshβ and OmpC). Panel A: 1, Tshβ; 2, cross-linked Tshβ; Panel B: 1, cross-linked Tshs; 2, Tshs; Panel C: 1, OmpC; 2, cross-linked OmpC.
Formation of monomers by the purified Tsh passenger domain
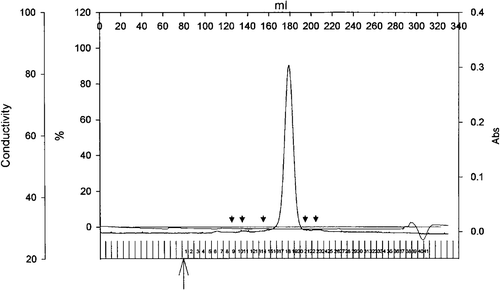
The quaternary structure of Tshs was assessed by cross-linking experiments (B) and size exclusion chromatography (). DSS-mediated cross-linking of Tshs indicated that the secreted domain of Tsh exists only as a 106-kDa monomer. No bands corresponding to higher molecular weight complexes were observed after separation on an SDS-PAGE. To further confirm these results we performed size exclusion chromatography using a gel filtration column with a fractionation range of 10–600 kDa. The obtained elution profile is shown in . Proteins of known molecular weight were separated on the column immediately prior to the purified Tshs-containing sample for generation of a standard curve. Tshs eluted in a single peak at 180 ml of buffer, and had an estimated mass of ∼110 kDa, which corresponds to the size of a monomer. Presence of Tshs in that fraction was confirmed by SDS-PAGE analysis (data not shown). There was no detectable peak corresponding to an oligomeric form of the secreted domain of Tsh. The results were confirmed with three independent preparations of Tshs separated on the column under the same conditions. The native state of Tshs throughout the experimental procedure was established by testing the protein's adhesive activities both before and after size exclusion chromatography as described before Citation[12]. These findings demonstrate that Tsh remains in a monomeric state even after secretion.
Figure 6. Elution profile of Tshs by size exclusion chromatography. Purified Tshs (6.4 mg) was loaded on a Superdex 200 26/60 column. The flow rate was 1 ml/min and protein presence was monitored at 280 nm. The arrowheads indicate elution volumes of standards. From left to right, standards included thyroglubulin (MW 669 kDa), ferritin (MW 440 kDa), catalase (MW 232 kDa), albumin (MW 67 kDa), ovalbumin (MW 43 kDa). Tshs eluted in a single peak that corresponds to an estimated molecular weight of 110 kDa.
Discussion
Autotransporters represent a growing family of secreted proteins with diverse virulence functions. They contribute to the pathogenicity of several Gram-negative bacteria such as N. meningitidis, N. gonorrhoeae, Haemophilus influenzae, Shigella flexneri, Bordetella pertussis, Chlamydia pneumoniae, and Salmonella spp. Citation[4], Citation[28], Citation[29]. For this reason, the investigation of the mechanism of autotransporter secretion and activation can provide new opportunities for vaccine and drug development against various human and animal infections.
Autotransporters share a common architecture. Namely, they consist of an N-terminal cleavable signal sequence, a secreted passenger domain, and a transmembrane C-terminal domain. They are divided into two main structural classes based on the length of their C-terminal domain: trimeric and conventional autotransporters. Trimeric autotransporters are characterized by a very short C-terminal domain, less than 90 amino acids, insufficient to form a complete β-barrel Citation[5], Citation[30]. Hia of H. influenzae, NhhA of N. meningitidis, YadA of Yersinia sp. are a few examples of trimeric autotransporters Citation[5]. In contrast, conventional autotransporters possess a C-terminal transmembrane domain, ranging from 250–300 amino acids, sufficient to form a complete transmembrane β-barrel. NalP of N. meningitidis is an example of this class of autotransporters. Based on the length of C-terminal domain the SPATEs are considered to be conventional autotransporters. They exhibit a conserved serine protease motif, GDSGS, in their secreted domains and share 35–55% amino acid identity Citation[8].
