Abstract
Bovine leukocyte adhesion deficiency (BLAD) and bovine citrullinaemia (BC) are autosomal recessive genetic diseases in Holstein cattle breeds and both result in death of homozygous animals. The aim of this study was to investigate the presence of BLAD and BC alleles in Holstein cows reared in the Antalya region of Turkey. In this study, blood samples were collected from a total of 504 Holstein breed randomly selected from the populations raised in different parts of Antalya. The polymerase chain reaction–restriction fragment length polymorphism analysis was used to identify individuals with these diseases. To cut the target regions TaqI and AvaII restriction enzymes were used to investigate the BLAD and BC alleles, respectively. In this study, from a total of 504 samples examined, homozygous recessive (mutant) BLAD and BC alleles were not determined. In addition there was no BC carrier animal in the populations from the Antalya region but the frequency of BLAD carriers (heterozygous) were found to be 2% in the same populations.
1. Introduction
Genetic diseases or inherited disorders occur in all animal species. In animal breeding, genetic disorders are one of the most important points of attention for breeders because of their negative impact on health and productivity of farm animals. At this time, there are identification records for Bulldog (achondroplasia), Mule Foot (syndactylism), bovine leukocyte adhesion deficiency (BLAD), complex vertebral malformation, Prolonged Gestation, Hairless, Dwarfism, Imperfect Skin, deficiency of uridine monophosphate synthase and Pink Tooth (congenital porphyria) (Citek and Blahova Citation2004).
In cattle, the autosomal recessive genetic disorders are breed-specific. In Turkey, there are few studies related to these genetic diseases (Akyüz and Ertuğrul Citation2006; Akyüz et al. Citation2010; Meydan et al. Citation2010; Öner et al. Citation2010; Karslı et al. Citation2011). Some of the genetic disorders are Holstein-specific, which mainly include: BLAD (Nagahata et al. Citation1997) and bovine citrullinaemia (BC) (Healy et al. Citation1991; Grupe et al. Citation1996). Due to international trade in semen for artificial insemination in dairy cattle, the risk of spread of genetic disorders has emerged. Holstein cattle breed has an important place for cattle breeding in Turkey. Antalya has around 25,567 farms and 105,574 heads of cattle, of which 95% belong to Holstein breeds (CBAT Citation2012).
BLAD is a recessive autosomal inherited disorder in Holstein cattle including several species of mammals. It is caused by a point mutation in the gene encoding bovine CD18, producing a substitution of guanine to adenine and of an aspartic acid to glycine at amino acid 128 (Nagahata et al. Citation1997). BLAD, which is characterised by lack of expression of adhesion molecules of the CD11/CD18 family on the leukocyte surface, results in death of the homozygous animal that is unable to defend itself from pathogens (Shuster et al. Citation1992).
BC is an autosomal recessive error of urea metabolism as a result of a deficiency of the activity of the urea cycle enzyme, argininosuccinate synthase (ASS). BC was disseminated starting from an American-born Holstein bull, named Linmack Kriss King, one of the most widely used sires for stockbreeding programmes in Holstein cattle, that has been identified as a heterozygous carrier for citrullinaemia (Healy et al. Citation1991).
Animals affected by BC lack ASS, which is crucial to the urea cycle. The mutation responsible for citrullinaemia has been characterised as a single-base substitution (C–T) in exon 5 of ASS, which converts the CGA codon that codes for arginine-86 to TGA, a translational termination codon. This conversion results in a truncated peptide product (85 amino acids instead of 412) lacking in functional activity. Clinical signs are the results of hyperammonaemia. Calves are clinically normal at birth but within 24 h they display poor feeding ability and become depressed, wandering aimlessly or with their head pressing with apparent blindness and odontoprisis. Within 4–5 days they are recumbent, convulsing, collapsing and dying (Grupe et al. Citation1996).
