Abstract
Present study evaluated the presence of a sexually active male buck upon the influence of an estradiol implant in ovariectomised (OVX) goats exposed to a 6-month-long controlled photoperiod in a range of 13.4–10.6 light-hours per day. Mexican-native (Criollo) goats (n=20) were randomly assigned into 2 groups: (1) goats exposed to a sexually active male buck (n=10); (2) goats not exposed to a male buck (n=10). Each experimental group of goats included ovariectomised goats (OVX, n=5), and ovariectomised and estradiol-implanted goats (OVX + E2, n=5). Blood samples were taken from OVX and OVX + E2 goats, every four weeks, during 6 h at 15 min intervals (i.e., 24 samples/day). Frequency, amplitude, and concentration of luteinising hormone (LH) were determined. While the OVX + E2 goats depicted an increased LH pulse frequency (2.0±0.5 vs. 0.7±0.1), the presence of a sexually active male increased frequency, amplitude and concentration of LH in OVX goats compared to goats not exposed to males (frequency: 3.2±0.4 vs. 0.7±0.1 pulses/6 h; amplitude: 1.6±0.1 vs. 0.8±0.3 ng/mL; concentration: 5.3±0.6 vs. 2.0±0.9 ng/mL) (P<0.001). In conclusion, the presence of a sexually active male increase LH pulse frequency, amplitude and concentration in OVX-goats (Criollo), irrespective of a controlled photoperiodic regime.
Introduction
A global world demands an increased goat production and implementation of reproductive technologies (Aréchiga and Rincón, Citation1998; Aréchiga et al. Citation2008). Criollo goats display reproductive seasonality, even in subtropical regions such as Mexico, as demonstrated by evaluating reproductive tracts (Valencia et al. Citation1986, Citation1990), measuring serum progesterone levels in non-pregnant goats (Escobar et al. Citation1997) or by evaluating reproductive activity patterns (Chemineau et al. Citation2004). During seasonal anestrous, male bucks have shown a reproductive activity and a reduced sexual stimulus. Photoperiodic treatments have proved to be successful by increasing sexual activity in goats under subtropical latitudes (Delgadillo et al., 2002; Delgadillo et al., Citation2003; Delgadillo et al., Citation2004b) as well as in Criollo bucks exposed to alternating light–dark photoperiodic cycles (16 h L:8 h D); either with or without melatonin treatment (Delgadillo et al. Citation1995; Delgadillo et al. Citation2001; Delgadillo et al. Citation2002; Delgadillo et al. Citation2004a; Delgadillo et al. Citation2006). A combination of photoperiod and male effect has been proposed to regulate goat reproductive seasonality (Delgadillo et al., Citation2003; Delgadillo et al., 2004; Delgadillo et al., Citation2006; Delgadillo et al., Citation2009). In fact, the ‘male effect’ has been recognised as a valuable technique for inducing a synchronised fertile ovulation during seasonal and post-partum anoestrous both in goats and sheep (Restall Citation1992; Walkden-Brown et al. Citation1999; Gelez and Fabre-Nys Citation2004; Scaramuzzi and Martin Citation2008). Sexually active males exposed to prepuberal (Amoah and Bryant Citation1984; Mellado et al. Citation2000) and adult females goats synchronised reproductive activity under either lactational or seasonal anoestrous (Véliz et al., Citation2002, Citation2006a, Citationb; Pellicer-Rubio et al. Citation2007), and also in ewes (Ungerfeld et al. Citation2004). A high proportion of goats exposed to a sexually active male showed estrus behaviour two or three days after joining with the buck (Chemineau Citation1983, Citation1987) and ovulated in response to a male by increases in both GnRH and LH secretion (Chemineau et al. Citation1986a, Citationb, Citation2006).
