Abstract
In avian medicine, non-steroidal anti-inflammatory drugs are used in the treatment of various musculoskeletal inflammatory disorders. Although usage of acetylsalicylic acid suggested in birds and continued to be use in the treatment of certain clinical conditions nevertheless, the studies relating to the potential toxic effects of this drug upon repeated oral administration in birds are rarely reported. Thus, a study was designed to assess the toxic effects of acetylsalicylic acid in chickens. For the purpose of the study two groups (n=10) of broiler chickens were used. Group I served as normal control and received distilled water (1.0 ml/day, p.o.) and group II received acetylsalicylic acid (10 mg/kg/day, p.o.) daily at 24 h interval for five consecutive days. Clinical signs of toxicity such as hypo activity, disinclination to move and watery droppings accompanied with blood mixed mucous observed in group II birds. These birds showed significant (P<0.01) decrease in serum total protein concentration and significant (P<0.05) decrease in the total erythrocyte count, haemoglobin concentration, per cent PCV and platelet count. Besides, gross and histopathological lesions were observed in small intestine. Thus, based on the results, it was concluded that acetylsalicylic acid (10 mg/kg) upon repeated oral administration daily for a period of five days found toxic in chickens.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are used commonly for the treatment of various musculoskeletal inflammatory disorders in human and veterinary practice. In veterinary medicine, they are used to overcome certain clinical conditions like spondylitis, laminitis, mastitis, endotoxic shock and colic in the horse and for the control of pain associated with trauma or surgery (Boothe Citation2001). Similar to mammals, prostaglandins are involved in the modulation of pain responses in birds (Nicol et al. Citation1992). Besides, NSAIDs are also indicated in birds (Bauck Citation1990) that are very much essential to manage pain and inflammatory conditions in food-producing birds (chickens, ducks, turkeys, geese, swans, quails, guinea fowls, ostrich, emu, etc), pet birds as well as in zoo birds (Mohan Citation2010). Although a large number of NSAIDs are being discovered following the initial breakthrough of aspirin, comparatively a very small number of NSAIDs are being used in birds unlike in other domestic animals or human beings. Sodium salicylate and acetaminophen found to possess anti-nociceptive properties in pigeons (Brune et al. Citation1974). Acetylsalicylic acid was used in duck production for tenosynovitis and in turkey industry for certain leg problems (Jouglar and Benard Citation1992). A moderate reduction in ascites was reported for acetylsalicylic acid treated broiler chickens kept in a hypobaric chamber to mimic high altitude (Balog et al. Citation2000). Self-selection of feed containing an analgesic drug, carprofen was found much higher in lame broiler chickens than sound birds (Danbury et al. Citation2000). Supplementation of acetyl salicylic acid at a rate of 20 ppm to layer diets during summer season improved both production and reproduction performance (Galil Citation2004). The findings of these studies suggest that the prostaglandins and cyclooxygenases (COXs) participate in avian nociception and as a result, the use of NSAIDs has found to produce analgesia in birds (Machin et al. Citation2001). Acetylsalicylic acid, popularly known as aspirin, is commonly used in human medicine. Since this drug is easily available at over the counter, there is all likelihood that pet owners, sometimes caretakers of pet birds and veterinarians use this drug for treating certain clinical conditions (febrile status, joint-related problems and other inflammatory conditions) in birds. Although usage of acetylsalicylic acid is suggested in birds nevertheless, the studies relating to the potential toxic effects of this drug upon repeated oral administration in birds are rarely reported. Hence, the present study was designed to assess the toxic effects of acetylsalicylic acid in chickens.
Materials and methods
Experimental birds
Twenty apparently healthy, unsexed broiler chickens, aged five weeks with body weight ranging from 1.4 to 1.6 kg procured from commercial poultry farm were used for the study. Live bird procedures used in this study were approved by the University Institutional Animal Ethics Committee (IAEC). All the birds were caged individually in experimental animal house maintained under standard laboratory housing conditions. Medication-free feed (free from any antibiotics or coccidiostats) procured from the university poultry farm was fed ad libitum with free access to potable water. All birds were acclimatised to the laboratory housing condition for a period of five days.
