Abstract
Mitochondria isolated from engineered mice lacking Cyclophilin D (CypD), a component of the Permeability Transition Pore (PTP) complex, can still undergo a Ca2 + -dependent but Cyclosporin A-insensitive permeabilization of the inner membrane. Higher Ca2 + concentrations are required than for wild-type controls. The characteristics of the pore formed in this system were not known, and it has been proposed that they might differ substantially from those of the normal PTP. To test this hypothesis, we have characterized the PTP of isogenic wild-type and CypD− mouse liver mitochondria in patch clamp experiments, which allow biophysical characterization. The pores observed in the two cases, very similar to those of rat liver mitochondria, are indistinguishable according to a number of criteria. The only clear difference is in their sensitivity to Cyclosporin A. CypD is thus shown to be an auxiliary, modulatory component of the “standard” PTP, which forms and has essentially the same properties even in its absence. The observations suggest that Ca2 + , CypD, and presumably other inducers and inhibitors act at the level of an activation or assembly process. Activation is separate and upstream of the gating observable on a short or medium-term time scale. Once the pore is activated, its molecular dynamics and biophysical properties may thus be predicted not to depend on the details of the induction process.
| Acronyms | ||
| ANT | = | Adenine Nucleotide Translocator |
| CL | = | Cardiolipin |
| CSA | = | Cyclosporin A |
| CypD | = | Cyclophilin D (mitochondrial cis-trans peptidyl-prolyl isomerase) |
| Erev | = | current reversal potential |
| MLM | = | Mouse Liver Mitochondria |
| MMC | = | Mitochondrial MegaChannel |
| PT | = | Permeability Transition |
| PTP | = | Permeability Transition Pore |
| RLM | = | Rat Liver Mitochondria |
| Ub0 | = | Ubiquinone 0 |
| VDAC | = | Voltage-Dependent Anion Channel (mitochondrial porin) |
Introduction
The discovery Citation[1–4] that Cyclosporin A (CSA) inhibited the mitochondrial permeability transition (PT) with high affinity led to the conclusion that the permeability transition pore was formed by proteins. Electrophysiological experiments Citation[5], Citation[6] soon thereafter confirmed this deduction. A well-supported current biochemical model of the PTP envisions its formation within a complex comprising the adenine nucleotide translocator (ANT), mitochondrial porin (VDAC), and other proteins of the mitochondrial envelope Citation[7], Citation[8]. Convincing evidence has been provided that the effect of CSA is mediated by CypD, the mitochondrial member of the family of cis-trans peptidyl-prolyl isomerases (PPIases) known as cyclophilins Citation[8–12]. CSA-unrelated Sanglifehrin, which also binds and inhibits CypD, also inhibits the PT with high affinity Citation[13].
CypD has been shown to reversibly bind, in a CSA-inhibitable fashion, to the inner mitochondrial membrane Citation[14–19], and in particular to the adenine nucleotide translocator Citation[20], Citation[21]. Whether PT prevention by CSA involves separation of CypD from the PTP complex, or blockage of the enzymatic activity of bound CypD, is uncertain [e.g., 8,12–14,19,22]. Nonetheless, there is little doubt that CypD is a component of the (open-pore) PTP complex. But how important a component? The PT can be CSA-insensitive [e.g., 7,Citation[23–26], perhaps implying that PTP variants lacking a functioning CypD can form. Klingenberg's group has shown that purified ANT does not need CypD to form Ca2 + -induced channels Citation[27], Citation[28]. On the other hand, CSA can inhibit Ca2 + -induced mitochondrial swelling in ANT-less mitochondria Citation[29]. CypD binding therefore must not be very specific, and its action may be a “generic” catalysis of conformational change, as appropriate for a chaperonin. Recent studies have addressed the question of CypD function via generation of CypD− mice Citation[22], Citation[30–32]. The data show that CypD− mitochondria can still undergo a permeability transition, although with a decreased sensitivity to Ca2 + . Basso et al. Citation[30] found that swelling (in the presence of high [Ca2 + ]) could still be elicited by depolarization or some SH reagents, and inhibited by Ub0 and low pH, as it happens with wild-type mitochondria. On the other hand, atractyloside and H2O2, two other well-characterized PT inducers, reportedly did not trigger the PT in CypD-deficient mitochondria (but, importantly, no Ca2 + had been added) Citation[31]. H2O2-induced PT in primary cell cultures was suppressed by lack of CypD, and reconstituted by reintroduction of the gene Citation[22]. CSA-insensitive (“unregulated”) types of PTP, perhaps consisting of aggregates of oxidized proteins, have been proposed to form under certain conditions, including high [Ca2 + ] Citation[25], Citation[33], Citation[34]. The distinct possibility thus remained that such (a) pore(s) may be involved in PT induction in CypD− mitochondria (see supplementary material in Citation[22]).