Besides that, clear links exist between metabolic fuel (glucose, pyruvate and lactate) availability and the reproductive function (Ebling Citation2005). In fact, changes in the blood levels of metabolic hormones are important signals that inform the nutritional status of mammals (Meza-Herrera et al. Citation2007a, Citationb, Citation2008; Gamez-Vazquez et al. Citation2008).An explanation is that the response to a feed supplementation alters glucose, insulin, leptin or IGF-I and probably other metabolic hormones which in turn may affect reproductive function (Meza-Herrera et al. Citation2004, Citation2008, Meza-Herrera et al. Citation2010a, Meza-Herrera et al. Citation2010b; Scaramuzzi et al. Citation2006; Guerra-García et al. Citation2009). Also, supplementation under grazing conditions has improved ovulation and pregnancy rates of Criollo goats exposed to the male effect (De Santiago-Miramontes et al., 2008; 2009; Fitz-Rodríguez et al., Citation2009). However, it has been emphasised that male ‘novelty’ could be more important than male isolation (Delgadillo et al. Citation2009). Male presence has also induced reproductive activity in melatonin-implanted Mediterranean female goats, causing a small retardation in the reactivation of reproductive activity during the natural breeding season (Zarazaga et al. Citation2009). Also, it has been observed a 2–3 month interval from summer solstice (21 June) to ovarian activity resumption in goats exposed to either natural photoperiod or controlled cycles of artificial photoperiod 6–12 months long (Escobar et al., 1997). Thus, caprine might expend two to three months to develop refractoriness. Therefore, these three major environmental factors (nutrition, photoperiod and ‘male effect’) may interact synergistically and/or antagonistically, but the precise nature of these interactions and their significance to reproductive outcomes are still not well understood (Scaramuzzi and Martin Citation2008).
Present work intended to evaluate the effect of presence of a sexually active male buck on LH secretion patterns of ovariectomised (OVX) and ovariectomised and estradiol implanted (OVX + E2-implanted) Criollo goats exposed to a controlled photoperiod regime at the subtropical Northern-Central Mexico (23° NL). Results might help to understand the male effect stimulus on the neuroendocrine responses exerted by the female Criollo goats during the seasonal reproductive state.
Materials and methods
Location, animals and treatments
The study was carried out at the University of Zacatecas located in north-central Mexico at 23° 00′ North latitude and 102° 44′ West longitude, and 2150 m above the sea level. The experiment include Criollo goats, (n=20) from a subtropical region located in arid and semiarid areas of Zacatecas State. These non-pregnant goats had an average body weight of 39±4.5 kg and were exposed to a sexually active male buck by alternated monthly photoperiodic treatments of 8 h light:16 h dark and vice versa (Delgadillo et al., Citation1993; Delgadillo et al., Citation1995; Delgadillo et al., Citation2001; Delgadillo et al., Citation2003).
Animals were located in controlled-photoperiodic chambers (i.e., controlling light-hours per day) to provide 350 lux directly over goat eyes (height). All goats were exposed to a 90-day alternating pattern of artificial long (13.4L/10.6D) for the entire study during a period of two years [six photoperiodic cycles: ascending (n=3); and descending (n=3) cycles]. The main purpose was to simulate occurrence of two consecutive natural photoperiods during a year and to evaluate the effect of a specific photoperiod upon reproductive function of goats.
Experimental design
Goats were randomly assigned into two groups: Goats non-exposed to presence of a sexually active male buck (Control group, n=10) and goats exposed to a sexually active male buck (Treated group, n=10). Within each group, there were ovariectomised goats (OVX-goats, n=5) and ovariectomised + estradiol-implanted goats (OVX + E2 goats, n=5). Both groups were exposed to a 6-month-long controlled artificial photoperiods. Thus, annual variations in light-hours per day were included in a 6-month-long controlled artificial photoperiod in order to obtain two photoperiodic cycles within a year and to evaluate variations in response to the presence or absence of a sexually active male buck.
The buck was also exposed to monthly alternated long–short photoperiodic (light–dark) treatments [long: 16 h L/8 h D; and short: 8 h L/16 h D] as previously reported by Delgadillo et al. (Citation1995). The sexually active buck was introduced to the treated group (treated goats exposed to a male buck) wearing a harness, beginning at the second photoperiodic cycle and remained with the goats throughout the day for 8 to 10 h depending upon the photoperiodic treatment received. Goats in the control group were kept permanently without a male presence.
Blood sample collection
Blood samples for LH determination were taken every 4 weeks during an intensive sampling (6 h, every 15 min; 24 samples per goat). Samples were obtained by jugular venipunction using Vacutainer® tubes and then centrifuged (1500×g, 15 min, 4°C). Blood serum was separated and stored at −20° C until the radioimmunoanalysis assay was performed.
Luteinising hormone (LH) radioimmunoassay
LH concentrations were determined by liquid phase radioimmunoanalyses in triplicate tubes. LH assay sensitivity was 0.1 ng/mL, and intra- and inter-analyses variation coefficients were 6.2% and 7.1%, respectively. LH pulse frequency, amplitude and concentration were determined using the Pulsar program developed by Merriam and Watcher, (Citation1982) and reported by Hoefler and Hallford (Citation1987).
Statistical analyses
Data were analysed using the GLM procedure of SAS as a 2×2 factorial design evaluating main effects of male presence stimulus (presence or absence) and estradiol effect (OVX and OVX + E2 implanted goats) in ovariectomised goats. When required, a Tukey test was performed in order to determine mean differences (SAS, Citation2000).
