Abstract
Canthaxanthin is a carotenoid pigment of physiological importance owing to potential modulation of the dynamic and structural properties of biomembranes. The effect of canthaxanthin on the organization of lipid membranes formed with dipalmitoylphosphatidylcholine (DPPC) was studied with application of monomolecular layer technique, FTIR spectroscopy and linear dichroism-FTIR. The specific molecular areas of the two-component monomolecular layers of canthaxanthin-DPPC show pronounced underadditivity in the concentration range below 2 mol% carotenoid with respect to the lipid, corresponding to the monomeric organization of the pigment. Additionally, the analysis of the FTIR spectra of the two-component monolayers deposited to the solid support shows that organization of the carotenoid in the lipid monolayer is governed primarily by van der Waals interactions between the pigment chromophore and lipid alkyl chains. This interaction is responsible for an ordering effect of canthaxanthin with respect to lipids. Analysis of FTIR spectra of two-component monolayers suggests the possibility of hydrogen bonding between the lipid polar headgroups and the keto groups of canthaxanthin via water bridges.
Introduction
Canthaxanthin (β,β-carotene-4,4’ dione), is a naturally occurring pigment which appears in many cases as an intermediate during the synthesis of astaxanthin. It can also be synthesized de novo by algae and plants and by certain bacteria and fungi or, as in the case of animals, be formed from other carotenoids ingested with the food Citation[1]. It has been shown that canthaxanthin can act as a strong antioxidant and prevent lipid peroxidation in various biomembrane systems Citation[2]. Although it does not show the provitamin A activity or involvement in light initiated biological processes its reported anti-cancer and anti-dermathosis Citation[3–5] abilities together with a very attractive color make it a highly demandable colorant in food and cosmetic industry. This molecule has been widespread applied as a food additive and a component of tanning creams and pills Citation[2], Citation[6]. The interest in this compound grows over the recent decades mainly because of the reported undesirable effects on human health caused by the formation of crystals within the macula lutea membranes of the retina Citation[7–10]. This process and the exceptionally low aggregation threshold, as compared to other carotenoid pigments Citation[11], Citation[12], is not fully understood. It has been found that this pigment highly interferes with the organization of biological membranes by exerting molecular restrictions to the segmental molecular motion of lipid molecules in the head group region as well as in the hydrophobic core and also promoting the extended configuration of alkyl lipid chains Citation[13]. Our previous studies on lipid vesicles show that the presence of canthaxanthin modifies the surface of the lipid membranes and promotes liposome aggregation Citation[13]. The formation of hydrogen bonds with canthaxanthin keto groups either directly or indirectly via water molecules is also of high probability and physiological interest. In this study we examine the interactions between canthaxanthin and lipids in monomolecular layers. Such a model system provides unique opportunity to address specific questions regarding lipid-carotenoid interaction in a single lipid monolayer which constitutes a lipid bilayer membrane. In order to study molecular interactions between DPPC and CAN, FTIR spectroscopy was applied. Canthaxanthin is a member of the C40 carotenoid pigment family that comprises also β-carotene (provitamine A) and lutein and zeaxanthin (present in the macula lutea of the membranes of the retina of primates). The present study may contribute to full understanding of organization of lipid membranes containing carotenoids. In particular, the results of the study on canthaxanthin-lipid interactions may shed light on the problems concerning molecular organization and orientation of carotenoid molecules in the lipid phase and influence of carotenoids on physical properties of lipid membranes.
Materials and methods
Chemicals
Dipalmitoylphosphatidylcholine (DPPC) has been purchased from Sigma Chem Co. (USA). Synthetic, crystalline canthaxanthin (β,β-carotene-4,4'dione, abbreviation-CAN, see ) was purchased from Fluka (Switzerland). Pigment was stored under argon in a freezer at the temperature of −30°C. Directly before use, CAN was re-purified by means of HPLC technique (column length 250 mm, internal diameter 4.6 mm, filled with Nucleosil C-18) in order to remove possible degradation products. The solvent mixture acetonitrile:methanol (72:8, v:v) has been used as a mobile phase. To avoid any organic contamination the water used in the monolayer experiments as a sub-phase was double distilled and distilled for the third time with the added KMnO4. The chloroform or chloroform-diethyl ether mixture (95:5, v:v) was used as a deposition solvent to form monomolecular layers of CAN and DPPC. The spreading solutions of CAN, DPPC and CAN-DPPC mixtures were prepared directly before measurements.
Monomolecular layers
CAN was dissolved in chloroform and centrifuged for 15 min at 5000 g in order to remove microcrystals, possibly remained in the solution. The molar concentration of CAN was evaluated spectrophotometrically; the molar extinction coefficient 1.2×105 M−1cm−1 was used Citation[14]. Monomolecular layers of pure CAN, DPPC and two-component monolayers were formed at the air-water interface in the Teflon trough with dimensions 37×4×1 cm, equipped with a Teflon compressing barrier. Monolayers were compressed with a constant speed of 1 mm/s along the longer side of the trough. The initial molecular area was 200 Å2/molecule in all the experiments. Surface pressure was monitored with a tensiometer, from Nima Technology, (Coventry, UK). Monolayers were formed and compressed at the constant temperature of 20±1°C in the argon atmosphere.
