Abstract
As the progeny of the two-line hybridisation of the Wanxi Goose and Sichuan Goose, Wanjiang white goose's fertility is unstable. To improve reproduction and select molecular marker associated with egg performance of Wanjiang white goose, PCR-SSCP was applied to detect SNPs in PRL intron 2. Five genotypes were found: AA, AB, BC, CC and DD genotypes. More than 30 SNPs were found in the PCR products Statistical results show that the polymorphism of the five genotypes is significantly related to the egg productivity. The differences of egg-laying level between BC and AA, AB, CC, DD genotypes are extremely significant (p<0.01). AA and AB genotypic geese yield more eggs than BC and DD genotypes. It could be used as a molecular marker for selecting high-productivity geese.
Introduction
Wanxi white goose and Sichuan white goose are national-protected poultry in China. However, there are many difficulties in the process of rearing. The reproduction rate of the Wanxi white goose is relatively low: the annual egg yield is merely around 25. As for the Sichuan white goose, its body is relatively small. All these negative impacts have directly lead to huge losses of poultry farm. Our research team has been committed to develop a new line for more than 20 years. Wanjiang white geese, the progeny of the two-line hybridisation of the Wanxi Goose and Sichuan Goose. It is larger than their parental generation, and its meat and productivity are better. However, its fertility is unstable at present, thus the productivity is limited. It is found that only part of Wanjiang white geese yield more than 90 eggs per year. It is urgent for scientists to screen the genetic marker associated with high-yield geese and set up a core group.
Marker-assisted selection (MAS) is an efficient way to resolve this problem, which helps us to find out genetic marker linked with QTL. Prolactin (PRL) is a polypeptide hormone, synthesised and secreted mainly by the eosinophile cells in the anterior pituitary gland (Bhatt et al. Citation2003; Dalrymple et al. 2000; Hu et al. Citation2009). There are 229aa precursor in the poultry, including 30aa signal peptide and 199aa mature protein (Cao et al. Citation2002). It can stimulate growth of mammalian galactophore and lactating, in addition, it is also involved in maternal behaviour, such as broodiness in the poultry and regurgitation-feeding in birds (Shi et al. Citation2001). PRL directly acts on granulosa cells and inhibits the activities of aromatase induced by FSH, then the synthesis of estradiol and ovulation are restrained, the broodiness and egg-laying performance are affected. In recent years, many researchers have done a lot of studies on the linkage between polymorphism of PRL and production performance in chickens and ducks (Jiang et al. Citation2005; Kansaku et al. Citation2005; Liu et al. Citation2008). However, little is known about the relationship between goose PRL and egg-laying performance. For this purpose, PCR-SSCP was used to explore the SNPs in PRL intron 2. So, it will provide molecular markers to select new Wanjiang white goose line and improve egg-yielding performance.
Materials and methods
Eighty-five hybrid adult geese (from Sanyuan Breeding Limited-liability Co. Ltd. in Wuwei, Anhui Province) with the same day-old (26 week) and similar physiological characters were picked out for this research, and their production indices (including fertilisation rate of eggs, hatching rate, average egg weight and annual egg yield) were recorded for biological statistics.
One millilitre blood samples was collected from wing venous of each goose and centrifuged 10 min at 1500 rpm at 4°C. Genomic DNA was extracted using conventional phenol-chloroform extraction method. DNA is preserved in TE buffer at −20°C after evaluated the purity through ultraviolet spectrophotometric method.
Three pairs of primers were designed to amplify PRL intron 2 according to the goose PRL gene in GenBank (Accession Number: DQ345782, DQ060077.1, DQ066733) with Primer Premier 5.0, The sequences, amplification sites, predicted PCR products size and annealing temperatures are listed in (). PCR amplification was carried out in a final volume of 25 µL, containing 100 ng of genomic DNA, 10 mM of each primer, 2.5 mL 10×PCR buffer, 25 mM MgCl2, 10 mM dNTPs and 0.5U of Taq DNA polymerase. The following cycles were applied: 94°C for 5 min; 30 cycles (94°C 45 s; 60.3, 56.2 and 61.6°C, respectively for 30 s; 72°C for 60 s); 72°C for 10 min. PCR products were electrophoresed in a 0.8% agarose gel stained with ethidium bromide.
Table 1. Three pairs of primers were used to amplify goose PRL intron 2.