Results and discussion
Luteinising hormone (LH) secretion
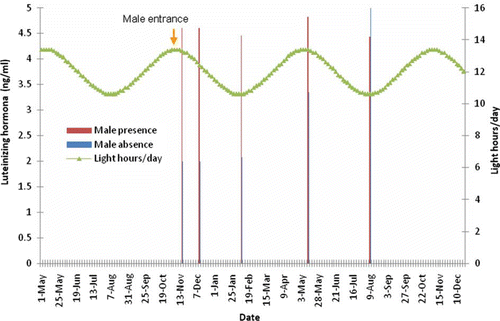
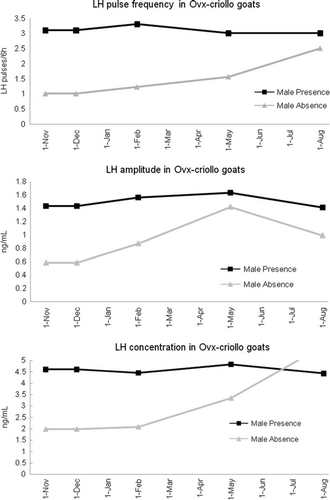
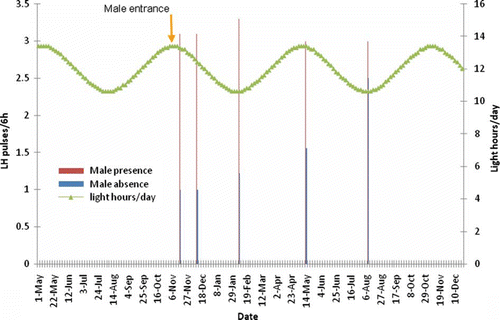
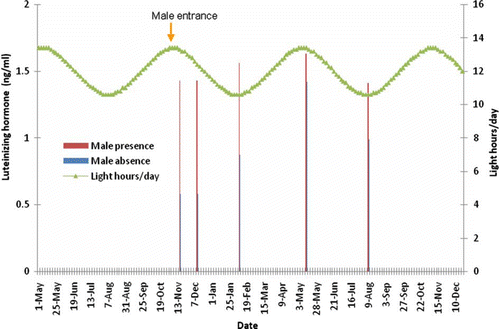
Presence of a sexually active male buck significantly increases (P<0.01) LH pulse frequency, amplitude and concentration in the OVX-goats exposed to an artificial photoperiod. However, there were no LH variations within artificial photoperiodic treatments (, , and ; , , and ).
Table 1. Effect of a sexually-active male buck presence on LH pulse frequency (F), amplitude (A) and concentration (C) (ng/ml) of ovariectomised goats exposed to controlled photoperiodic cycles (means±SD).
Ovariectomised goats
OVX-goats exposed to the presence of a sexually active male buck presented a higher LH pulse frequency (LH/6 h), amplitude and concentration, (P<0.01) than goats not exposed to a male buck (frequency: 3.2±0.4 vs. 0.7±0.1 LH pulses every 6 h; amplitude: 1.6±0.1 vs. 0.8±0.3 ng/mL; concentration: 5.3±0.6 vs. 2.0±0.9 ng/mL) (see , , and ).
Table 2. Effect of a sexually active male buck presence on LH pulse frequency (LH/6 h) on ovariectomised (OVX), and ovariectomised plus estradiol-implanted (OVX + E2) goats exposed to controlled photoperiodic cycles (means±SD).
OVX and E2-implanted goats
The OVX and E2-implanted goats (OVX + E2) exposed to a sexually active male buck only generate differences in the LH pulse frequency (P<0.05; ). In fact, no significant differences (P>0.05) were observed in relation to LH amplitude (1.2±0.2 vs. 1.1±0.5 ng/mL), or concentration (3.6±0.1 vs. 3.4±1.3 ng/mL) ( and ).
E2-implanted goats, exposed to a sexually active male buck, showed an increased LH secretion, similar to that occurred during reproductive seasonality. Thus, negative feed-back of estradiol (E2) on hypothalamus was absent during days of ascendant photoperiod (Karsch et al. Citation1984). LH concentration, pulse frequency and amplitude of OVX and E2-implanted goats were similar to those facing reproductive seasonality of goats (Chemineau Citation1983; Chemineau et al. Citation1988; Restall, Citation1992; Henniawati et al. Citation1995). Generally, presence of a sexually active male buck increased serum LH levels ().