Monomolecular layers for FTIR measurements were deposited to a solid support by means of the Langmuir-Blodgett (L-B) technique, with a speed of lift 5 mm/min at a constant, computer-controlled surface pressure (tensiometer KSV, Helsinki, Finland). Monolayer compression (trough dimensions: 28.2×7.5×1 cm, speed: 0.42 mm/s) and deposition was carried out at 26±1°C, above the liquid-like to solid-like phase transition for monolayers formed with DPPC Citation[15]. In order to remove residuals of bulk water, thin L-B films were placed in a vacuum for 1 h. Before measurements the samples were exposed for 30 min to air conditions (relative humidity 60%) in order to hydrate components of the monolayers (carotenoids and lipids).
Infrared absorption measurements (FTIR)
Infrared absorption spectra were recorded with the Fourier-transform infrared (FTIR) spectrometer, model Bio-Rad FTS 185, equipped with a MCT detector and KBr beamsplitter. Before measurements the instrument was purged with CO2-free dry air for 40 min. The attenuated total reflection (ATR) configuration was used with a 10-reflections Ge crystal (45° cut). Typically 200 interferograms were collected, Fourier transformed and averaged. Absorption spectra in the region between 4000 and 600 cm−1, at a resolution of one data point every 2 cm−1, were obtained using a clean crystal as a background. ATR crystals were cleaned with organic solvents and for 30 min by “Harrick” Plasma Cleaner. Glan-Taylor polarizer was used in the IR polarization experiments. Further details of our FTIR-linear dichroism measurements were described previously Citation[16]. Briefly, the dichroic ratio (R) can be calculated as a ratio of the integrated absorption bands corresponding to the selected vibrational modes in the parallel, AII and perpendicular A⊥ orientation of the electric vector of radiation with respect to the plane of incidence:1
The order parameter, that reflects orientational fluctuations of selected IR-active dipole transitions defined as:2 can be determined on the basis of the dichroic ratio measurements by employing the following relationship:
3 where θ is the average tilt angle between the arbitrary selected molecular director (usually the molecular axis defined by the shape of the molecule) and the axis perpendicular to the plane of the membrane (z axis) and α is the angle between the transition moment of the investigated vibration and the molecular director Citation[17], Citation[18]. In the case of the lipid chains, for example, α is 90° for the symmetric and antisymmetric CH2 stretching and 0° for the symmetric CH3 stretching. Ex, Ey and Ez are the components of the electric field of the evanescent wave in the three directions and they are expressed by the angle of incidence of the IR beam and refractive indices of the internal reflection plate, environment and of the sample
Citation[16–18]. Spectral analysis was performed with Grams 32AI software from ThermoGalactic (USA).
Results and discussion
Monomolecular layer experiments
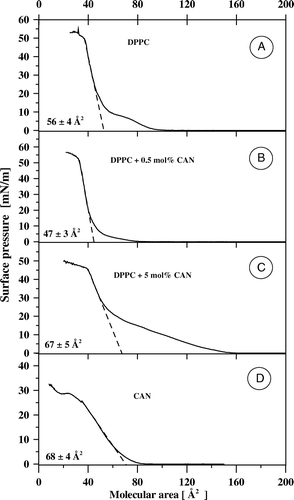
shows the exemplary isotherms of compression of monomolecular layers formed with pure DPPC (A), pure CAN (D) as well as two-component films formed at different molar concentrations (0.5 mol% CAN (B) and 5 mol% CAN (C). The shape of the isotherm recorded in the case of the pure lipid and the specific molecular area of 56±4 Å2 corresponds very well to those reported previously Citation[15], Citation[19–21]. As seen from A the DPPC monolayer undergoes the phase transition from the liquid-like to the solid-like state at the surface pressure of ca. 10 mN/m (for the specific discussion see Citation[15]). The specific molecular area of CAN in the monolayer formed at the air-water interface in the present study, 68±4 Å2, is bigger than in the case of monomolecular layers of CAN formed on the surface of the phosphate buffer pH 8 (ca. 54 Å2) Citation[22], phosphate buffer pH 7 (ca. 60 Å2Citation[23]) and was found to depend strongly on the rate of monolayer compression owing to aggregation of the pigment at the interface Citation[24]. A different specific molecular area of CAN, as compared to lutein and zeaxanthin, despite similar molecular structures of all these xanthophylls, suggests exceptional organization of this keto-xanthophyll pigment in the monolayer formed at the air-water interface. Despite the difference, the specific molecular area of CAN, determined in the present study, reflects roughly vertical orientation of the pigment molecules in the compressed film. As can be seen from B, addition of relatively small fraction of CAN to the DPPC film (0.5 mol%) results in a pronounced shift of the entire isotherm of compression towards lower molecular area values, without considerably affecting the collapse pressure of the monolayer (observed at ca. 50 mN/m). Moreover, the semi-plateau phase, observed in the course of compression of the pure DPPC monolayer, and corresponding to the process of ordering of the lipid alkyl chains within the film, disappears following the addition of CAN at 0.5 mol%. Ordering effect of the rigid, rod-like molecules of CAN with respect to the hydrocarbon lipid chains can be expected to be responsible for this mechanism via the van der Waals interactions.