The amplified fragments of the PRL gene were screened for mutations using PCR-SSCP analysis. The denatured buffer was added into PCR products for 5 min, then the reaction products were put on ice for 5 min and visualised on 12% polyacrylamide(180 V, 20 h), the gels were stained with AgNO3 to identify genotypes. The different genotypic individuals were selected for sequencing. The purified PCR products and vector pMD-18T were added into linkage system. The pMD-18T was transfected into E. coli. DH-5α and positive clones were selected for sequencing. The positive clones were sequenced in both directions on ABI PRISMTM 377. The identity and diversities of the sequences of PCR fragments in different genotypic individuals were analysed with the software of DNAstar 2.0 MegAlign. Nucleotide substitutions, deletions and insertions were also analysed. The frequencies of genotypes and genes were calculated, and the significant differences were tested by χ2. The results of calculation were used to analyse Hardy–Weiberg balance of mutation sites.
Data of the egg-laying capacity were not independent; therefore, the transformation of the square root was used to normalise the data of different genotypes to meet the requirements. The data were expressed as mean±SD. The data were analysed using one-way ANOVA analysis of variance. Certain data were transformed to ensure homogeneity of variance when necessary. LSD's multiple comparisons were used to identify differences among genotypes at the 5% and 1% significance level. Statistical tests were performed using SPSS17.0 package version.
Results and discussion
Results
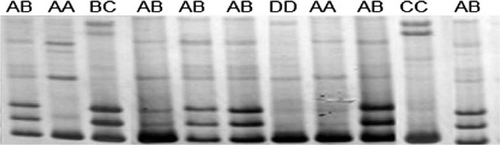
Polymorphism was found in PCR products amplified with primer 3 in this study. The product size was as expected (). Five genotypes are identified according to band patterns: AA, AB, BC, CC and DD (), and DD is the wild type. The sequencing and alignent results are shown in ( and ).
Figure 1. PCR products of the intron 2 of goose PRL gene. 1, 2, 3, 4, 5, 6, 7, 8: PCR products; M: Marker.
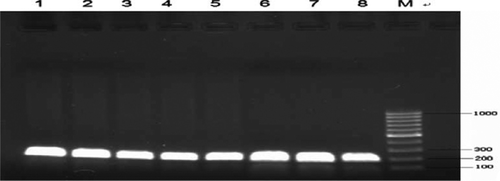
Figure 3. Five genotype sequence alignment with DNAstar 2.0 MegAlign. There are four SNP sites in AA genotype: 68 (T insertion), 148 (C/A), 36(GA simple repeat sequence deletion); 10 SNPs sites in BC genotype: 36(GA simple repeat sequence deletion), 50(C/T), 52(A/C), 56(A/T), 57(G/C), 104(G/A), 128, 134(A/G), 147(A deletion); only three SNPs in the CC genotype: 144–146(CCA deletion); 36 SNPs in the AB genotype: 46, 51, 52, 59, 86, 102, 106, 120, 133, 138, 140(A/T), 47, 146(A/C), 48, 99, 100, 136(T/C), 57, 96(G/A), 7, 67, 69(A deletion), 72, 128, 134(A/G), 77, 86, 97, 143(C/A), 84, 125, 142(T/G), 116, 139(T/A), 127(C/T), 131(G/C), 133(C deletion).
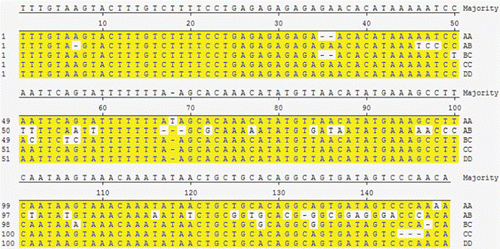
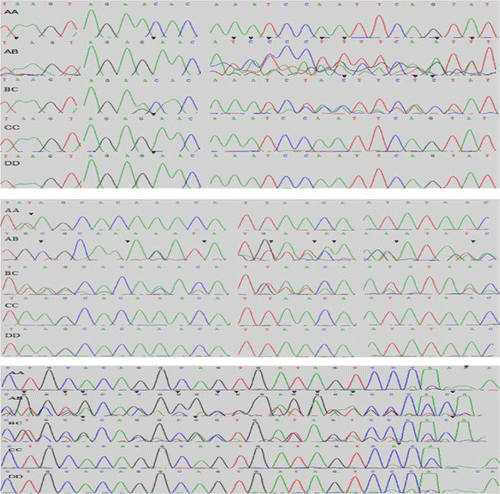
Figure 4. Waveform sequencing of five goose PRL genotypes. Mutation sites of different genotypes were shown in sequencing waveforms with black arrowheads, the results are consistent with .