The studies mentioned have largely relied on classical methods to assess the PT, using swelling and/or collapse of the Ca2 + retention capacity or of the transmembrane potential as indicators of the occurrence of the phenomenon. These techniques follow the behaviour of a population of organelles, and monitor the consequences of the initial opening of the PTP, providing little information on the finer properties and behaviour of the pore once the PT has been induced. They cannot therefore establish whether the PTP formed in the absence of CypD is essentially the same pore that forms in its presence, or a possibly quite different one. The purpose of the present study was to collect information on this aspect, using patch-clamp to study the gating and the biophysical characteristics of the channel corresponding to the PTP, i.e., the Mitochondrial MegaChannel (MMC) Citation[7], Citation[35], Citation[36]. We have thus recorded and compared the activity of CypD+ and CypD- mouse MMC channels to assess differences, or the lack thereof. In doing this, we have obtained a fuller biophysical characterization of the MMC/PTP than was previously available, and we report these data.
Materials and methods
Liver mitochondria were prepared from wild-type and CypD- mice as previously reported Citation[30]. Patch-clamp experiments on mitoplasts were carried out essentially as described Citation[37]. To mimic as much as possible the conditions of swelling experiments, the mitochondria were not exposed to hypotonic shock, but allowed instead to swell for 10–20 min in the Ca2 + -containing experimental medium (150 or 125 mM KCl, 0.5 mM CaCl2, 1 mM Pi, 20 mM Hepes, pH 7.35, unless otherwise specified) supplemented with 5 mM succinate. The mitoplasts attached to the chamber bottom were washed with the experimental medium and seals were established under symmetrical ionic conditions. For selectivity determinations, after the presence of channel activity was ascertained, bath [KCl] was increased, other components remaining constant. In all cases connection to the Ag/AgCl ground electrode was via a 1 M KCl agar bridge. In inhibition experiments the desired volume (µl range) of stock solution (ethanol or DMSO) was added, and the bath contents (1 ml) were thoroughly mixed. Voltage was controlled by an Axopatch 200 unit, and Axon pClamp software was used for voltage control and data analysis. The voltages reported are those applied to the patch-clamp pipette interior. Current (cations) flowing from the pipette to the ground electrode is considered as positive and plotted upwards. The experiments reported in this paper were conducted in the mitochondrion-attached configuration, as confirmed by the voltage-dependence of the “107 pS” anion-selective channels Citation[38], Citation[39] which were observed in approximately 1/3 of all patches and invariably activated at pipette-negative potentials (not shown). The presence of these channels furthermore confirms that the recordings were obtained from inner mitochondrial membrane patches.
Results
Comparison of the properties of the MMC in different systems is not straightforward, because channel characteristics can vary from experiment to experiment as well as within the same recording. This might hypothetically reflect differences in the make-up of the supramolecular complex (e.g., with different mitochondrial carriers; see Citation[29]), and in post-translational modifications (e.g., phosphorylation; phosphorylation of inner mitochondrial membrane proteins has been observed Citation[40]) and/or the adoption of different conformations by the current-conducting unit. The extent of this variability is illustrated below. The pore is nonetheless easily recognizable because of its Ca2 + -dependence, size, and characteristic substates and pharmacology.
Size and kinetic behaviour (gating modes)
Both wild-type and CypD− mitoplasts exhibit activity by channels strongly resembling the MMC previously studied in rat liver mitoplasts (). The frequency of observation of the MMC depended on [Ca2 + ]. With wild type MLM, for example, only one out of 58 patches exhibited this activity when [Ca2 + ] in the bath and pipette was 20 µM, increasing to 57 out of 133 (43%) with [Ca2 + ] = 500 µM. With CypD− MLM, at the same [Ca2 + ] (500 µM), MMC activity was observed in 44 out of 109 (40%) patches. It should be mentioned that these statistics do not necessarily quantitatively reflect the Ca2 + -dependence of PT onset in the two types of mitochondria, since seals were established on mitochondria (mitoplasts) which had already undergone the transition (see materials and methods).
Figure 1. Cyclophilin D-less mitoplasts exhibit mitochondrial megachannels indistinguishable from those of wild-type mouse and rat liver mitochondria. Representative current records (sampling: 5 KHz, filter: 1 KHz). In the examples, the application of voltage (30 mV, pipette-positive) induced channel closure via the characteristic “half-conductance” fast-gating substate. [KCl] was 150 mM for panels A and C, 125 mM for panel B.