The technological advances that have taken place in the fields of molecular genetics and bioinformatics during the last decades have allowed us to identify the genes responsible for a number of important genetics disorders with a monogenic origin in dairy cattle. Knowing the molecular basis of a defect, the direct detection of the heterozygous carriers is possible at the gene level after birth or even in embryos. Similarly, the detection of carriers of cytogenetic anomalies enables their exclusion from breeding and consequently, the maintenance of genetic health in the population.
The frequency of BLAD and BC carriers in Holstein populations varies depending on the country, year and policy of dairy cattle improvement, and the status of carriers has to be controlled by DNA testing methods. The aim of this study is to investigate the presence of BLAD and BC alleles in Holstein cows raised in the Antalya region of Turkey by using the polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method.
2. Materials and methods
Blood samples were randomly collected from 504 Holstein dairy cows belonging to nine farms in different parts of the Antalya province. Whole blood was collected from the jugular vein into a tube containing 5% EDTA and blood samples were stored at 4°C until DNA isolation. Genomic DNA was extracted from blood using the GenElute Blood Genomic DNA kit (Sigma Aldrich, St. Louis, MO, USA). Analysis on agarose gels and spectrophotometric methods were used to determine DNA quality and quantity. DNA was stored at 20°C until use. The purified DNA solution containing 50 ng/µl of DNA was used for all the subsequent analyses.
For BLAD, 2 µl of DNA solution was subjected to 35 cycles of PCR in a total volume of 50 µl containing 1 U of Taq polymerase, 4 µl of 10× PCR buffer, 0.8 nM dNTPs, 2 mM MgCl2, 1 µM of each primer (Forward: 5′-CCT GCA TCA TAT CCA CCA G-3′, Reverse: 5′-GTT TCA GGG GAA GAT GGA G-3′) that have been described by Kriegesmann et al. (Citation1997). The markers were subjected to a PCR amplification at the following conditions: initial denaturation step of 5 min at 94°C 35 cycles of 1 min at 94°C, at the annealing primer of 1 min at 57°C, extension of 30 s at 72°C and a final extension of 15 min at 72°C.
The PCR products, which are 343 bp, were analysed on 1.5% 1× TBE agarose gels and visualised under UV light after staining with ethidium bromide. A total of 10 µl of the resulting PCR products were subjected to digestion with 20 U of TaqI for 2 h at 65°C. The digestion products were separated by horizontal electrophoresis (60 V, 120 min) through 3% agarose gels. The digestion products amplified 343 bp product upon digestion by TaqI, to detect point mutation in a gene coding for BLAD, yielded two bands of 191 and 152 bp.
For BC, 3 µl of DNA solution was subjected to 35 cycles of PCR in a total volume of 50 µl containing 1 U of Taq polymerase, 6 µl of 10× PCR buffer, 0.8 nM dNTPs, 2 mM MgCl2, 1 µM of each primer (Forward: 5′-GGC CAG GGA CCG TGT TCA TTG AGG ACA TC-3′, Reverse: 5′-TTC CTG GGA CCC CGT GAG ACA CAT ACT G-3′) that have been described by Grupe et al. (Citation1996).
The markers were subjected to a PCR amplification using a mastercycler PCR at the following conditions: initial denaturation step of 5 min at 94°C, 35 cycles of 30 s at 94°C, at the annealing primer of 30 s at 57°C, extension of 30 s at 72°C and a final extension of 15 min at 72°C. The PCR products, which are 185 bp, were analysed on 1.5% 1× TBE agarose gels and visualised under UV light after staining with ethidium bromide. A total of 10 µl of the resulting PCR products were subjected to digestion with 20 U of AvaII for 2 h at 37°C. The digestion products were separated by horizontal electrophoresis (60 V, 120 min) through 3% agarose gels. The amplified 185 bp product upon digestion by AvaII, to detect point mutation in a gene coding for BC, yielded two bands of 103 and 82 bp.