Drug formulations
During the experimental period, fresh acetylsalicylic acid solution was prepared daily. The amount of acetylsalicylic acid required to prepare the formulation was based on the body weight of birds. The calculated amount of acetylsalicylic acid (Sigma–Aldrich, St. Louis, MO, USA) was dissolved in the Milli-Q water using an ultrasonicator (Powersonic-410, Daichen Labtech Co. Ltd., Korea).
Dose fixation
The dosage regimens reported for treatment in domestic chickens are mostly extrapolated from other species or sometimes identical to that of small mammals. Therefore, in view of the above premise and in order to evaluate the potential toxic effects of acetylsalicylic acid, the dose of 10 mg/kg (Thompson Citation2008) (therapeutic dose for small animals) was selected.
Experimental design
Twenty broiler chickens were randomly divided into two groups of 10 birds in each group (completely randomised design). The gavaging tubes of 10 cm length prepared from infant feeding tube (No. 8) fixed to two millilitre syringe were used to deposit the drug formulation directly into the crop of individual bird. Group I served as control and received Milli-Q water (1.0 ml/day, p.o.) and group II received acetylsalicylic acid (10 mg/kg/day, p.o.) at an interval of 24 h for a period of 5 days. After dosing, general clinical observations were made twice daily throughout the study period and birds were observed for general health condition, morbidity and mortality, if any, were recorded.
Blood sampling and analysis
The blood samples were collected from cutaneous ulnar vein by using two millilitre disposable syringe and needle (22 G, 1.5” length). The blood samplings were performed prior to treatment on day 1 and on subsequent days at 24 h interval for the period of five days. From each bird, approximately 1.5 ml blood was collected. From this, 0.2 ml blood was transferred to 1 ml storage vials containing 500 µg of Na2EDTA and this was used for estimation of total erythrocyte count (TEC), haemoglobin concentration (Hb), packed cell volume (PCV) and platelet count by using fully automatic blood cell counter (Model PCE-210, Erma Inc., Tokyo, Japan).
The left over blood in the syringe was transferred to clean, dry 3 ml glass tubes. The blood in the glass tubes was allowed to clot. Serum was separated from the clotted blood following centrifugation at 2500 rpm for 10 min at room temperature. The serum was transferred to storage vials of 1 ml capacity and stored at −20°C. The serum samples were analysed for aspartate aminotransferse (AST), alanine aminotransferase (ALT), creatinine, uric acid and total serum protein using commercially procured diagnostic kits from Merck (Ecoline®, Merck Specialties Limited, Kalyan Badlapur Road, M. I. D. C Area, Ambernath, India) by utilizing clinical chemistry analyzer-Microlab 300 (Vitalab Scientific, The Netherlands).
Specimen collection and processing
On day 6, all birds were subjected to detail necropsy and gross pathological changes if any were recorded. For histological examination, a representative tissue samples from proventriculus, small intestine, liver and kidney were collected in 10% neutral buffered formalin. They were processed by routine paraffin embedding technique and sections of 5 µ thicknesses were cut and stained by haematoxylin and eosin (Luna Citation1968).
Statistical analyses
The data were subjected to statistical analysis. Mean values and standard error of mean were calculated and all the values were expressed as Mean±SEM. The data were analysed by student's t-test using statistical software, GraphPad Prisim (Citation2004) and P<0.05 was considered as significant.
Results and discussion
Group II birds showed clinical signs of toxicity such as hypo activity and disinclination to move. These clinical signs were observed 2–3 h after daily dosing. In addition, they showed watery droppings accompanied with blood mixed mucous from day 3 onwards.