Table 3. Effect of sexually active male buck presence on LH pulse amplitude of ovariectomised (OVX) and ovariectomised plus estradiol-implanted (OVX + E2) goats exposed to controlled photoperiodic cycles (means±SD).
OVX goats showed reduced LH levels. However, when goats were E2-implanted, LH levels increased (pulse frequency, amplitude and concentration), while the presence of a sexually active male buck considerably increased LH levels (P<0.01). Therefore, it seems that ovarian estradiol plays a crucial role in a positive feedback and hypothalamic-hypophyseal-gonadal axis activation subsequent to the anestrous season in Criollo goats. However, such E2-stimulus was insufficient because such effect was triggered only when a sexually active male was present. Such effect could be a synergic effect on reproductive activity of Criollo goats, which seems to have developed timely dependent environmental adaptative mechanisms to better sort out environmental challenges (Meza-Herrera et al. Citation2006, Citation2007a).
Table 4. Effect of a sexually active male buck presence on LH (ng/ml) concentration of ovariectomised (OVX) and ovariectomised plus estradiol implanted (OVX + E2) goats exposed to controlled photoperiodic cycles (means±SD).
Artificial photoperiodic cycles (6 months long) were implemented to simulate two natural photoperiods in a year, through alternating ascendant and descendant photoperiodic cycles (Escobar et al. Citation1997). Range of light-hours per day used for present study was 13.4 and 10.6 h L/d and was determined based on previous studies using goats in the same location, observing a photoperiodic-dependent influence on the ovarian activity of these Criollo goats (Escobar et al. Citation1997).
A descending artificial photoperiod obtained in controlled-environmental chambers is able to stimulate reproductive function in Criollo goats. However, during the reproductive season there was no continuity in the estrous cycle progression. Introduction and presence of a sexually active male buck increase reproductive activity stimuli on goats. Male introduction during a summer-solstice artificial photoperiod generated ovarian activity in an average interval of 12.4 days. Similar results were observed in sheep (Martin et al. Citation1986) and goats under natural (Chemineau Citation1987) and artificially controlled (Chemineau et al. Citation1986b) photoperiods.
Figure 1. Effect of presence of a sexually active male buck on pulse frequency, amplitude and concentration of luteinising hormone (LH) in ovariectomised (OVX) Criollo goats.
Food availability, although not considered a critical or main regulating factor, becomes also a modulating factor on reproductive function of Criollo goats (Urrutia-Morales et al. Citation2009). Nevertheless, other authors have found an increase in reproductive activity and pregnancy rates by implementing a ‘male effect’ stimulus (De Santiago-Miramontes et al., Citation2009; Fitz-Rodríguez et al., Citation2009).
Thus, presence of a sexually active male buck allow to positively increase reproductive function of native goats independently of the photoperiodic treatment, as it has been previously reported by Malpaux et al. (Citation1997) and Delgadillo et al. (Citation2002). Such effect seems to be independent of the male–female proportion (Carrillo et al. Citation2007) as well as continuity or discontinuity of the ‘male effect’ treatment (Rivas-Muñoz et al. Citation2007). Although, recently, it has been more associated with ‘novelty’ of the male (Delgadillo et al. Citation2009).
Sexual activity induction in male bucks has been previously reported using different treatments. One treatment is based on alternate photoperiodic treatments (2 months long; 16 h L/8 h D) reported by Delgadillo and Chemineau (Citation1992) in northern Mexico (26° 23′N latitude; 104° 47′ W longitude). Delgadillo et al. (Citation1993) also reported sexual activation by controlled photoperiod and melatonin treatment in male bucks (Chemineau et al. Citation1999; Flores et al. Citation2000). For present trial, the male buck received a monthly alternated photoperiodic treatment of 16 h L/8 h D in order to prevent seasonal changes on the hypothalamic-hypophyseal-gonadal axis, and improved testicular weight and semen production (Delgadillo et al. Citation1995).
Figure 2. Effect of presence of a sexually active male buck on LH pulse frequency of OVX-goats exposed to a 6-month-long artificial photoperiod.
In a previous similar study, luteal phases increased as determined by the progesterone secretion patterns demonstrating a better estrous cycle progression, an increased number of estrous cycles as well as a better reproductive outcome; all of these irrespective of the photoperiodic regime (Rincon et al., 2011).
Male buck remained 8–10 h per day interacting with goats depending upon the photoperiodic treatment, promoting a constant ovarian activity. Also, a 2–3 months interval has been observed from summer solstice (21 June) to ovarian activity resumption in goats exposed to either natural photoperiod or controlled cycles of artificial photoperiod 6–12 months long (Escobar et al. Citation1997). The last suggests that goats might expend two to three months to develop refractoriness. Male presence have also induced reproductive activity in melatonin-implanted Mediterranean female goats, causing a small retardation in the reactivation of reproductive activity during the natural breeding season (Zarazaga et al. Citation2009).