3. Results and discussion
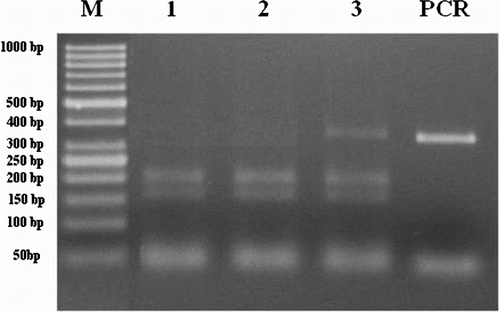
For BLAD, PCR amplification was successful in the 504 samples analysed and the expected 343 bp band was obtained. The restriction enzyme TaqI digested the PCR product into two fragments of 152 and 191 bp in normal homozygote animals. Heterozygote animals gave three fragments of 152, 191 and 343 bp. Homozygote recessive (mutant) animals had one long uncut segment because mutation lacks the digestion site, but were not found in this study (). BLAD analysis revealed that of the 504 Holstein cows genotyped, 493 were found normal homozygous and 11 were found heterozygous. The frequency of the BLAD-carriers in Turkish Holstein cows in Antalya region of Turkey was 2%.
Figure 1. Gel electrophoresis of PCR products after digested with TaqI restriction enzyme for BLAD gene polymorphism. M: 50 bp DNA ladder (Fermantase-SM0613). Line 1–2: homozygous normal animals showing two bands (191, 152 bp). Line 3: heterozygous carrier animals showing three bands (343, 191, 152 bp). PCR products of BLAD (343 bp).
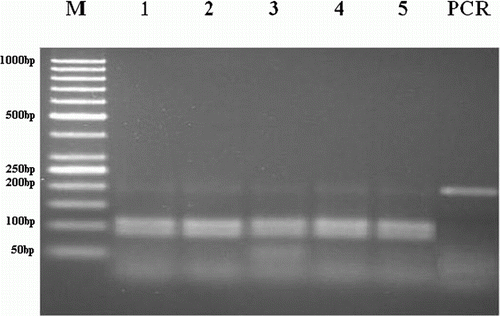
For BC, PCR amplification was successful in the 504 samples analysed and the expected 185 bp band was obtained. The restriction enzyme AvaII digested the PCR product into two fragments of 82 and 103 bp in normal homozygote animals. Heterozygote animals give three fragments of 82, 103 and 185 bp. None of the animals showed these three bands. No animal was found to be a mutant or carrier of BC (). As a result of electrophoretic analysis, no mutant animal has been detected for BLAD and BC.
Figure 2. Gel electrophoresis of PCR products after digested with AvaII restriction enzyme for BC gene polymorphism. M: 50 bp DNA ladder (Fermantase-SM0613). Line 1–5: homozygous normal animals showing two bands (103, 82 bp). PCR products of BC (185 bp).
The BLAD carriers in Holstein cattle populations have been reported in many countries such as: USA (Shuster et al. Citation1992), Germany (Biochard et al. Citation1995), France (Tainturier et al. Citation1995), Argentina (Poli et al. Citation1996), Japan (Nagahata et al. Citation1997), Brazil (Ribeiro et al. Citation2000), Poland (Czarnik et al. Citation2007), Iran (Norouzy et al. Citation2005), Turkey (Meydan et al. Citation2010) and India (Patel M et al. Citation2011).
In Turkey, the BLAD problem was first reported by Akyüz and Ertuğrul (Citation2006), the prevalence of BLAD carriers among Holstein bulls and bull candidates was found to be 0.84%. In another study, Meydan et al. (Citation2010) reported that the mutant allele frequency was found as 0.02 and the frequency of carriers was found to be 4.0%. Akyüz et al. (Citation2010) found that the prevalence of BLAD carriers was 2.2% in Turkish Holstein populations. On the other hand, Öner et al. (Citation2010) did not find any carrier individual for this disease. The reason for these differences is probably sampling of populations in different regions, although it may also be influences by the use of carrier sires over time.