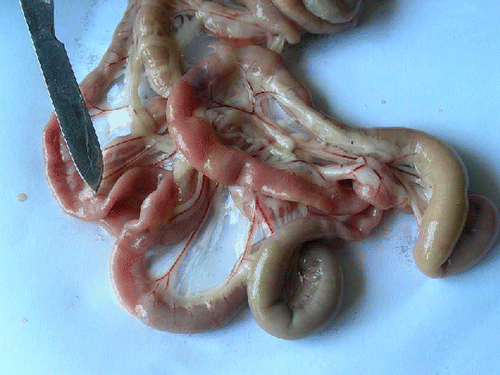
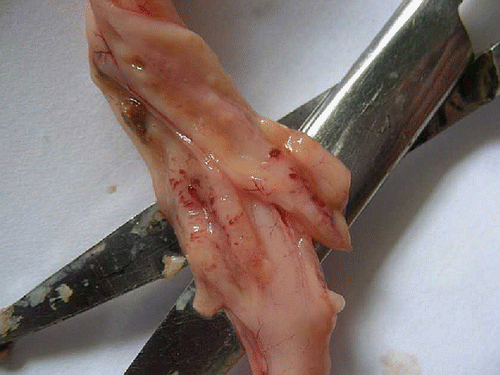
Significant (P<0.05) reduction in the TEC on day 4 and 5, haemoglobin concentration, PCV and platelet count on day 3, 4 and 5 was observed in the group II compared to group I (). Significant (P<0.01) decrease in serum total protein concentration on day 4 and 5 was observed in group II compared to group I (). At necropsy, group II birds showed severe congestion on serosal surface of small intestine (), petechial haemorrhages were observed on mucosal surface of ceca (). On microscopic examination of proventriculus sections of group II birds showed haemorrhages on the sub-mucosa or lamina propria, hyalinisation of villus epithelium accompanied with infiltration of inflammatory cells (). Sections of small intestine showed erosion, haemorrhages on the sub-mucosa or lamina propria and desquamation of villus epithelium into the lumen with infiltration of inflammatory cells (). No apparent histopathological lesions were observed in tissue sections of kidney or liver.
Figure 3. Group II-proventriculus section showing haemorrhages in the lamina propria accompanied with inflammatory cells (H&E 100X).
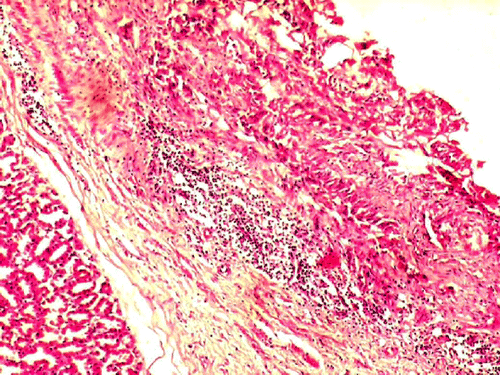
Figure 4. Group II-section of small intestine showing haemorrhage, degeneration and necrosis of villus epithelium (H&E 100X).
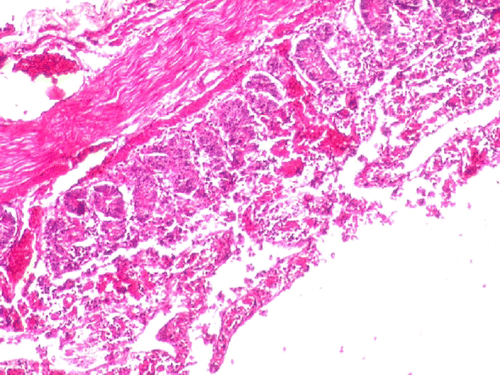
Table 1. Effect of acetylsalicylic acid on haematological parameters in broiler chickens.
Table 2. Effect of acetylsalicylic acid on serum biochemical parameters in broiler chicken.
The clinical signs of toxicity viz. hypo activity and disinclination to move observed in group II birds were associated with acetylsalicylic acid-induced toxicity and these toxic clinical signs found to be transitory and descended over a period of time (6–7 h after dosing). To correlate, NSAID-induced clinical signs of toxicity were also observed in the broiler chickens (Mohan et al. Citation2012) and Leghorn layers, 24 h post administration of diclofenac, wherein the affected birds appeared comatose and could not arouse (Naidoo et al. Citation2007).
The significant reductions in the TEC, haemoglobin concentration and PCV per cent of the blood samples observed in group II birds are attributed to the adverse effect exerted by this drug on haematopoietic system. The major function of the red blood cells is to transport haemoglobin, which in turn carries oxygen from the lungs to the tissues (Waugh and Grant Citation2001). Reduction in the haemoglobin is accompanied by a fall in the erythrocytes and PCV (Waugh and Grant Citation2001). Very low readings for TEC, haemoglobin concentrations and PCV per cent observed for group II birds are indication of anaemia due to heavy loss of erythrocytes on account of haemorrhage in the gastrointestinal tract.