![Figure 1. Cyclophilin D-less mitoplasts exhibit mitochondrial megachannels indistinguishable from those of wild-type mouse and rat liver mitochondria. Representative current records (sampling: 5 KHz, filter: 1 KHz). In the examples, the application of voltage (30 mV, pipette-positive) induced channel closure via the characteristic “half-conductance” fast-gating substate. [KCl] was 150 mM for panels A and C, 125 mM for panel B.](/cms/asset/12c18e24-7877-4e3b-a7f3-61b491d0b690/imbc_a_190683_f0001_b.gif)
As already observed in experiments on other membranes, the mouse MMC displayed substates and a variability of the maximal conductance in the 0.9–1.3 nS range, with most values falling close to 1 nS (in 150 mM KCl; approx. 0.85 nS in 125 mM KCl). Again as observed in other systems, the kinetic behaviour was also variable, with switches between well-behaved modes and irregular, noise-like flickering activity. The co-existence of these different modes is illustrated in . Fast, irregular gating is not simply due to “run-down”, since it can reversibly convert into more regular activity, and often is the mode observed at the beginning of a recording, soon after establishment of the tight seal. This type of activity is displayed in both wild-type and CypD− patches (as well as by RLM MMCs), and it may involve transitions between anion-selective and cation-selective states (see below). We could not identify a significant influence of CypD on this behaviour, on the probability of adopting (a) substate(s), or on the frequency of open/closed transitions. The concentration of Ca2 + also had little influence on the behaviour of the active channel. At low (<100 µM) [Ca2 + ] the probability of observing the MMC was lower, and the channels generally inactivated after a short time. While they were functioning, however, they could not be reliably distinguished from MMCs observed at higher [Ca2 + ].
Figure 2. The mitochondrial megachannel can alternate between well-behaved and unruly activity, regardless of the presence of Cyclophilin D. Representative current traces (sampling: 5 KHz; filter: 1 KHz). Segments indicated by lower case letters are shown at expanded time scale. (A) recording from a wild-type mitoplast. (B) from a CypD- mitoplast. [KCl]: 125 mM. Note in panel B the temporary appearance of the “half-conductance state” in the midst of noisy activity (arrow).
![Figure 2. The mitochondrial megachannel can alternate between well-behaved and unruly activity, regardless of the presence of Cyclophilin D. Representative current traces (sampling: 5 KHz; filter: 1 KHz). Segments indicated by lower case letters are shown at expanded time scale. (A) recording from a wild-type mitoplast. (B) from a CypD- mitoplast. [KCl]: 125 mM. Note in panel B the temporary appearance of the “half-conductance state” in the midst of noisy activity (arrow).](/cms/asset/fb685bf0-b00d-43ad-ab8a-372e571ff659/imbc_a_190683_f0002_b.gif)
CSA inhibits wild type, but not CypD− MMC activity
On the basis of the results with suspensions of mitochondria Citation[22], Citation[30–32] we expected the wild-type, but not the CypD− MLM MMC to be inhibited by CSA. These predictions were borne out: Cyclosporin inhibited MMC activity in wild-type mitoplasts in 5 out of 6 experiments (and time before loss of seal might not have been sufficient for diffusion of CSA to the site of action in the one negative case), while it had no effect in 4 experiments with CypD− MLM. shows representative experiments. Note, in panel 3A, that an increase of [Ca2 + ] can, in mouse as well as in rat liver Citation[41], resuscitate a CSA-inhibited MMC, counteracting the effect of CypD binding. This is coherent with the feasibility of PT induction in CypD− mitochondria. As shown by the current records, inhibition and reactivation in this case were rapid, as a first approximation “all-or-nothing” event. B shows an apparently somewhat different type of effect by CSA, which can induce a sort of incomplete inhibition with residual channel flickering which may perdure or give way to a complete silencing of the current. This latter behaviour was observed in 4 out of 5 inhibitions of wild type MLM MMC, and it has been commonly observed in experiments on RLM as well (not shown). The ability to cause development of a noisy, partially inhibited activity appears to be a peculiarity of CSA and, to a lesser extent, of Ub0 (see below). Other MMC inhibitors (used with RLM Citation[6], Citation[41]) typically induce a relatively abrupt closure, often involving passage through the “half-conductance state”, but no prolonged spiky activity. The CSA-induced mode is reminiscent of the unruly gating often exhibited by the untreated channel (). It must be emphasized, however, that the CypD− MMC did not show a noticeably greater tendency to behave in this manner.