The precise quaternary structure of conventional autotransporters is ambiguous. Veiga and colleagues performed experiments with the transmembrane domain of IgA1 protease Citation[16], Citation[17], Citation[31]. Size-exclusion chromatography indicated that IgA1 transmembrane domains come together as a 500-kDa complex Citation[16]. Their multimeric model for secretion of the conventional autotransporters argues that at least six C-terminal domains together to form the transmembrane pore (2 nm in diameter) which is large enough for transport of folded proteins Citation[16], Citation[17], Citation[31]. On the other hand, the solved crystal structure of the NalP transmembrane domain discloses a single 12-stranded β-barrel that forms a 10 by 12.5 Å pore partially blocked with α-helix. The size of resulting pore is enough to transport only the unfolded passenger domain Citation[15]. The small size of the transmembrane pore is consistent with the monomeric model for conventional autotransporters Citation[15].
Our current study focuses on the quaternary structure of Tsh, a SPATE autotransporter. We purified the natively folded C-terminal membrane domain () and the secreted domain of Tsh Citation[12]. The obtained Tshβ was of high purity and was folded into a heat and protease resistant β-barrel ( and ). This findings are consistent with the view that outer membrane proteins form stable transmembrane β-barrels in the outer membrane Citation[25]. Size-exclusion chromatography of the membrane proteins is often unreliable because these proteins associate with lipids and detergents. This leads to an overestimation of the actual protein size. To avoid this problem, we used several techniques to determine separately the quaternary structures of the secreted and membrane domains of Tsh autotransporter. For the membrane domain of Tsh we used the cross-linking experiment and BN-PAGE analysis, a technique specifically developed to resolve membrane proteins in their native state Citation[22]. To determine the quaternary structure of the secreted domain of Tsh, we used the cross-linking analysis and the gel filtration. Furthermore, we used OmpC, a known outer membrane trimeric protein as a control (, lanes 1, 2). Our results from BN-PAGE analysis (, lanes 2 and 4) are inconclusive and indicated that Tshβ could exist as either a monomer or a dimer. We performed cross-linking experiments with non cleavable DSS. OmpC was used as a control (C). We detected a single band at 33 kDa and there were no additional protein bands of higher molecular weight in the lane containing cross-linked Tshβ (A, lane 2) to indicate multimer formation. The results of our cross-linking experiment show that Tshβ exists as a monomer (A). Our studies were not limited to only the transmembrane autotransporter domain. We used cross-linking treatment (B), and size-exclusion chromatography () to explore the quaternary structure of Tshs. After cross-linking with DSS all of Tshs was in the band below 116 kDa band of molecular marker, as would be expected of 106-kDa monomer. We further confirmed cross-linking of Tshs results, with size-exclusion chromatography, a highly accurate technique for water-soluble proteins, such as secreted domains of autotransporters. Our data revealed that all Tshs eluted in a single peak at about 110 kDa, corresponding to a monomer (). Our data agree well with the work of Otto and colleagues showing that Hbp, a very close Tsh homologue, is also a monomer as determined by its crystal structure Citation[32]. Thus, the majority of our experiments indicate that Tsh is a monomeric protein. We found no evidence of multimer formation in any of our experiments.
Recent report on the AIDA-I translocator domain presents evidence that this autotransporter forms monomers and sometimes dimers, but not multimers Citation[27]. Despite the dimerization, AIDA-I autotransporter forms monomeric pores to transport its passenger domain across the outer membrane, as shown by co-expression experiments Citation[27]. Our studies of the quaternary structure of the Tsh autotransporter are in agreement with these findings.