Avian thrombocytes play a primary role in haemostasis in a manner similar to mammalian platelets. They also have a phagocytic function and participate in removing foreign material from the blood (Dieterlen-Lievre Citation1988; Campbell Citation1988). A normal thrombocyte count ranging between 20,000 and 30,000/µl of blood is used as a general reference for most of the birds (Jain Citation1993). Thrombocytopenias are usually indicative of excessive peripheral demand for thrombocytes, although a depression in thrombopoiesis should be considered (Campbell Citation1988). Thrombocytopenias are often seen in conditions where a combination of excessive peripheral demand for thrombocytes and in case of decreased thrombocyte production. A thrombocytosis may reflect a rebound response following haemorrhage or recovery from other conditions associated with excessive utilisation of thrombocytes (Campbell Citation1997). The significant reduction in the platelet count in blood samples observed on day 3, 4 and 5 in group II birds are due to impair platelet activity caused by impaired thromboxane synthesis. Particularly, acetylsalicylic acid irreversibly acetylates the platelet cyclo-oxygenase. Since, platelets unable to regenerate cyclo-oxygenases, platelet aggregation defects caused by acetylsalicylic acid found to last up to one week (Eyre and Burka Citation1979).
The most predictable and serious adverse effects associated with NSAIDs has reported in the gastrointestinal tract (Curry et al. Citation2005). Gastrointestinal perforation, ulceration and bleeding have been associated with NSAID-induced depression of normal PGE2 mediated and mucosal protective mechanisms (e.g., bicarbonate and mucous secretion, epithelialisation and maintenance of mucosal blood flow) (Bertolini et al. Citation2001). In the present study, toxic clinical signs such as blood and mucous mixed diarrhoea and lesions observed on the mucosa of intestine and ceca of the birds administered with acetylsalicylic acid are mainly due to the disruption of normal integrity of gastrointestinal mucosa largely mediated by inhibition of COX1 activity.
Non-steroidal anti-inflammatory drugs are well recognised for having potentially toxic effects on the gastrointestinal tract, which may lead to diarrhoea. The prostaglandins PGE2 and PGI2 are critical for the maintenance of normal mucosal blood flow within the gastrointestinal tract (Boothe Citation2001). Marked decrease in serum total protein concentration is suggestive of gastrointestinal and hepato-toxicity (Denman et al. Citation1983; Lumeij Citation1999). To corroborate, severe congestion and haemorrhages observed in the small intestine of group II birds was due to inactivation of the COX (cyclo-oxygenase) enzymes by acetylsalicylic acid. The inactivation of COX leads to a decrease in prostaglandin production, which, in turn, impairs mucosal blood flow and leads to mucosal inflammation and injury leading to protein loosing enteropathy (Boothe Citation2001). Thus, marked reduction in serum protein observed in group II attributed to the toxic effect exerted by acetylsalicylic acid on gastrointestinal tract.
Conclusion
In conclusion, acetylsalicylic acid at 10 mg/kg upon repeated oral administration daily for a period of five days found toxic in chickens.
Acknowledgements
The authors are grateful to Mr M. Narayana Gowda, Senior Lab Assistant, Department of Veterinary Pathology for his help in tissue sectioning and staining.
References
- Balog , JM , Huff , GR , Rath , NC and Huff , WE . 2000 . Effect of dietary aspirin on ascites in broilers raised in a hypobaric chamber . Poultry Science , 79 : 1101 – 1105 .
- Bauck L. 1990 . Analgesics in avian medicine . Proceedings of the 1990 Annual Conference of the Association of Avian Veterinarians, Phoenix, Arizona . 239 – 244 .
- Bertolini , A , Ottani , A and Sandrini , A . 2001 . Dual acting anti-inflammatory drugs: a reappraisal . Pharmacological Research , 44 : 437 – 450 . doi: 10.1006/phrs.2001.0872
- Boothe , DM . 2001 . “ The analgesic, antipyretic, anti-inflammatory drugs ” . In Veterinary pharmacology and therapeutics , 8th ed , Edited by: Adams , HR . 433 – 451 . Ames : Iowa State University Press .
- Brune , K , Bucher , K and Waltz , D . 1974 . The avian microcrystal arthritis II. Central verses peripheral effects of sodium salicylate, acetaminophen and colchicine . Agents Actions , 4 : 27 – 33 . doi: 10.1007/BF01965489
- Campbell , TW . 1988 . Avian hematology and cytology , Ames : Iowa State University Press .