Figure 3. Cyclosporin A inhibits the wild-type, but not the Cyclophilin D-less mitochondrial megachannel. Representative experiments. Trains of 1 sec/20 mV voltage pulses of alternating polarity, with a short interval at 0 mV between each pulse, were applied. Plots of the current amplitude, averaged over 1-sec periods, vs. time are shown. Some segments of the current record are also shown, and their position in the current amplitude vs. time plot above them is indicated (sampling rate: 5 KHz; filter: 1 KHz). Additions as indicated. [KCl]: 125 mM. (A),(B): liver mitoplasts from wild-type mice. (C): from a CypD− mouse.
![Figure 3. Cyclosporin A inhibits the wild-type, but not the Cyclophilin D-less mitochondrial megachannel. Representative experiments. Trains of 1 sec/20 mV voltage pulses of alternating polarity, with a short interval at 0 mV between each pulse, were applied. Plots of the current amplitude, averaged over 1-sec periods, vs. time are shown. Some segments of the current record are also shown, and their position in the current amplitude vs. time plot above them is indicated (sampling rate: 5 KHz; filter: 1 KHz). Additions as indicated. [KCl]: 125 mM. (A),(B): liver mitoplasts from wild-type mice. (C): from a CypD− mouse.](/cms/asset/81ab80b7-3efb-4522-b83c-6babe8a780d3/imbc_a_190683_f0003_b.gif)
Inhibition by Ub0
To confirm that the channels observed were a manifestation of the PTP, and that both types were sensitive to other PT antagonists, we tested the effect of Ub0, a specific inhibitor [e.g., Citation[35],Citation[42], Citation[43]. In both cases inhibition was observed (; n=4 and 5 for wild-type and CypD− MLM, respectively) at concentrations of Ub0 similar to those affecting the MMC of RLM Citation[35]. Ub0 sometimes also caused the partially inhibited, “flickering” behaviour often associated with CSA inhibition. This can be observed in the right-side panel of (CypD− MLM): addition of a high dose of Ub0 drives the channel into a fast, irregular gating mode (trace d), from which it recovers briefly only to close completely after a few seconds (trace e; residual current conduction is due to the presence of 107 pS channels, as shown with an expanded current amplitude scale in trace f). This behaviour might be linked to a gradual diffusion of CSA or Ub0 to their site of action, with irregular gating occurring at low local concentrations of the compounds.
Figure 4. Both the wild-type and Cyclophilin D-less mitochondrial megachannel are inhibited by Ubiquinone 0. Representative experiments performed and illustrated as in . [KCl]: 150 mM. (A) liver mitoplast from wild-type mice. The patch initially contained two active megachannels, one of which was inhibited during the addition and mixing period. (B) liver mitoplast from a CypD- mouse. See text for details.
![Figure 4. Both the wild-type and Cyclophilin D-less mitochondrial megachannel are inhibited by Ubiquinone 0. Representative experiments performed and illustrated as in Figure 3. [KCl]: 150 mM. (A) liver mitoplast from wild-type mice. The patch initially contained two active megachannels, one of which was inhibited during the addition and mixing period. (B) liver mitoplast from a CypD- mouse. See text for details.](/cms/asset/365976cd-51b5-4402-8451-bf1b890c191a/imbc_a_190683_f0004_b.gif)
Voltage-dependence
Megachannels in wild-type and CypD-less mitoplasts were also similarly modulated by voltage, as illustrated in . The pattern was analogous to that displayed by the RLM MMC: Application of transmembrane potentials above 10–20 mV often induced closures, with a dependence on the sign of the potential difference. Voltages of physiological polarity (pipette-positive) induced discrete, well-resolved closures. The sensitivity of the individual pores varied: Upon application of voltage “ramps” up to 30 mV, as in , some gated reversibly between fully open and partially or completely closed states (A, 5B); others were driven into a long-lasting closed or inactivated state (C, 5D). Complete and long-lasting closure could often be imposed on reluctant pores by increasing the voltage (not shown). Channel activity was usually regenerated by a short period at zero voltage (hence the use of trains of alternating pulses in inhibition experiments), but in other cases, such as those shown, reactivation was delayed or even blocked, i.e., the channels may exhibit hysteresis. Voltages of the opposite (unphysiological, pipette-negative) sign caused entry into a fast gating mode, as can be observed in the current traces recorded during trains of voltage pulses of alternating polarity ( and ) as well as in A and 5B.