Figure 3. Effect of presence of a sexually active male buck on LH pulse amplitude of OVX-goats exposed to a 6-month-long artificial photoperiod.
An artificially controlled photoperiodic treatment to be used in all goats seems complicated and impractical. However, a real alternative could be to expose the male buck into a photoperiodic treatment (16 h L/8 h D), since the male only requires a single controlled environmental chamber. Such management practices represent a real alternative management for goats under extensive production systems. The last, intending to control both, breeding and kidding seasons to the most profitable and optimal time of the year as required by goat producers.
Conclusion
Presence of a sexually active male buck increased LH pulse frequency, amplitude and concentration in ovariectomised Criollo goats irrespective of photoperiodic regime. Therefore, male presence in OVX and E2-implanted goats may induce an increase in LH pulse frequency as that naturally observed during the breeding season.
Acknowledgements
Research was partially supported by a SIVILLA-CONACYT Grant no. 1998401010-4 and a SAGARPA-CONACYT Grant.
References
- Amoah , EA and Bryant , M . 1984 . A note on the effect of contact with male goats on occurrence of puberty in female goat kids . Animal Production , 38 : 141 – 144 .
- Aréchiga , CF , Aguilera , JI , Rincón , RM , Méndez de Lara , S , Bañuelos , R and Meza-Herrera , CA . 2008 . Role and perspectives of goat production in a global world. Tropical and Subtrop . Agroecosystems , 9 : 1 – 14 .
- Aréchiga-Flores CF , Rincón-Delgado RM 1998 . Perspectives for implementation of reproductive technologies in goats . XIII Annual National Meeting on Goat Production . Universidad Autónoma de San Luis Potosí, San Luis Potosí, S.L.P., México 13 : 12 – 37 .
- Carrillo , E , Véliz , FG , Flores , JA and Delgadillo , JA . 2007 . A diminution in the male/female ratio does not reduce the ability of sexually active male goats to induce estrus activity in anovulatory female goats . Technical Pecu Mexico , 45 : 319 – 328 .
- Chemineau , P . 1983 . Effect on oestrus and ovulation of exposing creole goats to the male at three times of the year . Journal of Reproduction Fertility , 67 : 65 – 72 .
- Chemineau , P . 1987 . Possibilities for using bucks to stimulate ovarian and oestrus cycles in anovulatory goats . A Review of Livestock Production Science , 17 : 135 – 147 .
- Chemineau , P , Baril , G , Leboeuf , B , Maurel , M.C , Roy , F , Pellicer-Rubio , M , Malpaux , B and Cognie , Y . 1999 . Implications of recent advances in reproductive physiology for reproductive management of goats . Journal Reproduction Fertility Supplement , 54 : 129 – 142 .
- Chemineau , P , Daveau , A , Cognié , Y , Aumont , G and Chesneau , D . 2004 . Seasonal ovulatory activity exists in tropical Creole female goats and Black Belly ewes subjected to a temperate photoperiod . BMC Physiology , 4 : 1 – 12 .
- Chemineau , P , Levy , F and Thimonier , J . 1986a . Effects of anosmia on LH secretion, ovulation and oestrus behaviour induced by males in the anovular Creole goats . Animal Reproduction Science , 10 : 125 – 132 .
- Chemineau , P , Martin , GB , Saumande , J and Norman , TE . 1988 . Seasonal and hormonal control of pulsatile LH secretion in the dairy goat (Capra hircus) . Journal Reproduction Fertility , 83 : 91 – 98 .
- Chemineau , P , Norman , TE , Ravault , JP and Thimonier , J . 1986b . Induction and persistence of pituitary and ovarian activity in the out-of-season lactating dairy goat after a treatment combining a skeleton photoperiod, melatonin and the male effect . Journal Reproduction Fertility , 78 : 497 – 504 .
- Chemineau , P , Pellicer-Rubio , MT , Lassoued , N , Khaldi , G and Monniaux , D . 2006 . Male-induced short oestrous and ovarian cycles in sheep and goats: a working hypothesis . Review Reproduction Nutrition Devevelopment , 46 : 417 – 429 .
- De Santiago-Miramontes , MA , Rivas-Muñoz , R , Muñoz-Gutiérrez , M , Malpaux , B , Scaramuzzi , RJ and Delgadillo , JA . 2009 . The ovulation rate in anoestrous female goats managed under grazing conditions and exposed to the male effect is increased by nutritional supplementation . Animal Reproduction Science , 116 : 85 – 94 .