The frequencies of gene and genotype are shown in (), polymorphism information content (PIC) value is also listed in (). Significant differences (chi-square test, p<0.05) were detected among 85 geese. All allels and genotypes were in the Hardy–Weinberg imbalance due to BB absence. Statistic records of average annual egg production in different genotypic geese are shown in (). AA and AB genotypic geese yield more eggs than BC and DD genotypes.
Table 2. Genotype, allele frequencies and polymorphism information content.
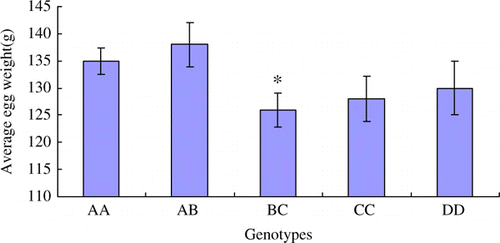
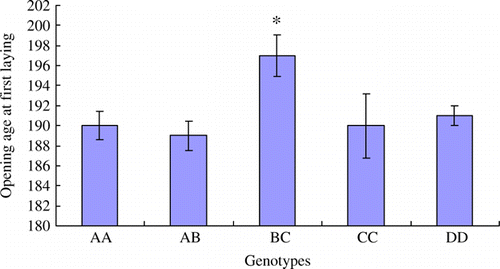
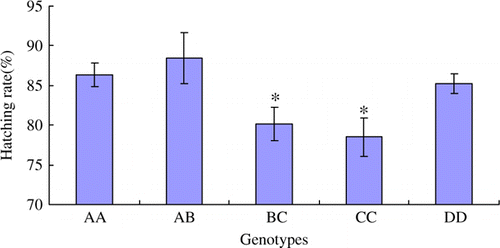
The fertilisation rate of eggs in BC genotype is the lowest among all genotypes, and the statistical difference is significant (p<0.05) (). The hatching rates of BC and CC genotypes are significantly lower than other genotypes (p<0.05) (). The average egg weight trait in BC is also significantly lower than other genotypes (p<0.05) (). The opening age of first laying in BC genotype is significant later than other genotypes (p<0.05) ().
Figure 5. The fertilisation rate of eggs (%) in different genotypes. The data are expressed as Means±SD. Asterisks indicate level of significant differences between different genotypes (*p<0.05).
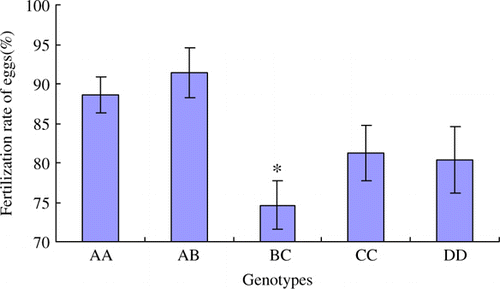
Figure 6. The hatching rate of eggs (%) in different genotypes. The data are expressed as Means±SD. Asterisks indicate level of significant differences between different genotypes (*p<0.05).
Discussion
Our research reveals that PRL intron 2 belongs to high polymorphism (P>0.5) and is closely related to egg yield of Wanjiang white goose. PRL intron does not encode amino acid and is sliced in the process of gene transcription, so its mutation will not affect the structure of protein. However, intron mutation may regulate gene transcription through certain mechanism (Gerbens et al., Citation2001; Schuelke et al. Citation2004), thus influence the expression of PRL. The expression level of PRL has a negative correlation with the egg-laying performance in chicken (Reddy et al. Citation2002). Therefore, it is suggested that the high mutation rate in intron 2 might largely affect the transcription and translation of PRL in geese.
Table 3. Least squares mean and standard error for egg yields size of different genotypes in Wangjiang white goose.
Although CC geese yield is the highest of all, it lower its applied prospect due to its scarcity. Our results of least-square analysis on egg-laying performance among different genotypes indicated that the egg-yielding levels of AA, AB genotypes are higher than those of BC and CD genotypes (p<0.05). The frequency of gene A(52.94%) turned out to be much higher than those of other genes, which is artificially selected from higher productivity family lines. Therefore, it is deduced that A gene should be the superior gene which affected the egg-laying quantity of the new strain.
