Abstract
A study was conducted to evaluate the potential of immature oocytes from the ovaries of yak. The effects of maturation times (23–30 h), hormones (FSH, LH, E2), sera (fetal calf serum, FCS, or oestrous cow serum, ECS) and culture system (co-culture with granulosa cell, GC, or with bovine oviduct epithelia cells, BOEC, tissue culture medium, TCM, 199 absence co-culture cell, and synthetic oviduct fluid with amino acids, SOFaa) on the in vitro development of in vitro matured and fertilised yak oocytes were examined. Immature oocytes surrounded with compacted cumulus cell were cultured for 23–30 h in TCM 199 supplemented with 10% FCS and hormones. In vitro fertilisation (IVF) was performed with frozen-thawed, caffeine and heparin treated spermatozoa from Datong yak. Oocytes were incubated with 1×106/ml spermatozoa for 16–18 h and then cultured in co-culture system with GC or BOEC and/or SOFaa for 7–8 days, respectively. Cleavage and development to blastocysts were recorded on days 2 and 8, respectively, after the start of culture. 27–28 h was superior to other culture times and addition hormones (FSH, LH, E2) were superior to absence either of them for oocyte maturation. The best maturation of oocyte in TCM 199 with 5.0 mg/L LH, 0.5 mg/L FSH, 1 mg/L E2. FCS was superior to ECS for development to blastocysts. Co-culture with GC was superior to SOFaa and TCM 199 absence co-culture cell for cleavage, development to blastocysts. The results show that choice of culture conditions has marked effect on the development of in vitro matured and fertilised yak oocytes. The present results indicate that the co-culture with GCs is the most important factor for IVF to development into blastocysts of yak oocytes matured in vitro.
Introduction
Yaks (Poephagus grunniens or Bos grunniens) are regarded as one of the world's most interesting domestic animals since they not only thrive in conditions of extreme harshness and deprivation but also provide respectable amounts of meat, milk, wool and draft power for people. A herbivore, the yak lives predominantly on the ‘roof of the world’ from 2500 to 6000 m above msl, as the Qinghai–Tibetan Plateau is often called. The world's total yak population is estimated to be approximately 14 million of which more than 90% is in China (Wiener et al. Citation2003). Although yaks are multi-purpose bovids and have large population, the animal suffers from certain inherent reproductive problems, such as (1) late maturity, (2) seasonality of oestrus, (3) long post partum calving intervals and (4) low reproduction, which limits its reproductive efficiency (Sarkar et al. Citation2008a, Citation2008b). So, how to faster multiplication and conservation of yak germplasm have got worldwide attention.
In vitro production (IVP) is a well-established embryonic biotechnology with a variety of applications in basic and applied sciences. The technology supports the production of embryos used for research investigations, for treating human infertility, for enhancing the productivity of food animals, and for conservation of endangered mammals (Bavister Citation2002). IVP is generally referred to as a three-step procedure, namely oocyte in vitro maturation (IVM), in vitro fertilisation (IVF) and in vitro culture (IVC) of the zygote (Balasubramanian et al. Citation2007). Although great progress has been made in IVP of cattle, which is becoming one of the most exciting and progressive procedures available for today's producers, the efficiency of yak IVP is still low (Li et al. Citation2007a, Citation2007b; Yan et al. Citation2007; Zi et al. Citation2008). There are still many problems need to be solved or improved, such as optimum culture time and medium for cytoplasm maturation of oocytes, unknown factors affecting embryo developmental potential, and so on.
In the present study, we first tested the effect of IVM time (23–30 h) of oocyte on the cleavage rate after fertilisation. We then compared the outcome of addition of different hormone combinations (FSH, LH, E2) in maturation medium to the cleavage rate after in vitro fertilisation. The effects of different sera (fetal calf serum, FCS, or oestrous cow serum, ECS) on the efficiency of IVP in yak were also done. Finally, we evaluated the efficiency of different embryo culture system (co-culture with granulosa cell, GC, or with bovine oviduct epithelia cells, BOEC, tissue culture medium, TCM, 199 absence co-culture cell, and synthetic oviduct fluid with amino acids, SOFaa).
