Abstract
Reproductive cycle in female and male Caspian brown trout (Salmo trutta caspius) was investigated by sampling blood plasma and gonadal tissue from 4-year-old fish. The reproductive cycles of both female and male Caspian brown trout are characterised by distinct variations in gonadal size and developmental stage and these are associated with changes in sex steroids. In females, ovarian development progressed merely until vitellogenesis stage while male fish completed testicular development and produced milt at January. Milt had good quality in terms of percentage and duration of motility, milt volume and sperm density. In females, the average diameter of the largest follicles increased steadily throughout the course of experiment and was coincident with changes in the concentration of sex steroids. Beginning in September, plasma testosterone (T) elevated from October to November and then remained unchanged until the end of the experiment. With a one month lag than T, the increasing trend of plasma estradiol (E2) was observed in September to October and then E2 levels decreased steadily for the rest of the experimental period. Plasma progesterone (P) levels were low throughout August to November, but sudden increases were observed during December and January. The concentrations of plasma calcium (Ca) increased significantly during October and November but then declined steadily until the end of experiment. In males, Plasma T increased rapidly, culminating in a transient peak in December. This peak was followed by a steady decline afterwards. Such increasing pattern was observed after October for P. Although E2 concentrations were low throughout the experiment, a weak peak was observed in October. The gonadosomatic index increased continuously for both female and male Caspian brown trout during gonadal development. In conclusion, our results showed that unlike 4-year-old female Caspian brown trout, the male fish can complete final maturation under cultural conditions in the hatchery. Probably, female fish would acquire this ability in ages more than 4 years.
Introduction
The Caspian brown trout, Salmo trutta caspius, is a critically endangered anadromous species that has been considered for a biological conservation programme in the southern part of the Caspian Sea (Kiabi et al. Citation1999; Niksirat and Abdoli Citation2009). In recent years, because of dramatic declines in broodstock capture and subsequently of insufficient availability to sexual materials (sperm and ova), the aquaculturists have produced brooders from fertilised eggs in the cultural conditions in the hatchery. Nevertheless, the investigation of the efficiency of this strategy is necessary since hatchery conditions are apparently different from wild environment and may affect the process of gonadal maturation. For example, under culture condition, adult fishes are exposed to various types of stressors such as confinement, crowding, handling, biopsy, transportation and hormonally induced spawning. It is now clearly established that there is an inhibitory effect of stress on reproductive processes in teleost fishes, with effects ranging from depression of endocrine function to reduction of gamete and larval quality (Pankhurst and Van Der Kraak Citation1997). Several researchers have investigated fish maturation by histological and hormonal observations in the cultural environment to the evaluation of the efficiency of reproduction (Matsuyama et al. Citation1991; Kozul et al. Citation2001; Dahle et al. Citation2003). Dahle et al. (Citation2003) investigated the gonadal development and associated changes in sex steroids during the reproductive cycle of captive Atlantic cod, Gadus morhua L. Histological observations in Red porgy, Pagrus pagrus presented a good background of reproductive cycle from a multiple spawner species (Kokokiris et al. Citation2000). Reliable indicators of reproductive status are essential for good fisheries management and they are also important to evaluate the effects of different treatments on sexual maturation in fish farming. Consequently, the aim of this work was to describe changes in sexual steroids in relation to oocyte (size and appearance) and testicular germ cell development in Caspian brown trout.
Materials and methods
Brooders
Adults of cultured Caspian brown trout of 4 years old were used. These adults were larvaes that had been propagated simultaneously from wild fish and then cultured for 4 years in the outdoor tanks under natural photoperiod and water temperature at the experimental facilities of the Kalardasht Salmonid Reproduction Center (KSRC), Iran. In brooder pond, adult fishes were fed to satiation for 5 days a week with commercially available dry feed (BioMar).