In our study, the frequency of BLAD carrier was calculated as 2.0%. These findings are similar to the results of earlier studies in some countries, for example, Argentina (3.5%) (Poli et al. Citation1996), Poland (3.0%) (Czarnik et al. Citation2007), Iran (3.33%) (Norouzy et al. Citation2005), India (3.23%) (Patel RK et al. Citation2007) and Turkey (2.2%) (Akyüz et al. Citation2010).
The frequency determined in this study (2.0%) is much lower than the values reported in some other countries such as: Germany (13.5%), France (10%), USA (8.2%), Japan (8.1%), Brazil (5.7%) (Biochard et al. Citation1995; Tainturier et al. Citation1995; Powell et al. Citation1996; Nagahata et al. Citation1997; Ribeiro et al. Citation2000). On the other hand, other studies have found a very low or zero BLAD frequency in countries like Hungary, Czech Republic, Pakistan, Mexico and China (Fesüs et al. Citation1999; Citek et al. Citation2006; Nasreen et al. Citation2009; Riojas-Valdes et al. Citation2009; Li et al. Citation2011).
The mutation rate can be reduced by using different selection strategies. For example, in Germany, while the frequency of BLAD carriers was 13.5% in 1995 (Biochard et al. Citation1995), Schütz et al. (Citation2008) reported that the frequency of carriers was approximately 0.8–3.45% of tested cattle. In contrast, the mutation rate can be increased with the use of carrier bulls in artificial insemination. In India, the frequency of BLAD carriers was found as 3.23% in 2007 (Patel RK et al. Citation2007) increasing to 4.76% in 2011 (Patel M et al. Citation2011).
The incidence of BC was found for first time in Australia, where 13% of sires in one artificial insemination centre were found to be heterozygous for the mutation in 1989 (Healy Citation1996). In other countries, like the USA (3%) and Germany (17%), the incidence of the citrullinaemia was found to be low (Robinson et al. Citation1993; Grupe et al. Citation1996). Generally, the citrullinaemia mutation frequency is very low or zero, such as China (0.16%), India (0%) (Li et al. Citation2011; Patel RK et al. Citation2006). BC carrier was not detected in this study as was reported in the other studies in Turkey (Meydan et al. Citation2010; Öner et al. Citation2010). Thus, our results confirm the previous findings.
Recessive inherited disorders and abnormal karyotypes in cattle have very low frequency. Nevertheless, in some cases they can influence the economics of cattle breeding depending on the policy of dairy cattle improvement, country and year. The intensive use of artificial insemination and international trading of semen and breeding bulls has caused the spread to a large population of disease-associated recessive alleles. Therefore, there is a need for screening methods of various genetic defects, to allow producers and breeders to test their suspicious cows and all breeding bulls. This would mean the collection of pedigree information to prevent the at-risk mating by paying attention to disease carrier animals, especially in the dairy industry, which is presently dominated by the Holstein Friesian breed worldwide.
4. Conclusion
Molecular genetic methods can help to control the genetic disorders in animal populations. Incidence of these disorders has reduced as a result of the regular use of these methods worldwide. This study shows that the PCR–RFLP method based on DNA analysis is a powerful and reliable tool for identification of inherited disorders like BLAD and BC. Moreover, this method is very important in animal breeding allowing for a good and rapid identification of carriers.
The mutant animal for BLAD and BC was not found in Holstein cows reared in the Antalya region of Turkey by using the PCR–RFLP method. Thus, the situation regarding these genetic disorders in the Turkish Holstein cattle population seems to be good at present.
Acknowledgements
This research was financially supported by the University of Akdeniz Scientific Research Projects Council (Project number: 2008.01.0104.002).
References
- Akyüz , B and Ertuğrul , O . 2006 . Detection of bovine leukocyte adhesion deficiency (BLAD) in Turkish native and Holstein cattle . Acta Veterinaria Hungarica , 54 ( 2 ) : 173 – 178 .
- Akyüz , B , Ertuğrul , O and Ağaoğlu , ÖK . 2010 . Detection of bovine leukocyte adhesion deficiency (BLAD) allele in Holstein cows reared in Kayseri vicinity . Journal of Faculty of Veterinary Medicine University of Kafkas , 16 ( 2 ) : 519 – 521 .