Materials and methods
Collection of oocytes
The ovaries were obtained from yaks killed at a local slaughter house and were transported in physiological saline (0.9% (w/v) NaCl) at 32–38°C to the laboratory within 3 h during October–December. The cumulus-oocyte complexes (COCs) (4–8/ovary) were collected from follicles of 2–8 mm in diameter with an 18-gauge needle attached to a 10 ml disposable syringe. Only oocytes with an unexpanded cumulus oophorus and evenly granulated cytoplasm were cultured in a polystyrene cell culture dish (35 mm× 10 mm) containing maturation medium of 50 µl microdrop covered with mineral oil.
IVM of oocytes
After washed three times in modified Dulbecco's phosphate buffered saline supplemented with 1% (w/v) polyvinyl pyrrolidone (Sigma) and once with the maturation medium (TCM 199 with Earle's salts and L-glutamine, Gibco, Cat.31100-035, NY, USA) supplemented with 10% sera (FCS or ECS). The medium was also supplemented with FSH (Follicle stimulation hormone, Institute of zoology, Chinese academy of sciences), LH (Luteinizing hormone, Sigma, L7134-5mg Lot 066 K1569,) and E2 (β-Estradiol, Sigma, Lot# 110M0138V). The oocytes were introduced into 50 µl the maturation medium covered with mineral oil in a polystyrene cell culture dish (35 mm×10 mm) and cultured for 27–28 h at 38.5°C in a CO2 incubator under 5% CO2 in air and high humidity.
The evaluation of oocyte maturation was referred by Choi et al. (Citation1998). After culture, oocytes were fixed in acetic acid-ethanol (1:3), stained with 1% orcein and examined under a phase contrast microscopy (×400). Maturation has been assumed due to the presence of Metaphase II chromosome with the first polar body.
Experiment 1, 4 different culture time (23–24 h, 25–26 h, 27–28 h and 29–30 h) for oocyte maturation were designed in TCM 199 with Earle's salts and L-glutamine supplemented with 10% FCS, FSH, LH and β-Estradiol.
Experiment 2, 4 different content hormons (0.5 mg/L FSH + 1 mg/L E2, 5.0 mg/L LH + 0.5 mg/L FSH, 5.0 mg/L LH + 1 mg/L E2, 5.0 mg/L LH + 0.5 mg/L FSH + 1 mg/L E2) for oocyte maturation were defined in TCM 199 with Earle's salts and L-glutamine supplemented with 10% FCS.
Experiment 3, 2 different serum (10% FBS or 10% ECS) for oocyte maturation were prepared in TCM 199 with Earle's salts and L-glutamine supplemented with hormones (FSH, LH and E2) or absence.
Sperm preparation
Two 0.25 ml frozen straws of semen from Datong male yak were thawed at 38.5°C and were prepared for sperm capacitation. The thawed semen was layered under 1 ml BO medium ( Brackett and Oliphant Citation1975) (pH 7.4, capacitation medium) with 10 mM caffeine (Sigma, C-4144 Lot 69H1232) and 3 mg/ml BSA (Sigma, A6003-5G Lot# 010M7400V) in conical tubes for a swim-up procedure. The top 0.8 ml medium was then collected after incubator for 1 h at 38.5°C. The pooled medium containing spermatozoa was washed twice (400 g, 10 min) with capacitation medium. The final pellet of semen was re-suspended with 0.2 ml BO medium (pH 7.4, fertilisation medium) with 6 mg/ml BSA and 20 µg/ml Heparin (Sigma-ALDRICH, H4784-250MG, Lot 050M1153). The spermatozoa were incubated for 30 min at 38.5°C.