At present, there are four models to explain autotransporter secretion across the outer membrane: the hairpin, the threading, the multimeric, and the Omp85 models. The classical hairpin model states that a linker region in the interface of passenger/transmembrane domains temporary folds into a hairpin structure that passes through the pore and initiates transport and progressive extracellular folding of the passenger domain Citation[33–35]. The threading model states that the passenger domain translocation starts from the N-terminus Citation[15]. Only one peptide strand passes at a time through the β-barrel and the folding is thought to occur once the translocation is completed. The multimeric model argues that at least six transmembrane domains interact and form a 2-nm central pore, large enough to transport folded domains Citation[16], Citation[17], Citation[31]. The fourth proposed model involves the Omp85 complex Citation[15]. It argues that an autotransporter β-barrel serves as a recognition signal. Omp85 helps to transport the large hydrophilic loops of the transmembrane domain and inserts this domain into the outer membrane. It might be the pore formed by Omp85 rather than the transmembrane domain that transports the passenger domain to the extracellular space. Our results are consistent with all models discussed above, except the multimeric model.
In summary, our work provides evidence that Tsh, a conventional autotransporter of the SPATE group, is monomeric. Other autotransporters of the SPATE group are likely to exhibit a similar quaternary architecture. Future studies will reveal the further details of the secretion of the passenger domain through the outer membrane.
This research was supported by a research grant from the US Department of Agriculture and two research grants from the Robert Welch Foundation. We would like to thank Dr Anne Delcour for kindly donating AW741 E. coli strain, Dr Arnaud Basle for his assistance with gel filtration experiments, and Dr William Widger for valuable suggestions on BN-PAGE. We also thank Dr Ian Henderson and Dr Rachel Fernandez for critical reviews of the manuscript and M. Bhattacharya, A. Karkal, Y. Yen, Y. Yatsenko, S. Ranjan for their help with preparations of the manuscript.
Also, we would like to thank the reviewers for useful comments, which improved the quality of the manuscript.
References
- Kostakioti M, Newman CL, Thanassi DG, Stathopoulos C. Mechanisms of protein export across the bacterial outer membrane. J Bacteriol 2005; 187: 4306–4314
- Jacob-Dubuisson F, Fernandez R, Coutte L. Protein secretion through autotransporter and two-partner pathways. Biochim Biophys Acta 2004; 1694: 235–257
- Thanassi DG, Stathopoulos C, Karkal A, Li H. Protein secretion in the absence of ATP: the autotransporter, two-partner secretion and chaperone/usher pathways of Gram-negative bacteria. Molec Memb Biol 2005; 22: 63–72
- Newman CL, Stathopoulos C. Autotransporter and two-partner secretion: delivery of large-size virulence factors by gram-negative bacterial pathogens. Crit Rev Microbiol 2004; 30: 275–286
- Surana NK, Cutter D, Barenkamp SJ, St Geme JW, 3rd. The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J Biol Chem 2004; 279: 14679–14685
- Cotter SE, Surana NK, St Geme JW, 3rd. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol 2005; 13: 199–205
- Yen MR, Peabody CR, Partovi SM, Zhai Y, Tseng YH, Saier MH. Protein-translocating outer membrane porins of Gram-negative bacteria. Biochim Biophys Acta 2002; 1562: 6–31
- Dutta PR, Cappello R, Navarro-Garcia F, Nataro JP. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect Immun 2002; 70: 7105–7113
- Otto BR, van Dooren SJ, Nuijens JH, Luirink J, Oudega B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J Exp Med 1998; 188: 1091–1103
- Eslava C, Navarro-Garcia F, Czeczulin JR, Henderson IR, Cravioto A, Nataro JP. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun 1998; 66: 3155–3163