Gonads histology and blood sampling
Ten individuals of adult Caspian brown trout (Total weight: 110–251 g; Total Length: 10–15 cm) from bloodstock's pond (2 m×3 m×1 m with Water flow: 100 L/min; Temperature range: 2–15°C; Dissolved oxygen (mg/L): 9.2–9.5) were randomly collected monthly from August to January 2007 (Totally 60 fish). After anesthetisation with 100 ppm of MS222 (tricaine methane sulphonate, Sigma, Deisenhofen, Germany), fishes were measured for body weight (BW; g) and gonadal weight (GW; g). The GSI values were calculated as follows: GSI = GW/BW×100.
Ovaries and testes of monthly collected fish were fixed in Bouin's solution, embedded in paraffin wax, sectioned at 6 µm thick, and stained with hematoxylin and eosin. The slides of testicular and ovarian tissues were examined and classified into stages of spermatogenic and ovariogenic development based on criteria of Yamamoto et al. (Citation1965) and Bromage and Gumaranatunga (Citation1988). The blood samples were collected monthly by cutting of caudal peduncle and centrifuged (13,700g for 10 min) to separate the serum and this was stored at –20°C until hormonal analysis.
Steroid assays
Direct Radio Immuno Assay method (DRIA) which was a simplified method of Canario and Scott (Citation1989) was used method according to Stanczyk et al. (Citation2007). This does not incorporate a purification step (extraction) before quantification of the analysis. Assay kits of T, P and E2 were obtained from IMMUNOTECH, France.
Kit properties
Testosterone kit
Sensitivity: 0.04 ng mL−1; specificity: mainly cross-reactivity with 5α-dihydrotestosterone: 10%, 19-nortestosterone: 5%, 11β-hydroxytestosterone: 2%, methyltestosterone: 2%, 5α-androstane-3β, 17β-diol: 2%, 5β-androstane-3α, 17β-diol: 1%, 5α-androstane-3, 17β-diol: 1%, Δ4 androstenedione: 0.6%.
Progesterone kit
Sensitivity: 0.03ng mL−1; specificity: 5α-pregnanedione: 13.8%, 5β-pregnanedione: 7.7%, corticosterone: 1.81%, 20α-dihydroprogesterone: 0.96%, 11-deoxycorticosterone: 0.96%, 16α-hydroxyprogesterone: 0.85% pregnenolone: 0.67%, 6β-hydroxyprogesterone.
Estradiol kit
Sensitivity: 6 pg mL−1, specificity: mainly Cross-reactivity with: Estrone: 2.3% Estriol: 0.26%, Estradiol 3-glucuronide: 0.033%, Estradiol 17-glucuronide: 0.033%, Estradiol sulphate: 13.5% with other 19 steroids, cross-reactivity was less than 0.1%.
Calcium assay
Calcium values were measured using arsenazo method. In this method, calcium reacts with Arsenazo III in a slightly alkaline medium to form a purple-coloured complex which absorbs at 650 nm. The intensity of the colour is proportional to the calcium concentration.
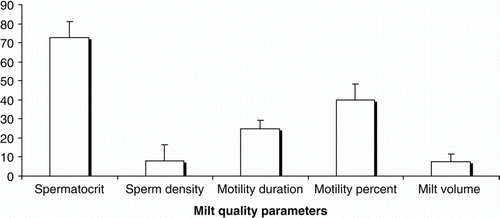
Milt quality assay
In January, the ripped males were stripped for milt collection. Snout and peduncle of each fish was held tightly by hand and then fishes were belly-massaged from the anterior portion of the testis towards the genital papilla. Care was taken to avoid contamination of the milt with water, mucus, blood cells, faeces and urine. A two-step dilution was used for motility activation according to the method suggested by Billard and Cosson (Citation1992). Firstly, the milt was prediluted with a saline solution (composed of 80 mM NaCl, 40 mM KC1, l mM CaC12 and 20 mM Tris-HCl per 1 L of distilled water (final pH = 9)) in a ratio of 1/100 and secondly, the prediluted milt was subjected to a second dilution in a physiological serum at a ratio of 1/20 and immediately 1 µL of solution was placed on the microscopic stage. Motility was analysed according to the methods of Rurangwa et al. (Citation2004) and recorded by a video camera coupled to the optical lens of microscope (HE ×40). At the end, the video recordings were reviewed and the motility was recorded as the percentage and duration of motility. The sperm motility was evaluates 5 s after activation and only forward-moving sperm were judged motile, those simply vibrating or turning on their axes were considered immotile (Aas et al. Citation1991). Milt volume was measured in scaled vials. Spermatocrit was determined by centrifuging milt for 10 min at 5000 rpm in a haematocrit centrifuge (D-78532, Tuttlingen Zentrifugen, Germany) according to Piironen (Citation1985) and sperm density in a haemocytometer counting chamber according to Caille et al. (Citation2006).