- De Santiago-Miramontes MA , Rivas-Muñoz R , Muñoz-Gutiérrez M , Malpaux B , Scaramuzzi RJ , Delgadillo JA . 2008 . The ovulation rate in anoestrous female goats managed under grazing conditions and exposed to the male effect is increased by nutritional supplementation . Animal Reproduction Science 105 : 409 – 416 .
- Delgadillo , JA , Carrillo , E , Moran , J , Duarte , G , Chemineau , P and Malpaux , B . 2001 . Induction of sexual activity of male creole goats in subtropical northern Mexico using long days and melatonin . Journal of Animal Science , 79 : 2245 – 2252 .
- Delgadillo , JA and Chemineau , P . 1992 . Abolition of the seasonal release of luteinizing hormone and testosterone in Alpine goats (Capra hircus) by short photoperiodic cycles . Journal of Reproduction Fertility , 94 : 45 – 55 .
- Delgadillo , JA , Cortez , ME , Duarte , G , Chemineau , P and Malpaux , B . 2004a . Evidence that the photoperiod controls the annual changes in testosterone secretion, testicular and body weight in subtropical male goats . Reproduction Nutrition Development , 44 : 183 – 193 .
- Delgadillo , JA , Fitz-Rodríguez , G , Duarte , G , Véliz , FG , Carrillo , E , Flores , JA , Vielma , J , Hernandez , H and Malpaux , B . 2004b . Management of photoperiod to control caprine reproduction in the subtropics . Reproduction Fertility Development , 16 : 471 – 478 .
- Delgadillo , JA , Flores , JA , Véliz , FG , Duarte , G , Vielma , J , Hernandez , H and Fernandez , IG . 2006 . Importance of the signals provided by the buck for the success of the male effect in goats . Review Reproduction Nutrition Development , 46 : 391 – 400 .
- Delgadillo , JA , Flores , JA , Veliz , FG , Hernández , HF , Duarte , G , Vielma , J , Poindron , P , Chemineau , P and Malpaux , B . 2002 . Induction of sexual activity in lactating anovulatory female goats using male goats treated only with artificially long days . Journal of Animal Science , 80 : 2780 – 2786 .
- Delgadillo , JA , Gelez , H , Ungerfeld , R , Hawken , PA and Martin , GB . 2009 . The ‘male effect’ in sheep and goats–Revisiting the dogmas . Review Behaviour Brain Research , 2009 : 304 – 314 .
- Delgadillo , JA , Hochereau-de Reviers , MT , Daveau , A and Chemineau , P . 1995 . Effect of short photoperiodic cycles on male genital tract and testicular parameters in male goat (Capra hircus) . Reproduction Nutrition Development , 35 : 549 – 558 .
- Delgadillo , JA , Leboeuf , B and Chemineau , P . 1993 . Maintenance of sperm production in bucks during a third year of short photoperiodic cycles . Reproduction Nutrition Development , 33 : 609 – 617 .
- Delgadillo-Sánchez , JA , Flores-Cabrera , JA , Véliz-Deras , FG , Duarte-Moreno , G , Vielma-Sifuentes , J , Poindron-Massot , P and Malpaux , B . 2003 . Control de la reproducción de los caprinos del subtrópico mexicano utilizando tratamientos fotoperiódicos y efecto macho . Veterinary Mexico , 34 : 69 – 79 .
- Ebling , FJ . 2005 . The neuroendocrine timing of puberty . Reproduction , 129 : 675 – 683 .
- Escobar FJ , Zarco L , Valencia J 1997 . Effect of photoperiod on reproductive seasonality of criollo goat in Mexico . XXII Annual Meeting of Academy for Reproductive Biology Research . Acapulco, Guerrero, México, AIBIR .
- Fitz-Rodríguez , G , De Santiago-Miramontes , MA , Scaramuzzi , RJ , Malpaux , B and Delgadillo , JA . 2009 . Nutritional supplementation improves ovulation and pregnancy rates in female goats managed under natural grazing conditions and exposed to the male effect . Animal Reproduction Science , 116 : 85 – 89 .
- Flores , JA , Veliz , FG , Perez-Villanueva , JA , Martinez de la Escalera , G , Chemineau , P , Poindron , P , Malpaux , B and Delgadillo , JA . 2000 . Male reproductive conditions is the limiting factor of efficiency in the male effect during seasonal anestrus in female goats . Biology of Reproduction , 62 : 1409 – 1414 .