Figure 5. Wild-type and Cyclophilin D-less mitochondrial megachannels have similar voltage-dependence. Plots of current records and of the applied voltage ramps vs. time are shown. The origin of the mitoplasts is indicated. (A) and (B) The channels close partially and briefly at modest applied transmembrane voltages. Note that the membrane patch of panel B contained two active channels. (C) and (D) The same voltages in other cases can induce long-lasting closures. [KCl]: (A,B): 150 mM; (C,D): 125 mM.
![Figure 5. Wild-type and Cyclophilin D-less mitochondrial megachannels have similar voltage-dependence. Plots of current records and of the applied voltage ramps vs. time are shown. The origin of the mitoplasts is indicated. (A) and (B) The channels close partially and briefly at modest applied transmembrane voltages. Note that the membrane patch of panel B contained two active channels. (C) and (D) The same voltages in other cases can induce long-lasting closures. [KCl]: (A,B): 150 mM; (C,D): 125 mM.](/cms/asset/da1b93f0-2424-43e3-91ca-9ee6bbacb314/imbc_a_190683_f0005_b.gif)
Selectivity
One of the most remarkable features of the MMC is that it can vary its selectivity in both quantitative and qualitative (anionic vs. cationic) terms. Preference for cations is most often associated with “unstable” states, i.e., with fast gating. This is illustrated in A, which shows an exemplificative record from an experiment on a wild-type mouse mitoplast. Salt concentration was higher in the bath (matrix side). While the voltage (lower trace) was held at zero, initially the current was positive, with fast transitions to negative values. Given the conventions and experimental conditions, positive current must have been carried by a net flow of Cl- ions from the matrix side into the pipette. Thus, the channel resided for most of the time in anion-selective state(s). At a certain point (arrow), the behaviour changed to very fast transitions between negative and less negative (or close to zero) current values. Negatives currents at zero applied voltage must correspond to a net flow of K+ ions into the pipette, i.e., to cation-selective state(s) of the channel. While the voltage was increasing, the MMC again changed its properties, adopting a state characterized by a lower gating frequency and a slightly anionic selectivity (an Erev of −5 mV, corresponding to a ratio of the permeability coefficients PCl/PK∼1.35, is extrapolated from the I-V curve in panel B).
Figure 6. The Selectivity characteristics of wild-type and Cyclophilin D-less mitochondrial megachannels are the same. The experiments presented are representative of 10 (WT) and 9 (CypD-) analogous ones. (A) and (C) Plots of the current (above) and voltage (below) records vs. time. Conditions as indicated. Sampling frequency: 1 KHz; filter: 200 Hz. (B) and (D) I-V plots of part of the data in (A) and (C) respectively. The lines in panels (B) and (D) were drawn by eye. See text for details. This Figure is reproduced in colour in Molecular Membrane Biology online.
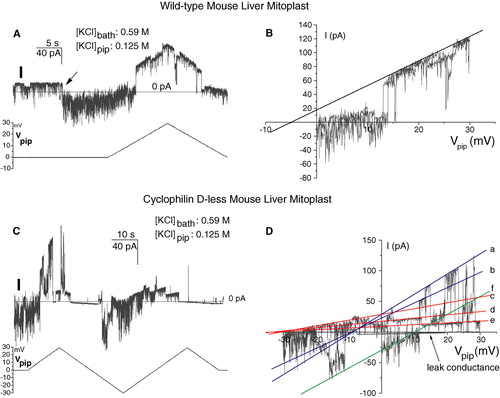
CypD− pores behaved in a completely analogous manner, as illustrated by the example in C–D. Strong gating to negative current values while Vpip was held at 0 (cationic selectivity) is evident at the beginning of the trace in panel C. In the I-V plot of the same data in panel D at least six discrete conductance states of the pore can be identified. Interpolation of the corresponding current trace segments allows the determination of three reversal potential values: lines a and b intercept the abscissa at about −9 mV, corresponding, if the correction for the small leak current is neglected, to PCl/PK∼1.7; lines c-e intercept at Erev∼ − 28.5 mV, corresponding to PCl/PK∼7.9; finally line f crosses the x axis at about +10.3 mV, identifying a cation-selective, rather unstable state with PCl/PK∼0.5. The experiments presented in are representative of 10 (wild-type) and 9 (CypD−) analogous ones.