Statistical analysis
The SPSS software (version 16) was used to analyse data. All parameters were normal according to Kolmogorov–Smirnov test, but data measured in percentage scale (spermatocrit, percentage of motile spermatozoa and GSI) were converted by angular transformation (arcsin√p) prior to analysis. The p<0.05 was used as significance level for data evaluation. One-way ANOVA was employed to analyse data. Then, means were compared by Tukey's test.
Results
Gonadal development
Females
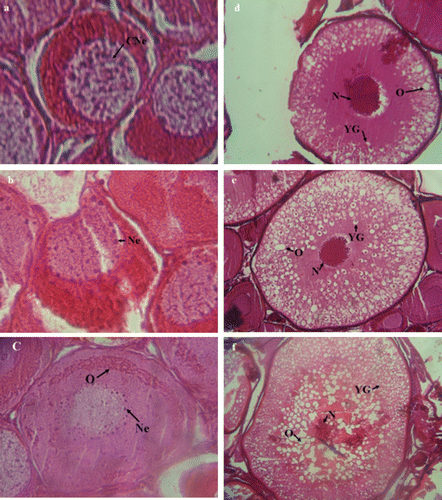
Histological observations indicated six main stages of ovarian maturity: stage I: most advanced oocytes at Chromatin nucleolus stage (a). They were 150–200 µm in diameter, with a spherical nucleus occupying his greater part. Stage II: most advanced oocytes at Perinucleolus stage (b). The Oocyte had 220–250 µm in diameter with cytoplasm strongly stained. The nucleus was less basophilic, with some nucleoli spread around periphery. Stage III: most advanced oocytes at Oil droplet stage (c). Oocyte 603–654 µm in diameter, with a cytoplasm less basophilic. Oil-droplets in cytoplasm appeared as an arch of unstained empty vacuoles around nucleolus. Phases IV: most advanced oocytes at Cortical alveoli stage (Early yolk globule stage) (d). the Oocytes had 902–1207 µm in diameter. The oil droplets were distributed throughout cytoplasm. The yolk globules start appearing in oocyte, first in peripheral cytoplasm as small granules stained with hematoxylin and after accumulating in cytoplasm filling spaces between oil droplets. Stage V: most advanced oocytes were at mid yolk globule stage (e) and they had 1000–1202 µm diameter. Due to the distribution and number of yolk globules throughout cytoplasm, the oocytes showed an increased in their size. Stage VI: The Oocytes were 1010–1250 µm in diameter and their size increased rapidly. Yolk accumulation increased and more Oil-droplets were accumulated around the nucleolus (f).
Figure 1. Cross section of ovary of Caspian brown trout (Salmo trutta caspius) at different stages of development. (a) Stage I: Chromatin nucleolus stage (in Out). (b) Stage II: Perinucleolus stage (in September). (c) Stage III: Oil droplet stage (in October). (d) Stage IV: Cortical alveoli stage (Early yolk globule stage) (in November). (e) Stage V: mid yolk globule stage (in December). (f) Stage VI: advanced mid yolk globule stage (in January). See text for description of stages. Sg, spermatogonia; Sc, spermatocyte; St, spermatid and Sz, spermatozoa. YG, yolk globule; N, nucleus’ CNe, chromatin nucleolus; Ne, nucleolus; O, oil droplets. HE ×40.