Figure 2. Isotherms of compression of monomolecular layers formed with DPPC (A), canthaxanthin (D) and two-component DPPC-canthaxanthin monolayers containing 0.5 mol% (B) and 5 mol% (C) of the pigment. The dashed lines fitted to the linear portions of the isotherms, extrapolated to zero surface pressure, point the specific molecular area (values±SD presented in each panel).
presents the analysis of molecular interactions in the two-component DPPC-CAN monolayers, in terms of the additivity rule, in the concentration range below 5 mol% carotenoid. The molecular areas of the two-component films formed with DPPC and other xanthophyll pigments (e.g., lutein and zeaxanthin) are overadditive Citation[19], Citation[20], which indicates that the mean molecular area values are located above the theoretical linear dependencies (such as that one presented in ), representing the ideal mixing. Such an effect was particularly pronounced at low molecular fractions of the pigments, below the aggregation threshold in the monolayers. Surprisingly, the molecular areas in the two-component CAN-DPPC monolayers, corresponding to the low molecular fractions of the pigment (below 1 mol%) practically follow the additivity rule, or are even lower than the expected values, at the surface pressures below 15 mN/m. The fact that the mean molecular areas in the two-component monolayer can be well described by a sum of the contributions of the components in the monocomponent monolayers is an indication of similar arrangement of the molecules both in the pure and mixed films. The underadditivity observed at low molecular fractions of CAN at low surface pressure values (below 15 mN/m, see ) corresponds to the effect of removal of the semi-plateau in the pure DPPC monolayer, observed below 15 mN/m. It is very likely that this mechanism represents molecular interactions of alkyl lipid chains and the rigid polyene chains of the carotenoid, leading to the ordering of the hydrocarbon lipid chains, on the one hand, and promoting vertical orientation of a certain fraction of CAN at the air-water interface, already at the relatively low surface pressures, on the other hand. This latter effect can be expected as responsible for the underadditivity observed, owing to the difference between the molecular cross-sections of the carotenoid oriented vertically and horizontally with respect to the surface of the monolayer. In contrast to the underadditivity observed at the low molar fractions of CAN, the pronounced overadditivity can be observed at all the surface pressure values, at carotenoid concentrations higher than 2 mol%. This effect can be related to a different orientation of the xanthophyll in the pure monolayer and in the two-component monolayer with DPPC. It is possible that a certain fraction of CAN, admixed to the lipid, adopts horizontal orientation, even at high surface pressure values, unlike in the mono-component films, owing to the interactions of the two keto groups of the pigment with the polar groups of two lipid molecules. Despite such a pronounced effect on mean molecular area, observed particularly at the surface pressures below 25 mN/m, a relatively good agreement between the specific molecular area values determined on the basis of the experimental isotherms of compression and those predicted on the basis of the additivity rule, in the same concentration range of CAN, can be observed ().
Figure 3. Mean molecular area vs. molar fraction of canthaxanthin in two-component DPPC-canthaxanthin monolayers at different surface pressure values, indicated. The dashed lines show the theoretical dependencies derived on the basis of the additivity rule.
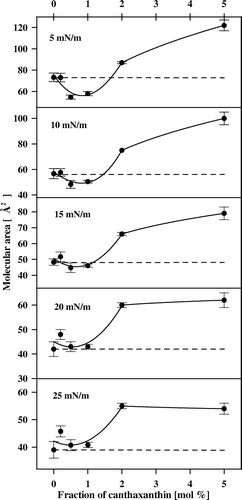
Figure 4. Specific molecular area vs. molar fraction of canthaxanthin in two-component DPPC-canthaxanthin monolayers. The dashed line shows the theoretical dependency, derived on the basis of the additivity rule.
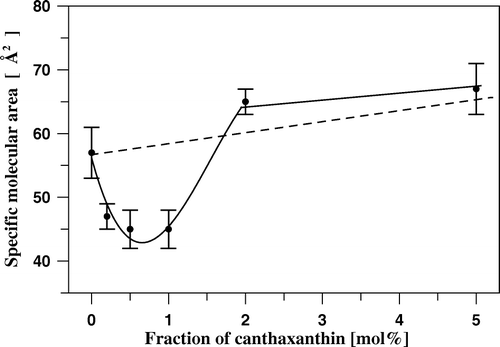
Such a result indicates that the fraction of horizontally oriented CAN, present at low surface pressures, remains relatively small in the so-called solid state film. In contrast, remarkable underadditivity in the specific molecular values, in the CAN concentration range below 2 mol% () is an expression of a pronounced ordering effect of the pigment with respect to the lipid phase. A relatively sharp change in the underadditivity in the concentration range between 1 and 2 mol% can be attributed to the CAN aggregation threshold in the lipid phase of the monolayer. The CAN concentration within the corresponding bilayer system would amount between 0.5 and 1 mol%. The same, exceptionally low aggregation threshold of CAN in the DPPC membranes, has been deduced recently on the basis of the results of 1H-NMR measurements Citation[13] as well as of the differential scanning calorimetry Citation[25]. CAN molecule, long enough to span the membrane, locates within the bilayer in such a way that the polar groups are anchored in the opposite membrane zones, constituting unique conditions in terms of modification of the membrane structure and dynamics.
The ordering effect of CAN on lipid monolayer was further analyzed with the application of infrared absorption spectroscopy.