- Gamez-Vazquez , HG , Rosales-Nieto , CA , Bañuelos-Valenzuela , R , Urrutia-Morales , J , Diaz-Gomez , MO , Silva-Ramos , JM and Meza-Herrera , CA . 2008 . Body condition score positively influence plasma leptin concentrations in criollo goats . Journal of Animal Veterinary Advances , 7 : 1237 – 1240 .
- Gelez , H and Fabre-Nys , C . 2004 . The “male effect” in sheep and goats: a review of the respective roles of the two olfactory systems . Hormone and Behaviour , 46 : 257 – 271 .
- Guerra-García , M , Meza-Herrera , CA , Sanchez-Torres-Esqueda , MT , Gallegos-Sanchez , J , Torres-Hernandez , G and Pro-Martinez , A . 2009 . IGF-1 and ovarian activity of goats in divergent body condition and supplemented with non-degradable ruminal protein . Agrociencia , 43 : 241 – 247 .
- Henniawati , H , Restall , BJ and Scaramuzzi , RJ . 1995 . Effect of season on LH secretion in ovariectomized Australian cashmere does . Journal Reproduction and Fertility , 103 : 349 – 356 .
- Hoefler , WC and Hallford , DM . 1987 . Influence of suckling status and type of birth on serum hormone profiles and return to estrus in early-postpartum, spring-lambing ewes . Theriogenology , 27 : 887 – 895 .
- Karsch , FJ , Bittman , EL , Foster , DL , Goodman , RL , Legan , SJ and Robinson , JE . 1984 . Neuroendocrine basis of seasonal reproduction . Recent Progress in Hormone Research , 40 : 185 – 232 .
- Malpaux , B , Viguié , C , Skinner , DC , Thiéry , JC and Chemineau , P . 1997 . Control of the circannual rhythm of reproduction by melatonin in the ewe . Brain Research Bulletine , 44 : 431 – 438 .
- Martin , G , Oldman , C , Cognie , Y and Pearce , D . 1986 . The physiological responses of anovulatory ewes to the introduction of rams . Review Livestock Production Science , 15 : 219 – 247 .
- Mellado , M , Olivas , R and Ruiz , F . 2000 . Effect of buck stimulus on mature and pre-pubertal norgestomet-treated goats . Small Rumenia Research , 36 : 269 – 274 .
- Merriam GR , Watcher KW . 1982 . Algorithms for the study of episodic hormone secretion . American Journal of Physiology 243 : E310 – E318 .
- Meza-Herrera , CA , Bocanegra , JA , Bañuelos , R , Aréchiga , CF , Rincón , RM , Ochoa-Cordero , MA , Juárez-Reyes , AS , Cerrillo-Soto , MA and Salinas , H . 2007a . Circannual fluctuations in serum cortisol and glucose concentrations and hair coat growth in goats . Journal of Applied Animal Research , 31 : 79 – 82 .
- Meza-Herrera CA , Gonzalez-Bulnes A , Kridli R , Mellado M , Arechiga-Flores CF , Salinas H , Luginbhul JM 2010b . Neuroendocrine, metabolic and genomic cues signaling the onset of puberty in females . Reproduction in Domestic Animals . doi: 10.1111/j.1439-0531.2009.01355.x
- Meza-Herrera , CA , Hallford , DM , Ortiz , JA , Cuevas , RA , Sanchez , JM , Salinas , H , Mellado , M and Gonzalez-Bulnes , A . 2008 . Body condition and protein supplementation positively affect periovulatory ovarian activity by non-LH mediated pathways in goats . Animal Reproduction Science , 106 : 412 – 420 .
- Meza-Herrera , CA , Martínez , L , Aréchiga , C , Bañuelos , R , Rincón , RM , Urrutia , J , Salinas , H and Mellado , M . 2006 . Circannual identification and quantification of constitutive heat shock proteins (HSP 70) in goats . Journal of Applied Animal Research , 29 : 9 – 12 .
- Meza-Herrera , CA , Ross , T , Hallford , DM , Hawkins , D and Gonzalez-Bulnes , A . 2007b . Effects of body condition and protein supplementation on LH secretion and luteal function in sheep . Reproduction in Domestic Animals , 42 : 461 – 465 .
- Meza-Herrera , CA , Sanchez , JM , Chavez-Perches , JG , Salinas , H and Mellado , M . 2004 . Protein supplementation, body condition and ovarian activity in goats. Preovulatory serum profile of insulin . South African Journal of Animal Science , 34 ( Suppl. 1 ) : 223 – 226 .