Males
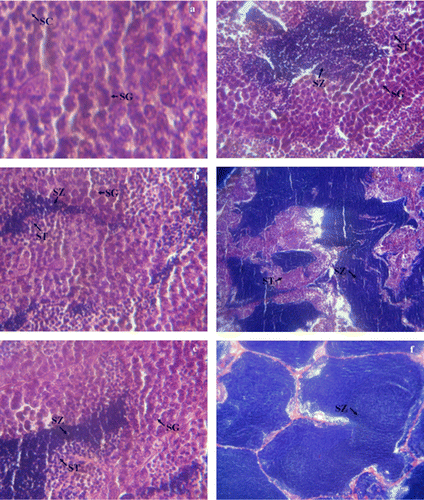
The relative abundance of each type of spermatogenic cyst (e.g., spermatogonia, spermatocytes, spermatids, spermatozoa) was considered for determination of testicular development stages. Male cultured Caspian brown trout completed all testicular development over the course of experiment. Histological observations indicated four main stages of testicular maturity: stage I (Spermatogonial proliferation): Only spermatogonia occupied all tissue of testicular lobules (a). In the Stage II (Mid spermatogenesis stage): the early spermatogenesis stage was not observed from September to October. It could be due to the prolifereation of transientional spermatogonia to more advanced stages of spermatogenesis. Various rations of testicular cysts including spermatogonia, spermatocytes, spermatids and spermatozoa were observed, although, spermatogonia decreased gradually during these two months (b–d). Stage III (Late spermatogenesis stage): in November, only spermatids and spermatozoa were observed in all the tissues examined (e). Stage IV (Prespermiation stage): It was observed in December, and testis tissues are only full filled with spermatozoa (f). In January, The cultured males produced milt and their quality parameters were shown in .
Figure 2. Cross section of testes of Caspian brown trout (Salmo trutta caspius) at different stages of development. (a) Stage I: Spermatogonial proliferation (in Out), (b) (c) and (d) stage II: Mid spermatogenesis stage (during September to October), (e) stage III: Late spermatogenesis stage (in November); (f) stage IV: Prespermiation stage (in December). See text for description of stages. Sg, spermatogonia; Sc, spermatocyte; St, spermatid; Sz, spermatozoa. HE ×40.
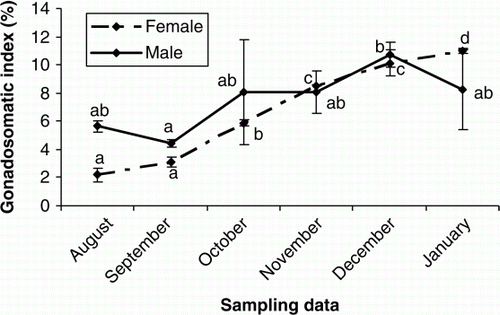
Changes in GSI during the experimental period
The GSI values increased continuously for both female and male Caspian brown trout during gonadal development (p<0.05), although in males it was not statistically significant from August to November (p>0.05) ().
Plasma steroids
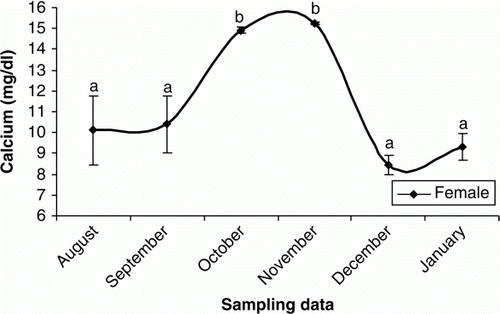
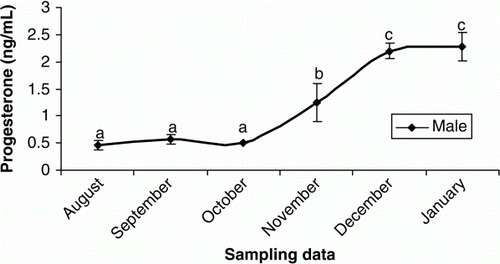
Changes of serum calcium in females and serum sex steroids during ovarian and testicular development
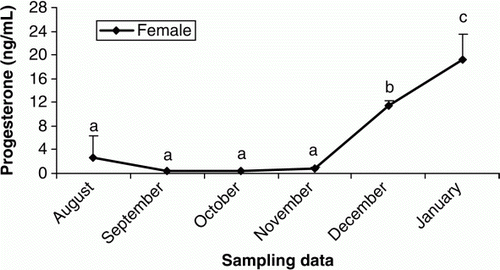
In females, plasma T was elevated from October to November and then remained unchanged until the end of the experiment (p < 0.05) (). The increasing trend of plasma E2 were observed in September to October (p < 0.05) and then E2 levels decreased steadily until the rest of the experimental period (p > 0.05) (). Plasma P levels were low throughout August to November (p > 0.05), but sudden increases were observed during December and January (p < 0.05) (). The concentrations of plasma Ca increased significantly during October and November (p<0.05) but then declined steadily until the end of experiment (p > 0.05) (). In males, the plasma testosterone T increased rapidly, beginning in October and culminating in a transient peak in December that was followed by a steady decline afterwards (p < 0.05) (). Such increasing pattern was observed after October for P (p<0.05) (). Although E2 concentrations were low throughout the experiment (p>0.05), a weak peak was observed in October (p<0.05) ().