FTIR measurements
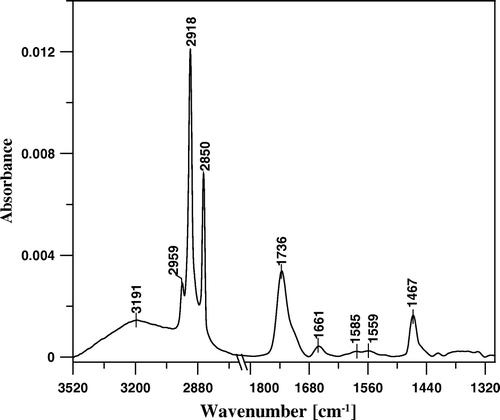
presents the IR absorption spectrum of a single monomolecular layer of CAN deposited to Ge crystal with application of the Langmuir-Blodgett technique. The spectrum comprises spectral features characteristic of CAN in a hydrated form, including the band centered at 3191 cm−1, characteristic of O-H stretching vibrations of water and the band with the maximum at 1661 cm−1, representing the antisymmetric stretching vibrations of two keto groups located at the 4 and 4’ positions of the pigment molecule, hydrogen-bonded to water molecules Citation[26]. The pronounced absorption band with the maximum at 1736 cm−1 represents the antisymmetric stretching vibrations of the keto groups that are not involved in the molecular interactions via hydrogen bonding. The skeletal vibrations of CAN are represented mainly by the broad absorption band representing the C = C stretching vibrations (main maxima recorded at 1585 and 1559 cm−1). Such a broad band is characteristic of C = C in a conjugated system and can be seen particularly in the case of polyenes remaining in the aggregated form Citation[16], Citation[26]. Other pronounced spectral features in the IR absorption spectrum of CAN represent: the CH2 group deformation vibrations (scissoring) mode at 1467 cm−1 and C-H stretching modes at 2850 cm−1 (CH2 symmetric), 2918 cm−1 (CH2 antisymmetric) and at 2959 cm−1 (CH3 antisymmetric) Citation[26].
Figure 5. FTIR absorption spectrum of monomolecular layer of canthaxanthin deposited to Ge crystal support at 25 mN/m.
Incorporation of CAN into the DPPC monolayers at relatively low concentration of the pigment (0.5 mol%) influences considerably the IR absorption spectrum of the pure lipid monolayer in all the spectral regions significant in terms of structural and dynamic properties of the lipid systems Citation[27]. present comparison of the IR absorption spectra of the L-B films deposited from the monocomponent monolayer of DPPC and the two-component monolayer containing 0.5 mol% CAN. As can be seen, the intensity of the band at 1737 cm−1, corresponding both to the keto group of CAN and to the ester carbonyl groups of DPPC is not essentially different in the two cases analyzed (see ). Interestingly, the new band can be observed at 1652 cm−1 upon incorporation of the pigment into the lipid system. This particular band can be assigned to the hydrogen-bonded keto groups of CAN and such a result indicates that all the pigment molecules incorporated into the DPPC monolayer are involved in the hydrogen bonding via the keto groups. A clear difference in the spectral region between 1538 and 1592 cm−1 corresponds directly to the conjugated C = C bonds, present in CAN but not in the lipid component. Interestingly, the intensity of the spectral bands assigned to the CH2 scissoring vibrations (1467 cm−1) and to the so-called umbrella deformation vibrations of the CH3 group (1378 cm−1) increases considerably upon incorporation of the carotenoid. Very similar effect has been observed in the case of CAN incorporation to the DPPC multibilayers Citation[13]. Such an increase may not be satisfactory explained in terms of a direct effect of the additive (such as in the case of the intensity of the band attributed to the antisymmetric C = O stretching vibrations). The condensing effect of CAN with respect to lipids, even at the solid-like state, can be applied to explain this phenomenon. Such an interpretation is supported by the CAN-induced increase in the intensity of the band at 1494 cm−1, that can be assigned to the CH2 scissoring vibrations in the headgroup region. It is also possible that the ordering effect influences an average orientation of the transition dipole moments for the methyl and methylene group vibrations and thus contributes to the enhancement of infrared absorption intensity observed. In fact, a pronounced ordering effect of CAN with respect to the monolayer hydrocarbon zone emerges from the analysis of the methylene wagging band progression resulting from a number of coupled CH2 oscillators (1320–1370 cm−1). The analysis reveals carotenoid-induced elimination of the end gauche and double gauche conformers of lipid alkyl chains, as manifested by the intensity damping of the band progression components that appear at ca. 1340 and 1350 cm−1, corresponding to those steric forms, respectively Citation[27].
Figure 6. FTIR absorption spectra, presented in the spectral region between 1300 and 1800 cm-1, of mono-component monomolecular layer of DPPC (dashed line) and two-component DPPC-canthaxanthin monomolecular layer containing 0.5 mol% of the carotenoid (solid line) deposited to Ge crystal support at the surface pressure 25 mN/m.
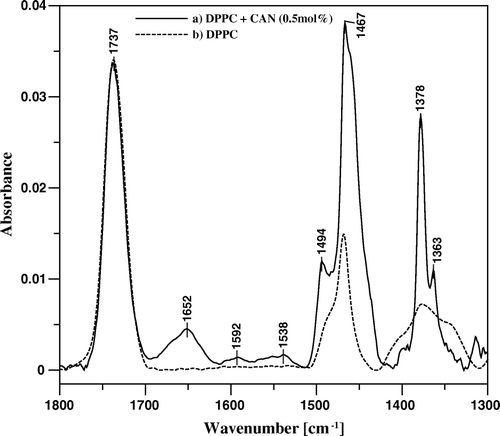
Figure 7. FTIR absorption spectra, presented in the spectral region characteristic of C-H stretching vibrations, of mono-component monomolecular layer of DPPC (A) and two-component DPPC-canthaxanthin monomolecular layer containing 0.5 mol% (B) and 5 mol% (C) of the carotenoid deposited to Ge support at the surface pressure 25 mN/m. The assignment of the main spectral bands is following: 2850 cm−1 νs (symmetric stretching) in CH2, 2884 cm−1 νs in CH3, 2918 cm−1 νas (antisymmetric stretching) in CH2, 2960 cm−1 νas in CH3. In order to perform an accurate deconvolution of the spectra a Gaussian-Lorentzian mixture has been applied. The fraction of a Gaussian component in the initial set applied for deconvolution was following: the component centered at 2832 cm−1: 1.0, 2850 cm−1: 0.4, 2861 cm−1: 1.0, 2884 cm−1: 1.0, 2900 cm−1: 1.0, 2918 cm−1: 0.47, 2932 cm−1: 0.77, 2960 cm−1: 0.6. The residuals are reported at the bottom of each panel.