- Meza-Herrera , CA , Veliz-Deras , FG , Wurzinger , M , Lopez-Ariza , B , Arellano-Rodriguez , G and Rodriguez-Martinez , R . 2010a . The kiss-1-kisspeptin-gpr54 complex: a critical modulator of GnRH neurones during pubertal activation . Journal of Applied Biomedical , 8 : 1 – 9 .
- Pellicer-Rubio , MT , Leboeuf , B , Bernelas , D , Forgerit , Y , Pougnard , JL , Bonné , JL , Senty , E and Chemineau , P . 2007 . Highly synchronous and fertile reproductive activity induced by the male effect during deep anoestrus in lactating goats subjected to treatment with artificially long days followed by a natural photoperiod . Animal Reproduction Science , 98 : 241 – 258 .
- Restall BJ 1992 . The male effect in goats . Wilson RT , Bourza TD . V International Conference on Goats . New Delhi, , India
- Rincón RM , Aréchiga CF , Escobar FJ , Silva JM , Aguilera-Soto JI , Lopez-Carlos MA , Rodríguez H , Meza-Herrera CA , Valencia J . 2011 . The male effect stimulus positively affected hypothalamic and ovarian function in Criollo goats irrespective of photoperiodic regime . Journal of Forestry and Environmental Science (in press)
- Rivas-Muñoz , R , Fitz-Rodríguez , G , Poindron , P , Malpaux , B and Delgadillo , JA . 2007 . Stimulation of estrous behaviour in grazing female goats by continuous or discontinuous exposure to males . Journal of Animal Science , 85 : 1257 – 1263 .
- SAS , 2000 . SAS/STAT® User's Guide (8.1 Edition) . SAS Inst. Inc. Cary, NC, , USA
- Scaramuzzi , RJ , Campbell , BK , Downing , JA , Kendall , NR , Khalid , M , Muñoz-Gutierrez , M and Somchit , A . 2006 . A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate . Reproduction Nutrition Development , 46 : 339 – 354 .
- Scaramuzzi , RJ and Martin , GB . 2008 . The importance of interactions among nutrition, seasonality and socio-sexual factors in the development of hormone-free methods for controlling fertility . Reproduction in Domestic Animals , 43 : 129 – 136 .
- Ungerfeld , R , Forsberg , M and Rubianes , E . 2004 . Overview of the response of anoestrous ewes to the ram effect . Reproduction Fertility Development , 16 : 479 – 490 .
- Urrutia-Morales , J , Meza-Herrera , CA , Escobar-Medina , FJ , Gamez-Vazquez , HG , Ramírez-Andrade , BM , Diaz-Gomez , MO and Gonzalez-Bulnes , A . 2009 . Relative roles of photoperiodic and nutritional cues in modulating ovarian activity in goats . Reproduction Biology , 9 : 283 – 294 .
- Valencia , J , González , JL and Díaz , J . 1986 . Actividad reproductiva de la cabra criolla en México en el examen postmortem del aparato genital . Veterinary México , 17 : 177 – 189 .
- Valencia , J , Zarco , L , Ducoing , A , Murcia , C and Navarro , H . 1990 . “ Breeding season of criollo and granadina goats under constant nutritional levels in the Mexican highlands ” . In International Atomic Energy Agency, Livestock Reproduction in Latin America , 321 – 333 . Viena : International Atomic Energy Agency .
- Véliz , FG , Moreno , S , Duarte , G , Vielma , J , Chemineau , P , Poindron , P , Malpaux , B and Delgadillo , JA . 2002 . Male effect in seasonally anovulatory lactating goats depends on the presence of sexually active bucks, but not estrous females . Animal Reproduction Science , 72 : 197 – 207 .
- Véliz , FG , Poindron , P , Malpaux , B and Delgadillo , JA . 2006a . Maintaining contact with bucks does not induce refractoriness to the male effect in seasonally anestrous female goats . Animal Reproduction Science , 92 : 300 – 309 .
- Véliz , FG , Poindron , P , Malpaux , B and Delgadillo , JA . 2006b . Positive correlation between the liveweight of anestrous goats and their response to the male effect with sexually active bucks . Reproduction Nutrition Development , 6 : 1 – 6 .
- Walkden-Brown , SW , Martin , GB and Restall , BJ . 1999 . Role of male-female interaction in regulating reproduction in sheep and goats . Journal of Reproduction Fertility Supplement , 52 : 243 – 257 .
- Zarazaga , LA , Gatica , MC , Celi , I , Guzman , JL and Malpaux , B . 2009 . Effect of melatonin implants on sexual activity in Mediterranean goat females without separation from males . Theriogenology , 72 : 910 – 918 .