Figure 5. Changes in plasma T of male and female cultured Caspian brown trout during the experimental period. Means with same superscripts are not significantly different (p>0.05).
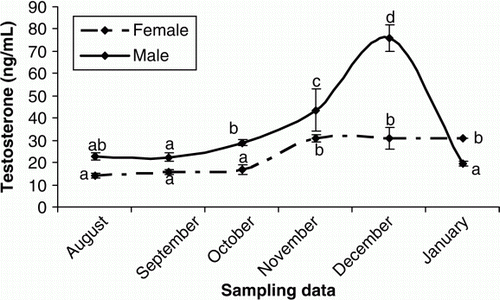
Figure 6. Changes in plasma E2 of male and female cultured Caspian brown trout during the experimental period. Means with same superscripts are not significantly different (p>0.05).
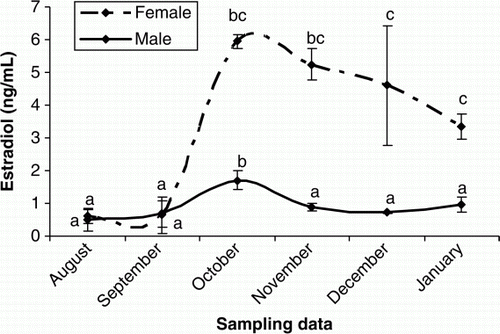
Figure 7. Changes in plasma P of female cultured Caspian brown trout during the experimental period. Means with same superscripts are not significantly different (p>0.05).
Discussion
Histological observations showed that male Caspian brown trout completed the testicular development stages during a period of six month and produced milt with desirable quality in terms of sperm density, milt volume, percentage and duration of motility. These findings suggest that male cultured Caspian brown trout acquire final maturation of testis with 4 years old. In contract, the follicular development progressed until vitellogenesis, indicating that female cultured Caspian brown probably acquired final maturation with more than 4 years. The increase of GSI confirmed the gonadal development for both female and male cultured Caspian brown trout.
In fish life cycle, the age at first maturity is in relation to genetic properties (Glebe and Saunders Citation1986; Ritter et al. Citation1988) and biological and environmental factors (Chadwick et al. Citation1986). A negative linear relationship between growth rate and age at first maturation was reported in rainbow trout, Oncorhynchus mykis (Sylven and Elvingson Citation1991) and Atlantic salmon, Salmo salar (Duncan et al. Citation2002). In this regard, as the growth rate increases, the gonad maturation occurs in younger ages. In this study, in spite of the age similarity between the fish of cultured and a group of wild Caspian brown trout in the hatchery, the cultured fish had lower body weight than wild individuals (data were not presented). In addition, the wild fish produced ova during the spawning season while the cultured females merely complete the ovarian development until vitellogenesis. These data suggest a negative relationship between growth rate and age at first maturation in cultured female Caspian brown trout as reported previously for rainbow trout (Sylven and Elvingson Citation1991) and Atlantic salmon (Duncan et al. Citation2002). Nevertheless, it is likely that such negative relationship is not much evident for cultured male Caspian brown trout since they produced milt at age-4+ in spite of their low weighs.