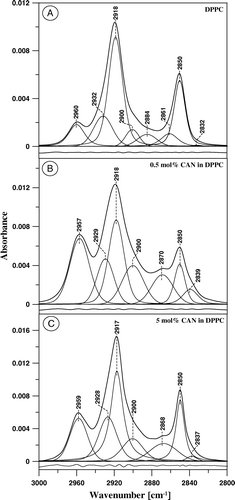
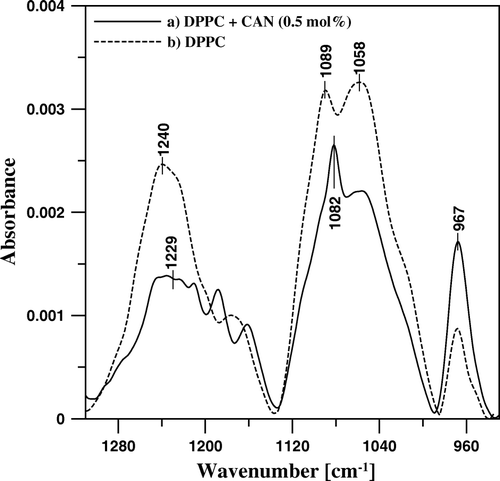
Figure 8. FTIR absorption spectra, presented in the spectral region characteristic of polar lipid headgroups, of mono-component monomolecular layer of DPPC (dashed line) and two-component DPPC-canthaxanthin monomolecular layer containing 0.5 mol% of the carotenoid (solid line) deposited to Ge support at the surface pressure 25 mN/m.
An effect of CAN on alkyl chains of DPPC can be also analyzed in the spectral region between 2800 and 3000 cm−1, corresponding to the C − H stretching vibrations of methyl and methylene groups (see ). presents the IR absorption spectra in this spectral region, recorded from the pure DPPC monolayer and from the monolayers containing CAN as a second component at 0.5 mol% and 5 mol%. The spectra were deconvoluted with the same initial set of the Gaussian-Lorentzian components, following the approach proposed by Szalontai and coworkers Citation[28]. Interestingly, the low-wavenumber component that centers below 2839 cm−1 (B) can be resolved in the region of the intensive band centered at 2850 cm−1. This minor band can be also assigned to the symmetric stretching vibrations of methylene groups. Such a low-wavenumber component corresponds to an ordered lipid phase Citation[28] and considerably gains intensity upon incorporation of CAN into the membrane, despite very low concentration of the additive (0.5 mol%, B). An ordering effect of CAN with respect to the hydrophobic zone of the DPPC monolayer, observed at low carotenoid concentration, is not as strongly pronounced at higher concentration of CAN (5 mol%, C). The same effect can be observed by following an intensity of the spectral component at 2900 cm−1, located in the lower wavenumber region with respect to the main band corresponding to methylene group antisymmetric vibrations at 2918 cm−1. The analysis of the surface pressure-molecular area isotherms of compression of monomolecular layers formed with DPPC and CAN at different concentrations (discussed above) supports interpretation according to which the reversibility of the ordering effect of CAN with respect to alkyl lipid chains, accompanying an increase in the concentration of the additive, is directly related to the pigment aggregation in the lipid phase. Interestingly, the intensity of the antisymmetric stretching vibrations of methyl groups, represented by the spectral component that centers in the region of 2960 cm−1 correlates with the intensity of the band most shifted towards lower wavenumbers, representing an ordered lipid phase (2839 cm−1). Such a correlation is a manifestation of the effect of CAN, dispersed in the lipid phase, on intensity of the absorption bands representing vibrations of the groups of atoms that form hydrophobic core of the membrane, as discussed above (). The ordering effect of CAN with respect to alkyl lipid chains is most probably based upon the hydrophobic van der Waals interactions with rigid carotenoid molecule containing conjugated double bond system.
presents the IR absorption spectra of the DPPC film and the lipid film modified with 0.5 mol% CAN, in the spectral region corresponding to vibrations of the functional groups, located in the polar headgroup region. In the case of pure DPPC, four principal bands are visible, characteristic of a hydrated lipid film: centered at 1240 cm−1 and corresponding to the antisymmetric stretching of the group, at 1089 cm−1, corresponding to the symmetric stretching of the same group, at 1058 cm−1, corresponding to the C-O-P-O-C stretching vibrations and at 967 cm−1, corresponding to the antisymmetric N+-(CH3)3 bending. Incorporation of CAN into DPPC monolayer is associated with the shift of the center of the band assigned to
antisymmetric stretching vibrations towards lower wavenumbers by 11 cm−1 and the band assigned to the symmetric stretching vibrations of the same group by 7 cm−1, in contrast to the band representing the vibrations of the choline group centered at 967 cm−1, position of which is insensitive to the presence of CAN in the monolayer. The spectral shift observed can be interpreted in terms of a hydrogen bonding to the phosphate groups. It is very likely that water molecules are involved in these interactions. It is also possible that water bridges link the keto groups of CAN with the polar groups of DPPC, including ester carbonyl groups and phosphate groups, and they are responsible for polar interactions of the carotenoid with the lipid matrix.