Fertilisation
The microdrops of 50 µl fertilisation medium were prepared in 35 mm×10 mm polystyrene cell culture dish using sterile tips. The microdrops were covered with mineral oil. The dishes put in incubator to equilibrate at least 2 h. The COCs were treated by 2% Hyaluronidase (Sigma H4272). After 3 min, oocytes were washed three times with fertilisation medium. Then maturation oocytes were transferred into the microdrop (about 20 oocytes/microdrop). And 20–50 µl of the fertilisation medium treated spermatozoa were also add to the microdrop to give the final sperm concentration of 1×106 cells/ml. After in vitro insemination, oocytes and spermatozoa were incubated for 16–18 h at 38.5°C under 5% CO2 and high humidity.
Subsequent culture
For examining the developmental capacity to blastocyts, the same procedures for in maturation and fertilisation were carried out. After 16–18 h of fertilisation, all embryos in the four different groups were transferred to a co-culture medium (TCM 199 supplemented with 10% FCS) with BOEC or GC. BOEC was isolated by opening the oviduct longitudinally and scraping the mucosal epithelial layer with a sterile glass slide, and was further processed as described by Reischl et al. (Citation1999). In brief, cells were collected in 2.5 ml Hepes-buffered TCM 199 with 10% FCS, and were poured from three oviducts before being washed twice by centrifugation at 200 g for 5 min each. The cell pellet was incubated in 2 ml 0.25% (w/v) trypsin −0.02% (v/v) EDTA solution (GIBCO, Canada Lot 939422) for 8 min at 38.5°C. Finally, the cells were washed in TCM 199 with 10% FCS, centrifuged at 170 g for 5 min and counted before plating (Rief et al. Citation2002). The GCs were collected from antral follicles of about 10 mm in diameter after dissection and washed (500 g, 5 min) 2 times with TCM 199 addition 10% FCS with 5×106 cells/ml. After 48 h the GC layer was formed and attached to the bottom of culture dish. Then 20 embryos in each well were cultured for 8 days at 38.5°C under 5% CO2 in air and high humidity. The incubation medium was replaced half with new medium every 48 h.
All of the culture media used were supplemented with antibiotics (100 iu penicillin/ml and 100 µg streptomycin/ml).
Observation and statistics
Some of oocytes used were randomly picked up for the calculation for maturation and fertilisation rates. Maturation rate was examined 27–28 h after maturation and fertilisation rate was examined 47–48 h after fertilisation. The early embryos were examined under the microscope every 24 h after cultured. The data obtained were subjected to SPSS 16.0 analysis.
Results
Effects of culture time on IVM of yak oocyte
The medium of oocyte maturation was made of TCM 199 with Earle's salts and L-glutamine supplemented with 10% sera, FSH, LH and β-Estradiol. The maturation rate of oocytes examined after 28 h of incubation was 84.3%. There were significant differences compared with 23–24 h and 29–30 h. The fertilisation rate examined after 18 h of insemination was 46.5%. The cleavage rate was significantly higher than those of 23–24 h and 29–30 h. At the same time, the rates of maturation and fertilisation of 27–28 h is higher than those of 25–26 h. 25% of matured oocytes used developed to at least the eight cell stage by 3–4 days after IVF in TCM 199 at 38.5°C under 5% CO2 in air and high humidity. The results of the maturation rate and cleavage rate in different culture time are shown in .
Table 1. Effects of culture time on yak oocytes IVM.
Effects of hormone on IVM and development of yak oocyte
Different hormones added to culture media, the maturation and cleavage rates of yak oocytes were different. The rate of oocyte maturation was lower than other groups in culture medium with LH and FSH except E2. Adding FSH, LH and E2 conditions, the rates of matured and cleaved was highest (P<0.05) than FSH and E2, LH and E2, and FSH and LH, respectively. The results showed in about effects of FSH, LH and E2 to oocytes maturation and fertilisation.
Table 2. Effects of different hormons (FSH, LH and E2) added to culture media on IVM and development into 2-cell of yak oocytes.