According to our histological observations, cultured males completed testicular development in six month and produced milt in January. In December, all male fishes were in prespermiation stage, that is entire testicular tissue was full filled with non-motile spermatozoa. The prespermiation stage is the appropriate time for artificial induction of fish spawning since in this stage brooders show best response to hormonal treatments such as gonadotropin releasing hormone (GnRHa) or pituitary preparation treatment (Mojazi Amiri et al. Citation1996; Etiënne et al. 2004). December was the appropriate time for artificial spawning induction of cultured male Caspian brown trout, producing a synchronisation that is an efficient tool for synchronisation of spawning and the induction of fishes that that are not capable to spawn naturally. Synchronisation of spawning is essential strategy for successful management of brooders and saving of propagation costs in aquaculture.
In male teleosts is well recognised that T And 11-KT are the predominant androgens. T is a precursor of 11-KT and could be involved in spermatogenesis since 11-KT acts in the initiation of spermiation (Nagahama Citation1983; Yaron Citation1995). In our study, the elevation of plasma T from October to December was coincident with the increase in number of spermatids and spermatozoa, suggesting the important role of this steroid in the spermatogenic cycle. The low T levels observed in January could be due to the shift of steroidogenesis pathways from androgens to progestins during the final maturation. In female Caspian brown trout, T showed an increasing trend during the experimental period, although T concentrations were lower. Studies on some teleosts demonstrated that T has probably a key role in formation of oil droplets in oocytes and stimulation of follicle stimulating hormone (FSH) involved in vitellogenesis (Crim and Evans Citation1979). On the other hand, it was recognised that T can act as precursor of E2 in follicular layers (Kagawa et al. Citation1982, Citation1983). In female teleosts, sexual maturation is driven by a gonadotropin-induced increase in plasma E2 levels. E2 stimulates the liver production of vitellogenin (VTG) which is a yolk protein precursor that is released to the circulation, producing an increase of total plasma. VTG is subsequently sequestered by the oocytes, processed and stored for the nutrition of the embryo (Nagahama Citation1987; Persson et al. Citation1998). In fact, there is a positive relationship between VTG concentration and plasma Ca during vitellogenesis stage (Linares-Casenave et al. Citation2003). Similar change trends in the plasma E2 and Ca were identified in our study. According to histological observations, E2 increases were coincident with initiation of vitellogenesis stage. Thus, it is likely that twin elevation of E2 and Ca from October to November could be in relation to the VTG production. The P hormone is usually predominant in female teleosts that acts as a precursor of other steroids in the steroidogenic pathways (Manire and Rasmussen Citation1997; Gelsleichter Citation2004; Henningsen et al. Citation2008). In female Caspian brown trout, the plasma P levels were low from August to November, while the E2 and T were in their maximum levels, suggesting that the metabolic conversion rate of P to T and E2 was higher during this period.
Conclusion
The gonad histologic observations and the associated changes in sex steroids and GSI showed that the male cultured Caspian brown trout can complete testicular maturation in the hatchery conditions at age-4+. December was the appropriate time for artificial induction of spawning by hormonal treatments due to all examined males was in prespermiation stage. In the female cultured Caspian brown trout, the ovarian development progressed merely until vitellogenesis stage, indicating that they acquired the ability of spawning in ages more than 4 years old.
Acknowledgements
The authors express their sincere appreciation to the people who gave their time, advice and support to this study, including: the manager (Mr Rezvani) and staff of the Kalardasht Salmonids Reproduction Center (KSRC), Iran.
References
- Aas , GH , Refstie , T and Gjerde , B . 1991 . Evaluation of milt quality of Atlantic salmon . Aquaculture , 95 : 125 – 132 .
- Billard , R and Cosson , MP . 1992 . Some problems related to the assessment of sperm motility in freshwater fish . Journal of Experimental Zoology , 261 : 122 – 131 .
- Bromage , N and Gumaranatunga , R . 1988 . “ Egg production in the rainbow trout ” . In Recent advances in aquaculture , Edited by: Miur , J and Roberts , RJ . 63 – 138 . London : Croom Helm .