- Biochard D , Coquereau JA , Amiques Y. 1995 . Effect of bovine leukocyte adhesion deficiency genetic defect in Holstein cattle under farm condition . 46th Annual Meeting of the European Association for Animal Production ; Sep 4–7 ; Prague .
- Cattle Breeders' Association of Turkey [CBAT] (TR) . 2012 . Statistics . Journal of Cattle Breeders . 14 2 : 13 .
- Citek , J and Blahova , B . 2004 . Recessive disorders—a serious health hazard? . Journal of Applied Biomedicine , 2 : 187 – 194 .
- Citek , J , Rehout , V , Hajkova , J and Pavkova , J . 2006 . Monitoring of the genetic health of cattle in the Czech Republic . Veterinarni Medicina , 51 ( 6 ) : 333 – 339 .
- Czarnik , U , Grzybowski , G , Kaminski , S , Prusak , B and Zabolewicz , T . 2007 . Effectiveness of a program aimed at the elimination of BLAD—carrier bulls from Polish Holstein–Friesian cattle . Journal of Applied Genetics , 48 : 375 – 377 .
- Fesüs , L , Zsolnai , A , Anton , I , Barany , I and Bozo , S . 1999 . BLAD genotypes and cow production traits in Hungarian Holsteins . Journal of Animal Breeding and Genetics , 116 : 169 – 174 .
- Grupe , S , Dietle , G and Schwerin , M . 1996 . Population survey of citrullinaemia on German Holsteins . Livestock Production Science , 45 : 35 – 38 .
- Healy , PJ . 1996 . Testing for undesirable traits in cattle: an Australian perspective . Journal of Animal Science , 74 : 917 – 922 .
- Healy , PJ , Dennis , JA , Camilleri , LM , Robinson , JL , Stell , AL and Shanks , RD . 1991 . Bovine citrullinemia traced to sire of Linrnack Kriss King . Australian Veterinary Journal , 1 ( 68 ) : 155
- Karslı , T , Şahin , E , Karslı , BA , Alkan , S and Balcıoğlu , MS . 2011 . Identification of alleles for factor XI (FXID) and uridine monophosphate synthase (DUMPS) deficiencies in Holstein cows reared in Antalya . Journal of Faculty of Veterinary Medicine University of Kafkas , 17 ( 3 ) : 503 – 505 .
- Kriegesmann , B , Jansen , S , Baumgartner , BG and Brenig , B . 1997 . Partial genomic structure of the bovine CD18 gene and the refinement of test for bovine leukocyte adhesion deficiency . Journal of Dairy Science , 80 : 2547 – 2549 .
- Li , J , Wang , H , Zhang , Y , Hou , M , Zhong , J and Zhang , Y . 2011 . Identification of BLAD and citrullinemia carriers in Chinese Holstein cattle . Animal Science Papers and Reports , 29 ( 1 ) : 37 – 42 .
- Meydan , H , Yıldız , MA and Agerholm , JS . 2010 . Screening for bovine leukocyte adhesion deficiency, deficiency of uridine monophosphate synthase, complex vertebral malformation, bovine citrullinaemia, and factor XI deficiency in Holstein cows reared in Turkey . Acta Veterinaria Scandinavica , 52 ( 1 ) : 56
- Nagahata , H , Miura , T , Tagaki , K , Ohtake , M , Noda , H , Yasuda , T and Nioka , K . 1997 . Prevalence and allele frequency estimation of bovine leukocyte adhesion deficiency (BLAD) in Holstein–Friesian cattle in Japan . Journal of Veterinary Medical Science , 59 ( 4 ) : 233 – 238 .
- Nasreen , F , Altaf , MN , Naeem , RM and Anver , QJ . 2009 . Detection and screening of bovine leukocyte adhesion deficiency in Pakistan using molecular methods . Hereditas , 146 ( 2 ) : 74 – 78 .