Discussion
Our observations show that the PTP can form and function in the absence of CypD, a result consistent with the view that CypD acts as a regulator of the PTP, rather than as a obligatory component e.g., Citation[16],Citation[28],Citation[44]. The biophysical properties of the pore, once it is activated, are not noticeably influenced by the presence of the chaperonin. CypD-less MMCs apparently differ from wild-type ones only in that they are insensitive to CSA. Gating, voltage-dependence and selectivity, although variable in each case, are not detectably affected by the cyclophilin. The possibility that the PTP forming in the absence of CypD might be quite different from the CSA-sensitive one is thus negated. As mentioned, [Ca2 + ] also seems irrelevant for the channel's properties. Brustovetsky et al. Citation[28] reported that the properties of channels formed by purified reconstituted ANT also did not depend on [Ca2 + ].
The kinetics of MMC gating may warrant further comment. A priori, the dynamics of the pore, once formed or induced to open, might or might not be affected by CypD. Whether the presence or activity of the chaperonin influences the electrophysiological record will depend on whether it catalyzes the gating being observed, or an upstream, activity-permitting step. The facilitation presumably involves CypD binding (only), and/or a cis-trans isomerization at a proline, perhaps analogously to what appears to happen during ligand-gated channel opening Citation[45]. If this is only a preliminary, activating step, different in molecular terms from the conformational change(s) responsible for subsequent gating, the pores of wild-type and CypD-less mitochondria would be expected to be essentially undistinguishable when observed on a relatively fast time scale. On the other hand, if CypD activity is involved in determining all, or a large fraction of, the transitions between states with different conductance (including zero), CypD+ and CypD− pores would be expected to differ in their kinetics. Crompton's group suggested in one of the earliest papers dealing with the PT and CSA Citation[2] that Cyclosporin might induce a flickering behaviour of the channel, i.e., that binding of CSA might destabilize the fully open state of the pore. This prediction turns out to have been correct at least for part of the events (cf. B), although the effect might actually just reflect a low local concentration of the inhibitor, due to a slow diffusion to the site. In studying the Ca2 + -induced channel activity of reconstituted N. crassa ANT, Brustovetsky et al. Citation[28] conversely found that addition of purified Cyclophilin suppressed the gating observed at high potentials, i.e., stabilized the open state(s).
The available results may thus be rationalized in terms of a two-tiered model. One may distinguish between an activation (or assembly) process – a primary gate – cooperatively induced by Ca2 + and CypD, and the functioning of the channel, essentially insensitive to these agents. That Ca2 + and CypD can cooperate has been clearly shown by the fact that the PT of CypD− (or CSA-treated [e.g., 24,25]) mitochondria can still be elicited by increased [Ca2 + ], and by the observation of apparently competitive effects of Ca2 + and CSA on MMC activity (A; Citation[41]). MMC reactivation by increased [Ca2 + ] after inhibition by divalent cations, H+, ADP and Ub0 is also possible Citation[6], Citation[35], Citation[41], suggesting that these inhibitors also act on the primary gate. Cooperation of Ca2 + and depolarization in PT induction has also been observed Citation[46]. The persistent closures induced in some experiments by low potentials of physiological polarity (C, 5D) may represent an effect at this level (however, CypD− MMCs did not display a noticeably greater propensity to undergo them). Open/closed transitions on a relatively fast time scale, substate occupancy and selectivity seem to depend instead on parts of the protein or protein complex which do not “feel” the presence of the activating or inactivating agents. Fast gating (closures in the millisecond to second range, adoption of substates), apparently unaffected by the agents mentioned, would reflect internal dynamics of the protein (complex). Thus, the gating patterns and other properties of the active channel do not depend on whether the cells of origin expressed CypD or not, or on [Ca2 + ]. Based on this reasoning, we may predict that they should also not reflect the mode of Ca2 + -cooperative activation, i.e., the use of one or the other of the many PT inducers (provided that these agents act only on the “primary gate”).
It is unlikely that a direct competition between Ca2 + and the various disparate inhibitors is taking place at one well-defined site. The process may perhaps best be described in thermodynamic terms: Ca2 + , CypD and other inducers such as thiol oxidizing or modifying agents may all be considered to stabilize, via distinct processes or interactions, the activated state(s), or, equivalently, to cause a relative destabilization of the inactive state(s) relative to the activated one(s). Vice versa, inhibitors would act the opposite way. The adoption of the activated or inactivated state would depend on the difference in the energy levels of the two states (conformations, complexes, or families thereof), which can each be influenced by various effectors. Transitions between activated and inactivated state(s), or vice versa, would correspond to instances of channel disappearance/reappearance for prolonged periods. The probability of such transitions taking place would be predicted to be influenced by activating/deactivating agents. Closures induced by CSA, H+, Mg2 + etc., which can only be reversed by removing the inhibitor or increasing [Ca2 + ], may correspond to deactivations at this level. As already mentioned, spontaneous (but a voltage is generally applied) prolonged (or “irreversible”) closures are observed in our electrophysiological records, but they are relatively rare, suggesting that the two energy levels are normally separated by a high activation barrier. A proper kinetic analysis is prevented by the low rates of these processes, coupled to the limited lifetime of seals. The spiky, fast gating observed sometimes during inhibition by CSA (B) – as well as, perhaps, the fast and erratic activity exhibited at times by the untreated channel () – might also reflect processes at the level of the “primary gate”, associated with a decrease of the interconversion energy barrier(s).