- Caille , N , Rodina , M , Kocour , M , Gela , D , Flajshans , M and Linhart , O . 2006 . Quantity, motility and fertility of tench (Tinca tinca) sperm in relation to LHRH analogue and carp pituitary treatments . Aquaculture International , 14 : 75 – 87 .
- Canario , AVM and Scott , AP . 1989 . Synthesis of 20-hydroxylated steroids by ovaries of the dab (Limanda limanda) . General and Comparative Endocrinology , 76 : 147 – 158 .
- Chadwick , EMP , Randall , RG and Leger , C . 1986 . Ovarian development of atlantic salmon, Salmo salar smolts and age at first maturity. In: Meerburg DJ, editor. Salmonid age at maturity . Canadian Special Publication of Fisheries and Aquatic Sciences , 89 : 15 – 23 .
- Crim , LW and Evans , DM . 1979 . Stimulation of pituitary gonadotropin by testosterone in juvenile rainbow trout (Salmo gairdneri) . General and Comparative Endocrinology , 37 : 192 – 196 .
- Dahle , R , Taranger , GL , Karlsen , O , Kjesbu , OS and Norberg , B . 2003 . Gonadal development and associated changes in liver size and sexual steroids during the reproductive cycle of captive male and female Atlantic cod (Gadus morhua L.) . Comparative Biochemistry and Physiology Part A , 136 : 641 – 653 .
- Duncan , NJ , Thrush , MA , Elliott , JAK and Bromage , NR . 2002 . Sea water growth and maturation of atlantic salmon, Salmo salar transferred to sea at different times during the year . Aquaculture , 213 : 293 – 309 .
- Etiënne , LM , Vermeirssen Mazorra de Quero , C , Shields , RJ , Norberg , B , Kime , DE and Scott , AP . 2004 . Fertility and motility of sperm from Atlantic halibut (Hippoglossus hippoglossus) in relation to dose and timing of gonadotrophin-releasing hormone agonist implant . Aquaculture , 230 : 547 – 567 .
- Gelsleichter , J . 2004 . “ Hormonal regulation of elasmobranch physiology ” . In Biology of sharks and their relatives , Edited by: Carrier , JC , Musick , JA and Heithaus , MR . 287 – 324 . Boca Raton : CRC Press .
- Glebe BD , Saunders RL 1986 . Genetic factors in sexual maturity of cultured Atlantic salmon, Salmo salar parr and adults reared in sea cages . In: Meerburg DJ , Salmonid age at maturity. Canadian Special Publication of Fisheries and Aquatic Sciences 89 : 24 – 29 .
- Henningsen , AD , Murru , FL , Rasmussen , LEL , Whitaker , BR and Violetta , GC . 2008 . Serum levels of reproductive steroid hormones in captive Sandtiger sharks, Carcharias taurus (Rafinesque), and comments on their relation to sexual conflicts . Fish Physiology Biochemistry , 34 : 437 – 446 .
- Kagawa , H , Young , G , Adachi , S and Nagahama , Y . 1982 . Estradiol-17β production in isolated amago salmon (Oncorhynchus rhodurus) ovarian follicles and its stimulation by gonadotropins . General and Comparative Endocrinology , 47 : 361 – 365 .
- Kagawa , H , Young , G and Nagahama , Y . 1983 . Relationship between seasonal plasma estradiol-17β and testosterone levels and in vitro production by ovarian follicles of amago salmon (Oncorhynchus rhodurus) . Biology of Reproduction , 29 : 301 – 309 .
- Kiabi , BH , Abdoli , A and Naderi , M . 1999 . Status of fish fauna in the south Caspian Basin of Iran . Zoology in the Middle East , 18 : 57 – 65 .
- Kokokiris , L , Mourot , B , LeMenn , F , Kentouri , M and Fostier , A . 2000 . Endocrine changes during the annual reproductive cycle of the red porgy . Pagrus pagrus (Teleostei, Sparidae) , 23 : 1 – 11 .