Effects of serum on IVM and development of yak oocyte
Analysis of variance showed that the serum type did not affect cleavage or development into blasotocysts. The addition of hormones in both groups tended to reduce cleavage rates and there was no significant (P>0.05) (). But absence of hormones in both groups to increase cleavage rates and there was significant (P<0.05). The rates of maturation, fertilisation and blastocyst with FCS were higher than ones with ECS, but no significantly. Under absence of hormone conditions, the rates of maturation, fertilisation and blastocyst with FCS were lower than those with ECS (P>0.05), and there were significantly, respectively.
Table 3. Effects of different serum on yak oocytes IVM, fertilisation and development.
Effects of culture system on in vitro development of yak blastocyst
Development into blastocyst was significantly (P<0.05) affected by the addition of GCs and oviduct epithelial cells, but not serum or hormones. At 5–6 and 7–8 days after IVF development to morulae and blastocysts were 28.7% and 10.6% in co-culture system with GC (), respectively. The rate of blastocysts in co-culture system with GC is higher than those in co-culture with BOEC, but not significantly (P>0.05). The rates of morulae and blastocysts in co-culture system with GC or BOEC were higher than the culture system of TCM199 with 10% FCS absence GC or BOEC and the culture system of SOFaa with 3 mg/ml BSA, respectively.
Table 4. Effects of different culture medium on yak early embryo in vitro development.
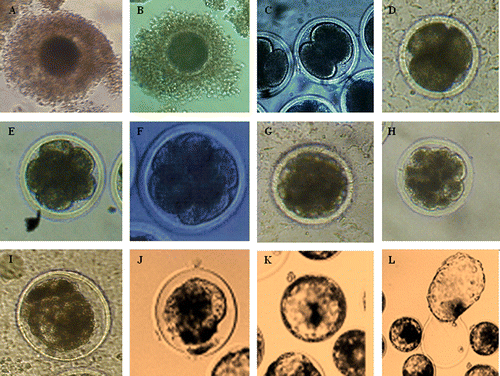
The results showed in about the photo of different development stage of oocyte and early embryo. From immature oocyte to matured oocyte, fertilised ovum to different stage of early embryo development, it needs 9–10 days in TCM 199 at 38.5°C under 5% CO2 in air and high humidity.
Figure 1. The photo of oocyte and embryo in different development stage for yak.
A: Immature oocyte (d −1); B: Matured oocyte (d 0); C: 2-cell embryo (d 1–2); D: 4-cell embryo (d 2); E: 8-cell embryo (d 2–3); F: 8–16 cell embryo (d 3); G: >16 cell embryo (d 3–4); H: Compact Morula (d 4–5); I: Compact morula-early blastocyst (d 5–6); J: Early blastocyst (d 6–7); K: Blastocyst (d 7–8); L: Hatching blastocyst (d 8–9).
Discussion
In vitro fertilisation of cow ova began in the 1970s (Brackett et al. Citation1978), and the first calf was born after IVF in 1981 (Brackett et al. Citation1982). Production in vitro offers a complementary strategy for enhancing and protecting the genetic diversity of rare or excellent populations. Although other calves have since been born (Brackett et al. Citation1984), the technology used has been considered unsuitable for field application. Few studies have scientific reported IVF of yak. In the present study we have obtained yak embryos that can be used of the introduction of new genetic information and a variety of other studies.
There are many factors effecting oocyte maturation and embryo cleavage in vitro in bovine. Some of the key investigations have focused on culture time for oocyte maturation. It is reported that 24 h was the idea culture time for oocyte IVM of bovine (Picco et al. Citation2010). But in this experiment, it is improper time for oocyte maturation of yak; 27–28 h was superior to 23–24 h, 25–26 h, 29–30 h for oocyte maturation of yak, respectively. In vitro maturation time of yak oocyte is longer than other cattle, it is possible to relation to living environment and physiological character of yak. We have obtained a high maturation rate (84.3%) in our culture system. This is partly due to the fact that cumulus cells surrounding the oocyte expanded well during the maturation process. The importance of cumulus expansion during the maturation process has been suggested by Ball et al. (Citation1984).