It is well known that mutations (including conversion, transversion, insertion and deletion) are fundamentals in the evolution (Murakami et al. Citation2002; Zhang et al. Citation2003; Denver et al. Citation2004). In the study, a large number of deletions were discovered, compared to AB, CC, DD genotypes, one GA repeat sequence was deleted in AA, BC genotypes (). Although little is known about underlying meaning of the specific (GA)n deletion, it provides us a new clue to further researches.
Acknowledgements
This study was supported by Anhui Natural Science and Technology Program of Anhui Province (10020303043 and KJ2009A039).
References
- Bhatt , R , Youngren , O , Kang , S and Halawani , M . 2003 . Dopamine infusion into the third ventricle increases gene expression of hypothalamic vasoactive intestinal peptide and pituitary prolactin and luteinizing hormone beta subunit in the turkey . Genetic Comparative Endocrinology , 130 : 41 – 47 .
- Cao , X , Wang , Q , Yan , JB , Yang , FK , Huang , SZ and Zeng , YT . 2002 . Molecular cloning and analysis of bovine prolactin full-long genomic as well as cDNA sequences . Yi Chuan Xue Bao , 29 : 768 – 773 .
- Dalrymple A , Edery M , Jabbour , HN. 2000 . Sequence and functional characterisation of themarmoset monkey (Callithrix jacchus) prolactin receptor: comparative homology with the human long-form prolactin receptor . Molecular Cellular Endocrinology 167 : 89 – 97 .
- Denver , DR , Morris , K , Michael , L and Thomas , WK . 2004 . High mutation rate and predominance of insertions in the Caenorhabditis elegans nuclear genome . Nature , 430 : 679 – 682 .
- Gerbens , F , Verburg , FJ , Van Moererkh , TB , Engel , B , Buist , W , Veerkamp , JH and Tepas , MF . 2001 . Associations of heart and adipocyte fatty acid-binding protein gene expression with intramuscular fat content in pigs . Journal of Animal science , 79 : 347 – 354 .
- Hu , XC , Lu , AJ , Chen , H , Gao , XY , Xu , HX , Zhang , CL , Fang , XT and Lei , CZ . 2009 . Preliminary evidence for association of prolactin and prolactin receptor genes with milk production traits in Chinese Holsteins . Journal of Applied Animal Research , 36 : 213 – 217 .
- Jiang , RS , Xu , GY , Zhang , XQ and Yang , N . 2005 . Association polymorphisms for prolactin and prolactin receptor genes with broody traits in chickens . Poultry Science , 84 : 839 – 845 .
- Kansaku , N , Ohkubo , T , Okabayashi , H , Guémené , D , Kuhnlein , U , Zadworny , D and Shimada , K . 2005 . Cloning of duck PRL cDNA and genomic DNA . General and Comparative Endocrinology , 141 : 39 – 47 .
- Liu , Z , Shi , ZD , Liu , Y , Li , MY , Huang , YM and Yao , BH . 2008 . Molecular cloning and characterisation of the Magang goose prolactin gene . General and Comparative Endocrinology , 155 : 208 – 216 .
- Murakami , H , Hohsaka , T and Sisido , M . 2002 . Random insertion and deletion of arbitrary number of bases for codon based random mutation of DNAs . Nature Biotechnology , 20 : 76 – 81 .
- Reddy , IJ , David , CG , Sarma , PV and Singh , K . 2002 . The possible role of prolactin in laying performance and steroid hormone secretion in domestic hen (Gallus domesticus) . Genetic Comparative Endocrinology , 3 : 249 – 255 .
- Schuelke , M , Wagner , KR , Stolz , LE , Hübner , C , Riebel , T , Kömen , W , Braun , T , Tobin , JF and Lee , SJ . 2004 . Myostatin mutation associated with gross muscle hypertrophy in a child . New England Journal of Medicine , 26 : 2682 – 2688 .
- Shi , ZD , Liang , SD and Bi , YZ . 2001 . Studies on the role of hypothalamic dopamine and 5-hydroxy tryptamine in regulation of broodiness in chicken hens . Acta Veterinaria Et Zootechnica Sinica , 31 : 487 – 492 .
- Zhang , GZ and Gerstein , M . 2003 . Patterns of nucleotide substitution, insertion and deletion in the human genome inferred from pseudogenes . Nucleic Acids Research , 31 : 5338 – 5348 .