- Kozul , V , Scaramuca , B , Glamuzina , B , Glavic , N and Tutman , P . 2001 . Comparative gonadogenesis and hormonal induction of spawning of cultured and wild Mediterranean amberjack (Seriola dumerili, Risso 1810) . Scientia Marina , 65 : 215 – 220 .
- Linares-Casenave Kroll , KJ , Van Eenennaam , JP and Doroshov , SI . 2003 . Effect of ovarian stage on plasma vitellogenin and calcium in cultured white sturgeon . Aquaculture , 221 : 645 – 656 .
- Manire , CA and Rasmussen , LEL . 1997 . Serum concentrations of Steroid hormones in the mature male bonnethead shark, Sphyrna tiburo . General and Comparative Endocrinology , 107 : 414 – 420 .
- Matsuyama , M , Adachi , S , Nagahama , Y , Kitajima , C and Matsuura , S . 1991 . Annual reproductive cycle of the captive female Japanese sardine Sardinops melanostictus: relationship to ovarian development and serum levels of gonadal steroid hormones . Marine Biology , 108 : 21 – 29 .
- Mojazi Amiri , BM , Maebayashi , S , Adachi , S and Yamauchi , K . 1996 . Testicular development and serum sex steroid profiles during the annual sexual cycle of the male sturgeon hybrid the bester . Journal of Fish Biology , 48 : 1039 – 1050 .
- Nagahama , Y . 1983 . “ The functional morphology of teleost gonads ” . In Fish Physiology , Edited by: Hoar , WS , Randall , DJ and Donaldson , EM . Vol. IXA , 223 – 275 . New York : Academic Press .
- Nagahama , Y . 1987 . Gonadotropin action on gametogenesis and steroidogenesis in Teleost gonads . Zoological Science , 4 : 209 – 222 .
- Niksirat , H and Abdoli , A . 2009 . On the status of the critically endangered Caspian Brown trout, Salmo trutta caspius, during recent decades in the southern Caspian Sea Basin . Zoology in the Middle East , 46 : 55 – 60 .
- Pankhurst , NW and Van Der Kraak , G . 1997 . “ Effects of stress on reproduction and growth of fish ” . In Fish stress and health in aquaculture , Edited by: Iwama , GK , Pickering , AD , Sumpter , JP and Schreck , CB . 73 – 93 . Cambridge : Cambridge University Press .
- Persson , P , Sundell , K , Björnsson , BTH and Lundovist , H . 1998 . Calcium metabolism and osmoregulation during sexual Maturation of river running Atlantic salmon . Journal of Fish Biology , 52 : 334 – 349 .
- Piironen , J . 1985 . Variation in the properties of milt from the Finnish landlocked salmon (Salmo salar m. Sebago Girard) during a spawning season . Aquaculture , 48 : 337 – 350 .
- Ritter , JA , Farmer , GJ , Misra , PK , Goff , TR , Bailery , JK and Baum , ET . 1988 . Parental influences and smolt size and sex ratio effects on seasonal variation of plasma lipids correlated with vitellogenesis . Comparative Biochemistry and Physiology , 118A : 183 – 190 .
- Rurangwa , E , Kime , DE , Ollevier , F and Nash , JP . 2004 . The measurement of sperm motility and factors affecting sperm quality in cultured fish . Aquaculture , 234 : 1 – 28 .
- Stanczyk , FZ , Lee , JS and Santen , RJ . 2007 . Standardization of steroid hormone assays: why, how, and when? . Cancer Epidemiology Biomarkers , 16 : 1713 – 9 .
- Sylven , S and Elvingson , P . 1991 . Comparison of rainbow trout, Oncorhynchus mykiss strain for body weight, length and age at maturity in different Swedish production systems . Aquaculture , 104 : 37 – 50 .
- Yamamoto , K , Oota , I , Takano , K and Ishikawa , T . 1965 . Studies on the maturing process of the rainbow trout, Salmo gairdneri irideus, maturation of the ovary of a one-year old fish . Bulletin of the Japanese Society of Fisheries , 31 : 123 – 132 .
- Yaron , Z . 1995 . Endocrine control of gametogenesis and spawning induction in the carp . Aquaculture , 129 : 49 – 73 .