Culture of yak zygotes to the blastocyst stage in the most often recommended option for testing new batches of medium, its components, sera, hormones, BSA, etc. The results of such tests should be interpreted with caution because they depend on multiple factors such as the genetic background of the yak and the type of the medium used. Under conventional conditions for IVM culture, which is supplemented with FCS and hormones such as gonadotrophins and steroids, bovine oocytes with cumulus cells matured after 23–24 h. It appears that hormone plays a significant role during IVC, and that hormone is mainly a problem in the presence of concentration in medium. In the experiment, FSH, LH and E2 play important roles in oocyte maturation. Hormones are necessary during IVM of yak oocytes for subsequent development. Serum is a common constituent of culture media. Significant research has been done regarding the role of sera as growth factors in culture medium for mammalian preimplantation embryos. Although fetal formation is the ultimate assessment of embryo viability after IVC, embryo transfer is not always practical and is affected by additional factors involved in the procedure.
Successful embryo culture depends on multiple parameters, with the genetic background of the embryos and the medium composition being the most important. It is well known that the culture of embryos in reduced volume of medium and/or in groups increases blastocyst development, blastocyst cell number and, most importantly, viability after transfer. The choice of the medium also depends on the actual purpose for which it is going to be used. Early formulations of embryo culture media based on balanced salt solutions, lacking co-culture cell and supplemented only with carbohydrates and BSA, supported development to term, but embryos exhibited a delayed cleavage rate and reduced viability after transfer. It had reported that the development of a two cell bovine embryo to the blastocyst stage had a block of development.
Historically, in vitro cultured bovine embryos struggled to pass the so-called ‘8- to 16-cell developmental block’, a culture-induced event during the maternal-to-zygotic transition (Eyestone and First Citation1991). In vitro culture systems resembling the reproductive tract environment were improved to support preimplantation embryo development to pass this block. BOEC, GC and SOF (synthetic oviduct fluid) and other simple media were capable of supplying the embryonic requirement in vitro. The general lack of understanding about the developmental block in vitro, as well as early developmental processed and metabolism of bovine embryos, led to the advent of co-culture systems using somatic cells of the reproductive tract, typically BOEC (Thibodeaux et al. Citation1992).
In summary, mammalian preimplantation embryo culture is a constantly developing field. The use of specific media for IVC of preimplantation stage embryos allowed studies of the early development and manipulation of the mammalian genome. These media were designed for somatic cell culture (TCM199 and Ham's F10) and embryo culture media (SOF and modified Parker's medium). First, the effect of common cell culture media ((TCM) 199 and Han's F10) and embryo culture media (SOF and minimum essential medium (MEM)) on differentiated BOEC or GC growth was compared. TCM199 has been traditionally used in successful IVF for many years. Because hypoxanthine content is low in M199, it is beneficial to oocyte development in livestock. So, TCM199 is the basic medium in the study. There are four different media to apply to study embryo development. SOFaa was unsuitable for culture early embryo of yak. In the present study the effects of BOEC or GC co-culture on yak IVP embryo development were evaluated to assess its suitability as a model for studying embryo-maternal communication. The beneficial effects on blastocyst development are thought to be due to embryotrophic factors provided by epithelial cells (Gandolfi et al. Citation1992) and glucose concentration by these cells (Bavister Citation1995). The effects of co-culture on embryo development are typically evaluated by rates of cleavage and development to the blastoyst stage. But as the co-culture system with GC is easier to establish than a co-culture system with BOEC. And in vitro development of yak embryo could also obtained beneficial factor from co-culture system with GC.