- Norouzy , A , Nassiry , MR , Shahrody , FE , Javadmanesh , A , Abadi , MRM and Sulimova , GE . 2005 . Identification of bovine leucocyte adhesion deficiency (BLAD) carriers in Holstein and Brown Swiss AI bulls in Iran . Genetica , 41 ( 12 ) : 1697 – 1701 .
- Öner , Y , Keskin , A and Elmaci , C . 2010 . Identification of BLAD, DUMPS, citrullinaemia and factor XI deficiency in Holstein cattle in Turkey . Asian Journal of Animal and Veterinary Advances , 5 ( 1 ) : 60 – 65 .
- Patel , M , Patel , RK , Singh , KM , Rank , DN , Thakur , MC and Khan , A . 2011 . Detection of genetic polymorphism in CD18 gene in cattle by PCR–RFLP . Wayamba Journal of Animal Science , 11 : 110 – 111 .
- Patel , RK , Singh , KM , Soni , KJ , Chauhan , JB and Sambasiva Rao , KRS . 2006 . Lack of carriers of citrullinaemia and DUMPS in Indian Holstein cattle . Journal of Applied Genetics , 47 ( 3 ) : 239 – 42 .
- Patel , RK , Singh , KM , Soni , KJ , Chauhan , JB and Sambasiva Rao , KRS . 2007 . Low incidence of bovine leukocyte adhesion deficiency (BLAD) carriers in Indian cattle and buffalo breeds . Journal of Applied Genetics , 48 ( 2 ) : 153 – 155 .
- Poli , MA , Dewey , R and Semorile , L . 1996 . PCR screening for carriers of bovine leukocyte adhesion deficiency (BLAD) and uridine monophosphate synthetase (DUMPS) in Argentine Holstein cattle . Zentralbl Veterinaermed A , 43 ( 3 ) : 163 – 168 .
- Powell , RL , Norman , HD and Cowan , CM . 1996 . Relationship of bovine leukocyte adhesion deficiency with genetic merit for performance traits . Journal of Dairy Science , 79 : 895 – 899 .
- Ribeiro , LA , Baron , EE and Martinez , ML . 2000 . PCR screening and allele frequency estimation of bovine leukocyte adhesion deficiency in Holstein and Gir cattle in Brazil . Genetics and Molecular Biology , 23 : 831 – 834 .
- Riojas-Valdes , VM , Carballo-Garcia , B , Rodriguez-Tovar , LE , Garza-Zermeno , MV , Ramirez-Romero , R , Zarate-Ramos , J , Avalos-Ramirez , R and Davalos-Aranda , G . 2009 . Absence of bovine leukocyte adhesion deficiency (BLAD) in Holstein cattle from Mexico . Journal of Animal and Veterinary Advances , 8 ( 9 ) : 1870 – 1872 .
- Robinson , JL , Burns , JL , Magura , CE and Shanks , RD . 1993 . Low incidence of citrullinaemia carriers among dairy cattle of the United States . Journal of Dairy Science , 76 : 853 – 858 .
- Schütz , E , Scharfenstein , M and Brenig , B . 2008 . Implication of complex vertebral malformation and bovine leukocyte adhesion deficiency. DNA-Based testing on disease frequency in the Holstein population . Journal of Dairy Science , 91 : 4854 – 4859 .
- Shuster , DE , Kehrli , MK Jr , Ackermann , MR and Gilbert , R . 1992 . Identification and prevalence of a genetic defect that causes leukocyte adhesion deficiency in Holstein cattle . Proceedings of the National Academy Sciences , 89 : 9225 – 9229 .
- Tainturier , D , Grobet , L , Brouwers , B , Bruyas , JF , Fieni , F , Battut , I , Lecoanet , J , Douart , A , Breyton , I and Duclos , P . 1995 . Observations on three cases of bovine leukocyte adhesion deficiency (BLAD) . Revue-de Medecine Veterinaire , 146 ( 3 ) : 189 – 195 .