FTIR-linear dichroism measurements
In order to determine orientation of CAN in the mono-component and two-component monolayers, IR absorption spectra have been recorded with application of linearly polarized radiation. presents the polarized absorption spectra recorded from the monomolecular layer of CAN deposited to the Ge support at 25 mN/m. The band intensities integrated between 1747 and 1721 cm−1 and between 1628 and 1525 cm−1 have been used to calculate the dichroic ratio corresponding to the C = O and C = C stretching vibrations (R = 1.01 and R = 1.05 respectively, see ). Let us define the molecular director of CAN, which is parallel to the normal to plane of the mono-component monomolecular film deposited to Ge support at the surface pressure 25 mN/m. The dichroic ratio values allowed to calculate the mean orientation angles between the dipole transitions that give rise to C = C and C = O vibrations, and the defined axis of the molecular director on the basis of Equations 2 and 3 (θ = 0 in Equation 2): αC = C=65° and αC = O=70° (see ). The average αC = C and αC = O angles determine unequivocally the orientation of the molecular director defined above with respect to the CAN molecule. The orientation angles determined indicate, that the CAN monomolecular layer is organized in such a way that the pigment chromophores and even axes connecting the opposite polar groups are tilted with respect to the normal to the plane of the membrane (as demonstrated schematically in ). Such a pigment orientation is consistent with formation of the H-type molecular aggregates in the lipid bilayer. One has to consider differences between the direction of the transition dipole associated with the C = C vibrations and the direction of the conjugated C = C system in order to determine actual orientation of the polyene fragment of CAN molecule. For example, an angle of 24° has been determined as a difference between the molecular axis of heptaene fragment of amphotericin B and the direction of the dipole transition of the C = C vibrations Citation[16]. The problem is even more complex in the case of CAN molecule, owing to the double bonds in the 5, 6 and 5’, 6’ positions, oriented differently with respect to the main conjugated double bond system of the pigment. The band corresponding to the C = C vibrations can be applied to a linear dichroism analysis of orientation of CAN in the two-component DPPC-CAN monomolecular layers, owing to the fact that this band is exclusively present in the carotenoid spectrum (C = C bonds are not present in the pure lipid).
Figure 9. FTIR absorption spectra of the monomolecular layer formed with pure canthaxanthin, deposited to Ge support at the surface pressure 25 mN/m, recorded with the electric vector of the radiation polarized perpendicular (A⊥, solid line) and parallel (A||, dashed line) with respect to the plane of incidence. The C = O and C = C bands integration limits are shown (1721–1747 cm−1 and 1525–1628 cm−1, respectively) along with the determined dichroic ratio values (R) and calculated mean orientation angle values αC = O and αC = C.
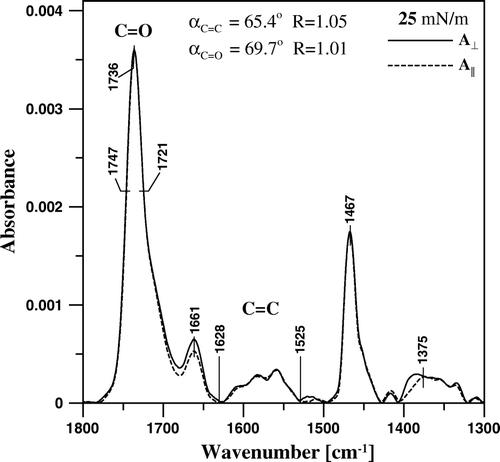
Figure 10. Schematic representation of the orientation of the canthaxanthin molecule in the monolayer formed at the air-water interface and deposited to the Ge support by the Langmuir-Blodgett technique. Z is the axis normal to the plane of the membrane. The angles αC = C and αC = C between the dipole transition of the C = C and C = O stretching vibrations and the axis Z are shown.