Taking into consideration of all these factors, the zygotes are cultured in defined conditions. Blastocyst formation, assessed at a specific time, should reach more than 80%. Yak are considered to be seasonally polyestrous breeding occurs from July to November. Oocytes collected at the end of breeding season or in anestrous season of yak is the main reason lead to low cleavage rates and blastocyst rates in the study. Although the percentage of embryos developed to blastocysts was low (10.6%), the results of this study have verified the viability of embryos (blastocysts) developed in a co-culture system with yak cumulus cells.
In conclusion, the present study shows that the success of a particular culture system depends on the correct combination of a number of parameters. One of the most important findings of this study was that the IVM/IVF oocytes from yak could be matured in vitro and inseminated with frozen-thawed semen. In our laboratory, we prefer to culture oocyte or embryo with GC, 10% FCS, and incubations are most often carried out in a 38.5°C, humidified atmosphere of 5% CO2, 95% air, regulated automatically.
Acknowledgements
The work was supported or partly supported by grants from Central Public-interest Scientific Institution Basal Research Fund (Contract No.1610322011002), China Agriculture Research System (Contract No. CARS-38), and Special Fund for Agro-Scientific Research in the Public Interest (Contract No. 201003061).
References
- Balasubramanian , S , Son , WJ , Mohana Kumar , B , Ock , SA , Yoo , JG , Im , GS , Choe , SY and Rho , GJ . 2007 . Expression pattern of oxygen and stress-responsive gene transcripts at various developmental stages of in vitro and in vivo preimplantation bovine embryos . Theriogenology , 68 : 265 – 275 . doi: 10.1016/j.theriogenology.2007.05.044
- Ball , GD , Leibfried , ML , Ax , RL and First , NL . 1984 . Maturation and fertilization of bovine oocyte in vitro . Journal of Dairy Science , 67 : 2775 – 2785 . doi: 10.3168/jds.S0022-0302(84)81634-3
- Bavister , BD . 1995 . Culture of preimplantation embryos: facts and artefacts . Human Reproduction Update , 1/2 : 91 – 148 . doi: 10.1093/humupd/1.2.91
- Bavister , BD . 2002 . Early history of in vitro fertilization . Reproduction , 124 : 181 – 196 . doi: 10.1530/rep.0.1240181
- Brackett , BG , Bousquet , D , Boice , ML , Donawick , WJ , Evans , JF and Dressel , MA . 1982 . Normal development following in vitro fertilization in the cow . Biology of Reproduction , 27 : 147 – 158 . doi: 10.1095/biolreprod27.1.147
- Brackett BG , Keefer LL , Troop LG , Donawick WJ , Bennett KA. 1984 . Bovine twins resulting from in vitro fertilization . Theriogenology 21 : Abstract 224 .
- Brackett BG , Oh YK , Evans JF , Donawick WJ. 1978 . In vitro fertilization of cow ova . Theriogenology 9 : Abstract 89 .