- Campbell , TW . 1997 . “ Hematology ” . In Avian medicine: principles and application , Abridged edition , Edited by: Ritchie , BW , Harrison , GH and Harrison , LR . Lake Worth , FL : Wingers Publishing .
- Curry , SL , Cogar , SM and Cook , JL . 2005 . Nonsteroidal anti-inflammatory drugs: a review . Journal of American Animal Hospital Association , 41 : 298 – 309 .
- Danbury , TC , Weeks , CA , Chambers , JP , Waterman-Pearson , AE and Kestin , SC . 2000 . Self-selection of the analgesic drug carprofen by lame broilers . Veterinary Record , 146 : 307 – 311 . doi: 10.1136/vr.146.11.307
- Denman , B , Hamdi , E , Fox , F and Frajola , W . 1983 . Carbon tetrachloride toxicity, hepatostructural and enzymatic change. Archives of . Environmental Health , 7 : 630 – 646 .
- Dieterlen-Lievre , F . 1988 . “ Birds ” . In Vertebrate blood cells , Edited by: Rawley , AF and Ratcliffe , A . 257 – 336 . New York : Cambridge University Press .
- Eyre , P and Burka , JF . 1979 . Hypersensitivity in cattle and sheep: a pharmacological review . Journal of Veterinary Pharmacology and Therapeutics , 1 : 97 doi: 10.1111/j.1365-2885.1978.tb00313.x
- Galil , MAAA . 2004 . Effect of using some anti-heat stress compounds on the performance of some local breeds of chicken under hot climatic condition . Egyptian Poultry Science , 24 : 417 – 427 .
- GraphPad Prism . 2004 . Version 4.01 for windows . San Diego , CA : GraphPad Software Inc .
- Jain NC. 1993 . Essentials of veterinary hematology . Philadelphia : Lea & Febiger .
- Jouglar , JY and Benard , G . 1992 . Indications, modalites pratiques et précautions particulières d'emploie des anti-inflammatoires chez les oiseaux . Recueil de Médecine Vétérinaire Special Anti-inflammatoires , 168 : 745 – 747 .
- Lumeij , JT . 1999 . “ Avian clinical biochemistry ” . In Clinical biochemistry of domestic animals , Edited by: Kaneko , JJ , Harvey , JW and Bruss , ML . 857 – 877 . Singapore : Harcourt Brace and Company .
- Luna , LG . 1968 . Manual of histopathological staining methods of the armed forces Institute of Pathology , New York : McGraw Hill Book Co .
- Machin , KL , Tellier , LA , Lair , S and Livingston , A . 2001 . Pharmacodynamics of flunixin and ketoprofen in mallard ducks (Anas platyrhynchos) . Journal of Zoo and Wildlife Medicine , 32 : 222 – 229 .
- Mohan K. 2010 . Safety evaluation of certain NSAIDs in birds [doctoral thesis] . [India]: Karnataka Veterinary Animal and Fisheries Sciences University, Bidar .
- Mohan , K , Jayakumar , K , Narayanaswamy , HD , Manafi , M and Pavithra , BH . 2012 . An initial safety assessment of hepatotoxic and nephrotoxic potential of intramuscular ketoprofen at single repetitive dose level in broiler chickens . Poultry Science , 91 : 1308 – 1314 . doi: 10.3382/ps.2011-01929
- Naidoo , V , Duncan , N , Bekker , L and Swan , G . 2007 . Validating the domestic fowl as a model to investigate the pathophysiology of diclofenac in Gyps vultures . Environmental Toxicology and Pharmacology , 24 : 260 – 266 . doi: 10.1016/j.etap.2007.06.003
- Nicol , GD , Klingberg , DK and Vasko , MR . 1992 . Prostaglandin E2 increases calcium conductance and stimulates release of substance P in avian sensory neurons . Journal of Neuroscience , 12 : 1917 – 1927 .
- Thompson , L . 2008 . “ Anti-inflammatory agents ” . In The Merck veterinary manual , 9th ed , Edited by: Kahn , CM and Line , S . Whitehouse Station , NJ : Merck & Co., Inc. (Electronic version) .
- Waugh , A and Grant , A . 2001 . Anatomy and physiology in health and illness , 59 – 70 . Churchill Livingstone : Elsevier Science Limited .