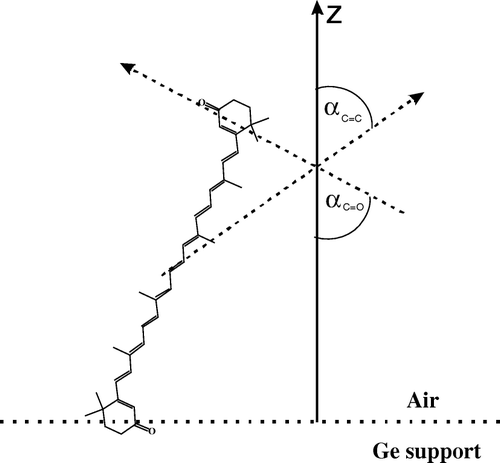
The results of the determinations of orientation based on the linear dichroism measurements are presented in . Assuming the average angle αC = C=65°, between the dipole transition of the C = C vibrations of CAN and the defined molecular director, one arrives to the angle between this particular axis and the normal to the plane of the monolayer as 29° in the case of 0.5 mol% CAN in DPPC and 48° in the case of 5.0 mol% CAN in DPPC. This means that CAN incorporated into the monomolecular film formed with DPPC adopts different orientation that in the case of the pure pigment monolayer and that the difference is lower at low pigment concentration (29° at 0.5 mol%) and bigger at a higher concentration (48° at 5.0 mol%). In the case of low pigment concentration, the θ angle defines an angular distribution of CAN in the lipid phase and reflects motional freedom of the pigment molecules. The θ angle determined for CAN present at a higher concentration in the DPPC monolayers compressed to the surface pressure close to the surface pressure of biomembranes (25 mN/m Citation[29]), reflects rather orientational distribution of aggregated structures of the pigment than single molecules of the pigment. Interestingly, very close orientation angle (47°) of the transition dipole of CAN molecules incorporated into the DPPC multibilayers have been determined in the case of 2 mol% CAN, above the aggregation threshold in the lipid phase Citation[13]. Such an agreement indicates, that a fitting of the CAN molecule to the thickness of the hydrophobic core of the lipid bilayer, considered as a main determinant of a localization and orientation of carotenoid molecules within the membrane Citation[11], Citation[12], Citation[30], is not as much important at high pigment concentrations. The findings suggest also that the lipid-carotenoid interactions, in the case of low carotenoid concentrations, and the pigment aggregation in the membrane environment, in the case of high concentrations, govern orientation of CAN in the lipid environment. An average orientation of CAN within the lipid bilayers and monolayers does not provide direct information regarding possibility that a certain fraction of the pigment molecules adopts the horizontal orientation. On the other hand, the relatively small orientation angle, that was determined in the case of the non-aggregated pigment, indicates that if such a pool of horizontally-oriented CAN molecules exists in the membrane, it has to be relatively small.
Table I. The results of linear dichroism-FTIR measurements (±SD: S [±0.05], α [±4°], θ [±5°]) of canthaxanthin in two-component monomolecular films composed of canthaxanthin and DPPC, deposited at 25 mN/m to Ge crystal.
presents the results of the linear dichroism determinations of molecular order in the lipid monolayers, based on the C-H stretching vibrations in the methyl and methylene groups. The average orientation angle θ, corresponds to the angle of the cone in which the segmental motions of the lipid alkyl chains take place (in principal the trans-gauche isomerization). As can be seen, in the case of the film formed with pure DPPC, the terminal CH3 groups of alkyl chains, that are not directly attached to the crystal support, remain in an unordered state (θ = 63°). An average orientations of the CH2 groups, determined on the basis of the most intensive band corresponding to the antisymmetric stretching vibrations (the component centered at 2918 cm−1) corresponds very well to that one determined for the methyl groups in the lipid bilayers in the ordered state (∼27° Citation[31], Citation[32]). Incorporation of CAN into the DPPC monolayer at low concentration has some small effect on the order parameter of the terminal CH3 groups of alkyl chains but very pronounced effect on the order parameter of the CH2 groups. Interestingly, the ordering effect of CAN, demonstrated by the decrease in the θ angle, is totally abolished in the case of methylene groups in the two-component monolayer containing high concentration of the carotenoid. Such an effect can be interpreted in terms of the van der Waals interactions between the rigid molecules of CAN and alkyl lipid chains, limited at high pigment concentration owing to the formation of molecular aggregates.
Table II. The results of linear dichroism-FTIR measurements (±SD: S [±0.03], θ [±4°]) of mono-component and two-component monomolecular films composed of canthaxanthin and DPPC, deposited at 25 mN/m to Ge crystal. Determinations are based on the deconvolution components of the spectra, as depicted in .
It is concluded that CAN present in the DPPC monolayer is miscible with lipids below 1 mol% but remains aggregated above 2 mol% at 20°C. Such a low aggregation threshold is the key determinant responsible for the observed crystallization of this pigment in the natural lipid systems. The carotenoid dispersed in the lipid phase exerts pronounced ordering effect with respect to the alkyl lipid chains. Interaction of CAN with a lipid matrix is based upon van der Waals interactions between the hydrophobic fragments of the lipid and the pigment molecules and upon hydrogen bonding with involvement of water bridges.