Translating a general description of this type into a biochemical model would obviously require definite knowledge of what proteins are involved, and the discussion is therefore bound to be hypothetical. A well-supported model of the PTP/MMC envisions its formation by the ANT or (in its absence Citation[29]) by another carrier of the inner mitochondrial membrane. The ANT requires 6 tightly bound cardiolipin molecules to operate Citation[47], and at least some of these are thought to be important in determining the interaction between the two monomers forming the dimeric, functionally active form of the carrier Citation[48]. Other carriers (including the phosphate carrier Citation[49]) and membrane proteins share this requirement for CL Citation[50]. CL complexation by Ca2 + has been proposed to represent a key step in the induction of the PT [e.g., Citation[27],Citation[51]]. This is an attractive hypothesis since CL is located in the inner leaflet of the inner mitochondrial membrane Citation[52], PT induction is elicited by accumulation of Ca2 + in the matrix, and CL (and perhaps other negatively charged phospholipids) could constitute an “infinite” reservoir of binding sites for Ca2 + , thus explaining the ability of this ion to act as a PT inducer at various accumulation (concentration) levels, depending on circumstances. Its progressive complexation by increasing levels of Ca2 + might destabilize the dimeric form of the ANT, causing it to form a pore, or facilitate an interaction of the ANT with porin, resulting in the formation of a permeation pathway by the latter. CypD might a priori act as a catalyst for this conversion, or stabilize the PT-inducing conformation via binding to a proline (Pro61 according to Citation[20]). Because of CL complexation or by another mechanism, increasing [Ca2 + ] might also make the binding site for CypD more accessible, thereby increasing its affinity for the chaperonin, i.e., the stability of the active-pore conformation. Antagonism by increasing [CSA] Citation[41] might be explained simply as a mass-law effect, i.e., a competition between CSA and the membrane protein site for CypD.
In summary, we have shown that very similar Permeability Transition Pores form in wild-type and CypD-less mitochondria. This excludes the possibility that the PT induced in the latter might rely on a molecularly different process, and establishes that CypD is an accessory component of the PTP, playing the role of a facilitator.
We thank Prof. Paolo Bernardi for useful discussions and the use of equipment, and Pranvera Hoxha and Lisa Fante for experimental help. This work was financed in part by grants from the Italian Association for Cancer Research (AIRC) to M.Z.
References
- Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr 1987; 19: 297–303
- Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 1988; 255: 357–360
- Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 1989; 264: 7826–7830
- Broekemeier KM, Pfeiffer DR. Cyclosporin A-sensitive and insensitive mechanisms produce the permeability transition in mitochondria. Biochem Biophys Res Comm 1989; 163: 561–566
- Szabò I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by Cyclosporin A. J Biol Chem 1991; 266: 3376–3379
- Szabò I, Zoratti M. The mitochondrial megachannel is the permeability transition pore. J Bioenerg Biomembr 1992; 24: 111–117
- Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta Rev Biomembranes 1995; 1241: 139–176
- Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem 2003; 10: 1507–1525
- Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 1997; 174: 167–172
- Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta 1998; 1366: 79–94
- Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie 2002; 84: 153–166
- Forte M, Bernardi P. Genetic dissection of the permeability transition pore. J Bioenerg Biomembr 2005; 37: 121–128
- Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 2002; 277: 34793–34799
- McGuinness O, Yafei N, Costi A, Crompton M. The presence of two classes of high-affinity cyclosporin A binding sites in mitochondria. Evidence that the minor component is involved in the opening of an inner-membrane Ca2+-dependent pore. Eur J Biochem 1990; 194: 671–679
- Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J 1994; 302: 321–324
- Connern CP, Halestrap AP. Chaotropic agents and increased matrix volume enhance binding of mitochondrial cyclophilin to the inner mitochondrial membrane and sensitize the mitochondrial permeability transition to [Ca2+]. Biochemistry 1996; 35: 8172–8180
- Andreeva L, Crompton M. An ADP-sensitive cyclosporin-A-binding protein in rat liver mitochondria. Eur J Biochem 1994; 221: 261–268