- Brackett , BG and Oliphant , G . 1975 . Capacitation of rabbit spermatozoa in vitro . Biology of Reproduction , 12 : 260 – 274 . doi: 10.1095/biolreprod12.2.260
- Choi , YH , Takagi , M , Kamishita , H , Wijayagunawardane , MPB , Acosta , TJ , Miyazawa , K and Sato , K . 1998 . Developmental capacity of bovine oocytes matured in two kinds of follicular fluid and fertilized in vitro . Animal Reproduction Science , 50 : 27 – 33 . doi: 10.1016/S0378-4320(97)00087-0
- Eyestone , WH and First , NL . 1991 . Characterization of developmental arrest in early bovine embryos cultured in vitro . Theriogenology , 35 : 613 – 625 . doi: 10.1016/0093-691X(91)90457-O
- Gandolfi , F , Brevini , TAL , Modina , S and Passoin , L . 1992 . Early embryonic signals: embryo maternal interactions before implantation . Animal Reproduction Science , 28 : 269 – 276 . doi: 10.1016/0378-4320(92)90113-R
- Li , Y , Dai , Y , Du , W , Zhao , C , Wang , L , Wang , H , Liu , Y , Li , R and Li , N . 2007a . In vitro development of yak (Bos grunniens) embryos generated by interspecies nuclear transfer . Animal Reproduction Science , 101 : 45 – 59 . doi: 10.1016/j.anireprosci.2006.09.018
- Li , Y , Li , S , Dai , Y , Du , W , Zhao , C , Wang , L , Wang , H , Li , R , Liu , Y , Wan , R and Li , N . 2007b . Nuclear reprogramming in embryos generated by the transfer of yak (Bos grunniens) nuclei into bovine oocytes and comparison with bovine-bovine SCNT and bovine IVF embryos . Theriogenology , 67 : 1331 – 1338 . doi: 10.1016/j.theriogenology.2006.10.022
- Picco , SJ , Anchordoquy , JM , de Matos , DG , Anchordoquy , JP , Seoane , A , Mattioli , GA , Errecalde , AL and Furnus , CC . 2010 . Effect of increasing zinc sulphate concentration during in vitro maturation of bovine oocytes . Theriogenology , 74 : 1141 – 1148 . doi: 10.1016/j.theriogenology.2010.05.015
- Reischl , J , Prelle , K , Schol , H , Neumuller , C , Einspanier , R , Sinowatz , F and Wolf , E . 1999 . Factors affecting proliferation and dedifferentiation of primary bovine oviduct epithelial cells in vitro . Cell and Tissue Research , 296 : 371 – 383 . doi: 10.1007/s004410051297
- Rief , S , Sinowatz , F , Stojkovic , M , Einspanier , R , Wolf , E and Prelle , K . 2002 . Effects of a novel co-culture system on development, metabolism and gene expression of bovine embryos produced in vitro . Reproduction , 124 : 543 – 556 . doi: 10.1530/rep.0.1240543
- Sarkar , M , Chakraborty , P , Sharma , BC , Deka , BC , Duttaborah , BK , Mohanty , TK and Prakash , BS . 2008a . Assessment of superovulatory responses in terms of palpable corpora lutea and embryo recovery using plasma progesterone in yaks (Poephagus grunniens L.) . Research in Veterinary Science , 85 : 233 – 237 . doi: 10.1016/j.rvsc.2007.11.011
- Sarkar , M , Sengupta , DH , Dutta Bora , B , Rajkhoa , J , Bora , S , Bandopadhaya , S , Ghosh , M , Ahmed , FA , Saikia , P Mohan , K . 2008b . Efficacy of Heatsynch protocol for induction of estrus, synchronization of ovulation and timed artificial insemination in yaks (Poephagus grunniens L.) . Animal Reproduction Science , 104 : 299 – 305 . doi: 10.1016/j.anireprosci.2007.02.010
- Thibodeaux , JK , Menezo , Y , Roussel , JD , Hansel , W , Goodeaux , LL , Thompson , DL Jr and Godke , RA . 1992 . Coculture of in vitro fertilized bovine embryos with oviductal epithelial cells originating from different stages of the estrous cycle . Journal of Dairy Science , 75 : 1448 – 1455 . doi: 10.3168/jds.S0022-0302(92)77900-4
- Wiener , G , Jianlin , H and Ruijun , L . 2003 . The Yak , 2nd ed , Bangkok , , Thailand : RAP publication 2003/06, FAO .
- Yan , P , Xu , BZ , Guo , X , Pan , HP and Yang , BH . 2007 . In vitro maturation of yak oocytes . Chinese Journal of Veterinary Science , 27 : 130 – 133 .
- Zi , XD , Lu , H , Yin , RH and Chen , SW . 2008 . Development of embryos after in vitro fertilization of bovine oocytes with sperm from either yaks (Bos grunniens) or cattle (Bos taurus) . Animal Reproduction Science , 108 : 208 – 215 . doi: 10.1016/j.anireprosci.2007.08.005