References
- Ausich RL. Commercial opportunities for carotenoid production by biotechnology. Pure Appl Chem 1997; 69: 2169–2173
- Mayne ST, Parker RS. Antioxidant activity of dietary canthaxanthin. Nutr Cancer 1989; 12: 225–236
- Chew BP, Park JS, Wong MW, Wong TS. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res 1999; 19: 1849–1853
- Macdonald K, Holti G, Marks J. Is there a place for beta-carotene/canthaxanthin in photochemotherapy for psoriasis?. Dermatologica 1984; 169: 41–46
- Wennersten G. Carotenoid treatment for light sensitivity: a reappraisal and six years’ experience. Acta Derm Venereol 1980; 60: 251–255
- Lober CW. Canthaxanthin – the “tanning” pill. J Am Acad Dermatol 1985; 13: 660
- Goralczyk R, Buser S, Bausch J, Bee W, Zuhlke U, Barker FM. Occurrence of birefringent retinal inclusions in cynomolgus monkeys after high doses of canthaxanthin. Invest Ophthalmol Vis Sci 1997; 38: 741–752
- Goralczyk R, Barker FM, Buser S, Liechti H, Bausch J. Dose dependency of canthaxanthin crystals in monkey retina and spatial distribution of its metabolites. Invest. Ophthalmol Vis Sci 2000; 41: 1513–1522
- Espaillat A, Aiello LP, Arrigg PG, Villalobos R, Silver PM, Cavicchi RW. Canthaxanthin retinopathy. Arch Ophthalmol 1999; 117: 412–413
- Daicker B, Schiedt K, Adnet JJ, Bermond P. Canthaxanthin retinopathy. An investigation by light and electron microscopy and physicochemical analysis. Graefes Arch Clin Exp Ophthalmol 1987; 225: 189–197
- Gruszecki WI. Carotenoids in membranes. The photochemistry of carotenoids, HA Frank, AJ Young, G Britton, RJ Cogdell. Kluwer Academic Publishing, Dordrecht 1999; 363–379
- Gruszecki WI, Strzalka K. Carotenoids as modulators of membrane physical properties. Biochim Biophys Acta 2005; 1740: 108–115
- Sujak A, Gabrielska J, Milanowska J, Mazurek P, Strzalka K, Gruszecki WI. Studies on canthaxanthin in lipid membranes. Biochim Biophys Acta 2005; 1712: 17–28
- Britton, G. 1995. UV/Visible spectroscopy. In:. G Britton, Liaaen-Jensen, S, Pfander, H. Carotenoids Vol. 1B: Spectroscopy. Basel: Birkhauser Verlag. pp 13–62.
- Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Quart Rev Biophys 1975; 8: 185–235
- Gagos M, Gabrielska J, Dalla Serra M, Gruszecki WI. Binding of antibiotic amphotericin B to lipid membranes: Monomolecular layer technique and linear dichroism-FTIR studies. Molec Memb Biol 2005; 22: 433–442
- Tamm LK, Tatulian SA. Infrared spectroscopy of proteins and peptides in lipid bilayers. Quart Rev Biophys 1997; 30: 365–429
- Harrick NJ. Internal reflection spectroscopy. Harrick Scientific Corporation, Ossining, NY 1979
- Milanowska J, Polit A, Wasylewski Z, Gruszecki WI. Interaction of isomeric forms of xanthophyll pigment zeaxanthin with dipalmitoylphosphatidylcholine studied in monomolecular layers. J Photochem Photobiol B: Biol 2003; 72: 1–9
- Sujak A, Gruszecki WI. Organization of mixed monomolecular layers formed with the xanthophyll pigments lutein or zeaxanthin and dipalmitoylphosphatidylcholine at the argon-water interface. J Photochem Photobiol B: Biol 2000; 59: 42–47
- Wójtowicz K, Gruszecki WI, Walicka M, Barwicz J. Effect of amphotericin B on dipalmitoylphosphatidylcholine membranes: calorimetry, ultrasound absorption and monolayer technique studies. Biochim Biophys Acta 1998; 1373: 220–226
- Diarra A, Hotchandani S, Max J-J, Leblanc RM. Photovoltaic properties of mixed monolayers of chlorophyll a and carotenoid canthaxanthin. J Chem Soc Faraday Trans 1986; 82: 2217–2231
- Sielewiesiuk J, Veeranjaneyulu K, Leblanc RM. Cryptoxanthin, echinenone and hydroxyechinenone films at the air-water interface. Annales UMCS Sectio AAA 2002; 57: 123–135
- Sielewiesiuk J. Model studies on carotenoids as photoprotectors. Maria Curie-Sklodowska University [in Polish], Lublin 1988
- Sujak, A, Strzalka, K, Gruszecki, WI. 2006. Thermotropic phase behaviour of lipid bilayers containing carotenoid pigment canthaxanthin: a differential scanning calorimetry study. Chem Phys Lipids, 145: 1–12.
- Bernhard, K, Grosjean, M. 1995. Infrared spectroscopy. In:. G Britton, Liaaen-Jensen, S, Pfander, H. Carotenoids Vol. 1B: Spectroscopy. Basel: Birkhauser Verlag. pp 117–134.
- Lewis RNAH, Mcelhaney RN. Fourier transform infrared spectroscopy in the study of hydrated lipids and lipid bilayer membranes. Infrared spectroscopy of biomolecules, HH Mantsch, D Capman. Wiley-Liss Inc, New York 1996; 159–202
- Varkonyi Z, Masamoto K, Debreczeny M, Zsiros O, Ughy B, Gombos Z, Domonkos I, Farkas T, Wada H, Szalontai B. Low-temperature-induced accumulation of xanthophylls and its structural consequences in the photosynthetic membranes of the cyanobacterium Cylindrospermopsis raciborskii: an FTIR spectroscopic study. Proc Natl Acad Sci USA 2002; 99: 2410–2415
- Roux B. Commentary: surface tension of biomembranes. Biophys J 1996; 71: 1346–1347
- Gruszecki WI. Carotenoid orientation: role in membrane stabilization. Carotenoids in health and disease, NI Krinsky, ST Mayne, H Sies. Marcel Dekker, New York 2004; 151–163
- Menestrina G, Cabiaux V, Tejuca M. Secondary structure of sea anemone cytolysins in soluble and membrane bound form by infrared spectroscopy. Biochem Biophys Res Comm 1999; 254: 174–180
- Binder H, Gutberlet T, Anikin A. Biaxial ordering of terminal diene groups in lipid membranes: an infrared linear dichroism. J Molec Struct 1999; 510: 113–129