- Andreeva L, Tanveer A, Crompton M. Evidence for the involvement of a membrane-associated cyclosporin-A-binding protein in the Ca2+-activated inner membrane pore of heart mitochondria. Eur J Biochem 1995; 230: 1125–1132
- Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem 1996; 271: 2185–2192
- Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J 1998; 336: 287–290
- Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem 1998; 258: 729–735
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW II, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005; 434: 658–662
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol 1990; 258: C755–C786
- Brustovetsky N, Dubinsky JM. Limitations of Cyclosporin A inhibition of the permeability transition in CNS mitochondria. J Neurosci 2000; 20: 8229–8237
- He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function?. FEBS Lett 2002; 512: 1–7
- Zoratti M, Szabò I, De Marchi U. Mitochondrial permeability transition: how many doors to the house?. Biochim Biophys Acta Bioenerg 2005; 1706: 40–52
- Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+. Biochemistry 1996; 35: 8483–8488
- Brustovetsky N, Tropschug M, Heimpel S, Heidkämper D, Klingenberg M. A large Ca2+-dependent channel formed by recombinant ADP/ATP carrier from Neurospora crassa resembles the mitochondrial permeability transition pore. Biochemistry 2002; 41: 11804–11811
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004; 427: 461–465
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 2005; 280: 18558–18561
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005; 434: 652–658
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 2005; 102: 12005–12010
- Kowaltowski AJ, Castilho RF, Vercesi AE. Opening of the mitochondrial permeability transition pore by uncoupling or inorganic phosphate in the presence of Ca2+ is dependent on mitochondrial-generated reactive oxygen species. FEBS Lett 1996; 378: 150–152
- Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett 2001; 495: 12–15
- Martinucci S, Szabò I, Tombola F, Zoratti M. Ca2+-reversible inhibition of the mitochondrial megachannel by ubiquinone analogues. FEBS Lett 2000; 480: 89–94
- Loupatatzis C, Seitz G, Schonfeld P, Lang F, Siemen D. Single channel currents of the permeability transition pore from the inner mitochondrial membrane of rat liver and a human hepatoma cell line. Cell Physiol Biochem 2002; 12: 269–278
- Campello S, De Marchi U, Szabò I, Tombola F, Martinou J-C, Zoratti M. The properties of the mitochondrial megachannel in mitoplasts from human colon carcinoma cells are not influenced by Bax. FEBS Lett 2005; 579: 3695–3700
- Sorgato MC, Keller BU, Stühmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature 1987; 330: 498–500
- Schonfeld P, Sayeed I, Bohnensack R, Siemen D. Fatty acids induce chloride permeation in rat liver mitochondria by activation of the inner membrane anion channel (IMAC). J Bioenerg Biomembr 2004; 36: 241–248
- Salvi M, Brunati AM, Toninello A. Tyrosine phosphorylation in mitochondria: a new frontier in mitochondrial signaling. Free Rad Biol Med 2005; 38: 1267–1277
- Szabò I, Bernardi P, Zoratti M. Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem 1992; 267: 2940–2946
- Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. J Biol Chem 1998; 273: 12662–12668
- Walter L, Miyoshi H, Leverve X, Bernardi P, Fontaine E. Regulation of the mitochondrial permeability transition pore by ubiquinone analogs. A progress report. Free Radic Res 2002; 36: 405–412
- Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem Soc Symp 1999; 66: 167–179
- Lummis SC, Beene DL, Lee LW, Lester HA, Broadhurst RW, Dougherty DA. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature 2005; 438: 248–252
- Petronilli V, Cola C, Massari S, Colonna R, Bernardi P. Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J Biol Chem 1993; 268: 21939–21945
- Hoffmann B, Stockl A, Schlame M, Beyer K, Klingenberg M. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J Biol Chem 1994; 269: 1940–1944
- Nury H, Dahout-Gonzalez C, Trezeguet V, Lauquin G, Brandolin G, Pebay-Peyroula E. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett 2005; 579: 6031–6036
- Kadenbach B, Mende O, Kolbe HV, Stipani I, Palmieri F. The mitochondrial phosphate carrier has an essential requirement for cardiolipin. FEBS Lett 1982; 139: 109–112
- Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta 1992; 1113: 71–133
- Grijalba MT, Vercesi AE, Schreier S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry 1999; 38: 13279–13287
- Daum G. Lipids of mitochondria. Biochim Biophys Acta 1985; 822: 1–42