- Villaseca JM, Navarro-Garcia F, Mendoza-Hernandez G, Nataro JP, Cravioto A, Eslava C. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect Immun 2000; 68: 5920–5927
- Kostakioti M, Stathopoulos C. Functional analysis of the Tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect Immun 2004; 72: 5548–5554
- Stathopoulos C, Provence DL, Curtiss R, 3rd. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect Immun 1999; 67: 772–781
- Provence DL, Curtiss R, 3rd. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun 1994; 62: 1369–1380
- Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P. Structure of the translocator domain of a bacterial autotransporter. Embo J 2004; 23: 1257–1266
- Veiga E, Sugawara E, Nikaido H, de Lorenzo V, Fernandez LA. Export of autotransported proteins proceeds through an oligomeric ring shaped by C-terminal domains. Embo J 2002; 21: 2122–2131
- Veiga E, de Lorenzo V, Fernandez LA. Structural tolerance of bacterial autotransporters or folded passenger protein domains. Mol Microbiol 2004; 52: 1069–1080
- Bullock WO, Fernandex JM, Short JM. Xl1-Blue: A high efficiency plasmid transforming recA Escherichia coli strain with Beta-Galactosidase selection. biotechniques 1987; 5: 376–378
- Ingham C, Buechner M, Adler J. Effect of outer membrane permeability on chemotaxis in Escherichia coli. J Bacteriol 1990; 172: 3577–3583
- Kramer RA, Zandwijken D, Egmond MR, Dekker N. In vitro folding, purification and characterization of Escherichia coli outer membrane protease ompT. Eur J Biochem 2000; 267: 885–893
- Thanassi DG, Saulino ET, Lombardo MJ, Roth R, Heuser J, Hultgren SJ. The PapC usher forms an oligomeric channel: implications for pilus biogenesis across the outer membrane. Proc Natl Acad Sci USA 1998; 95: 3146–3151
- Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 1991; 199: 223–231
- Thanassi DG, Hultgren SJ. Assembly of complex organelles: pilus biogenesis in gram-negative bacteria as a model system. Methods 2000; 20: 111–126
- Touze T, Hayward RD, Eswaran J, Leong JM, Koronakis V. Self-association of EPEC intimin mediated by the beta-barrel-containing anchor domain: a role in clustering of the Tir receptor. Mol Microbiol 2004; 51: 73–87
- Mogensen JE, Tapadar D, Schmidt MA, Otzen DE. Barriers to folding of the transmembrane domain of the Escherichia coli autotransporter adhesin involved in diffuse adherence. Biochemistry 2005; 44: 4533–4545
- Nikaido H. Porins and specific diffusion channels in bacterial outer membranes. J Biol Chem 1994; 269: 3905–3908
- Muller D, Benz I, Tapadar D, Buddenborg C, Greune L, Schmidt MA. Arrangement of the translocator of the autotransporter adhesin involved in diffuse adherence on the bacterial surface. Infect Immun 2005; 73: 3851–3859
- Vandahl BB, Stensballe A, Roepstorff P, Christiansen G, Birkelund S. Secretion of Cpn0796 from Chlamydia pneumoniae into the host cell cytoplasm by an autotransporter mechanism. Cell Microbiol 2005; 7: 825–836
- Wing HJ, Yan AW, Goldman SR, Goldberg MB. Regulation of IcsP, the outer membrane protease of the Shigella actin tail assembly protein IcsA, by virulence plasmid regulators VirF and VirB. J Bacteriol 2004; 186: 699–705
- Roggenkamp A, Ackermann N, Jacobi CA, Truelzsch K, Hoffmann H, Heesemann J. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J Bacteriol 2003; 185: 3735–3744
- Veiga E, de Lorenzo V, Fernandez LA. Probing secretion and translocation of a beta-autotransporter using a reporter single-chain Fv as a cognate passenger domain. Mol Microbiol 1999; 33: 1232–1243
- Otto BR, Sijbrandi R, Luirink J, Oudega B, Heddle JG, Mizutani K, Park SY, Tame JR. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J Biol Chem 2005; 280: 17339–17345
- Pohlner J, Halter R, Beyreuther K, Meyer TF. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 1987; 325: 458–462
- Jose J, Jahnig F, Meyer TF. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol Microbiol 1995; 18: 378–380
- Henderson IR, Navarro-Garcia F, Nataro JP. The great escape: structure and function of the autotransporter proteins. Trends Microbiol 1998; 6: 370–378