Abstract
Vascular density of the yolk sac membrane (YSM), protein and mRNA expression of hypoxia-inducible factor 2alpha (HIF-2α), vascular endothelial growth factor (VEGF-A) and its type 2 receptor (FLK-1) in the YSM were compared in subjects incubated at two different altitudes: 355 and 1378 metres above sea level (masl) in Colombia. On days 3 or 4 of incubation, higher vascular density of YSMs (p<0.05 both of the days), relative expression of HIF-2α mRNA (p<0.01 and p<0.001 days 3 and 4, respectively), VEGF-A mRNA (p<0.01 and p<0.001 days 3 and 4, respectively) and FLK-1 mRNA (p<0.001 and p<0.0001 day 3 and 4, respectively) were encountered in embryos incubated at 1378 masl as compared to those incubated at 355 masl. Information is presented about immunoexpression of HIF-2α, VEGF-A and its receptor FLK-1 in the endoderm, mesoderm and ectoderm in the YSM on days 3 and 4, and it is demonstrated that endothelial cells in the YSM on day 3 of incubation express HIF-2α, which had not been previously reported.
Introduction
The nutrition for the growth and development of the avian embryo depends on the transfer of nutrients from the yolk through the enveloping yolk sac membrane (YSM) (Romanoff Citation1967). The gas exchange of an incubating avian egg is performed across the YSM early during development. Between 3 and 6 days of incubation, the vascular area in the YSM acts as part of the respiratory organ (Cirotto & Arangi Citation1989) and also provides nutrition to the embryo. In the YSM, between days 2 and 4 of incubation, two morphological areas can be distinguished: a central zone surrounding the embryo, the area pellucida and external to it the area opaca (Eyal-Giladi & Kochav Citation1976). The first blood vessels in the YSM are formed between the above-mentioned areas; they represent the so-called area vasculosa, which rapidly spreads throughout the entire YSM (Augustine Citation1970). The external region to the area vasculosa, which is still free of blood vessels, is called the area vitellina (Flamme Citation1989). Early in their development, the YSM vessels are present in the form of a capillary plexus and shortly after the onset of perfusion, the area vasculosa is composed of radially growing vessels (vitelline arteries) with lateral connecting vessels to the outer edge (Allen & Wilson Citation1993), the sinus terminalis (Mayer & Packard Citation1978), which collects blood from distal capillaries (Baumann & Meuer Citation1992). From the sinus terminalis, blood is taken to the embryo through the anterior and posterior vitelline veins (Ribatti Citation1995). The first vascular network in the YSM is established by the process of vasculogenesis, which represents de novo formation of blood vessels from mesodermal derived cells in the YSM, the so-called hemangioblasts, which give rise to the blood islands (Pardanaud et al. Citation1989). Fusion of blood islands results in the formation of the primary vascular plexus. From the above-mentioned pre-existing vessels, new ones are formed by a process known as angiogenesis (Poole & Coffin Citation1989). Angiogenesis occurs by sprouting and non-sprouting mechanisms. The first mechanism involves breaking out of capillaries from the pre-existing vessels, and the last one includes intussusception (septation) and elongation of already formed vessels (Risau Citation1997). Vascular endothelial growth factor (VEGF) and its receptors (VEGFR) are the most critical drivers of vessel formation (Eichmann et al. Citation2005). The VEGF family currently includes the classical VEGF (VEGF-A), VEGF-B, VEGF-C, VEGF-D, VEGF-E and placental growth factor (PlGF); they bind the following tyrosine kinase family cell-surface receptors: VEGFR-1 (FLT-1), VEGFR-2 (FLK-1) and VEGFR-3 (FLT-4) (Veikkola & Alitalo Citation1999). VEGF-A and its receptor FLK-1 are implicated in most aspects of vascularisation (Carmeliet et al. Citation1996; Ferrara et al. Citation1996). It is known that certain genes as the hypoxia-inducible factor (HIF) regulate the expression of VEGF-A (Ema et al. Citation1997) and its receptor FLK-1 (Kappel et al. Citation1999; Elvert et al. Citation2003). HIF is a transcription factor that regulates the cellular response to hypoxia (Wang & Semenza Citation1995). There are three currently recognised HIF isoforms: HIF-1α, HIF-2α and HIF-3α (Wang & Semenza Citation1995; Tian et al. Citation1997; Hara et al. Citation2001), but only HIF-2α is expressed in the extraembryonic tissues (Ota et al. Citation2007).
Tengerdy et al. (Citation1970) and Visschedijk (Citation1985) found that chick embryos exposed to hypoxic conditions reduced their hatchability, which suggests that decreasing the availability of oxygen (O2) impairs embryonic survival (Mortola Citation2009), and in non-fatal cases, hypoxia delays embryonic growth, especially in the early (<10 days) and late stages (>16 days) of incubation (Taylor et al. Citation1971).
Vascularisation of extraembryonic membranes reacts to ambient O2 conditions; however, most studies about the effect of hypoxia on vascular development of the chicken extraembryonic membranes have been performed in the chorioallantoic membrane (CAM) and few of them include the YSM (Adair et al. Citation1987; Hoper and Jahn Citation1995; Wang et al. Citation2007).
Embryo deaths occur throughout the entire incubation period, but mortality is especially high between days 3 and 4 (Payne Citation1919; Hamilton Citation1952, Christensen Citation1978, Citation2001). From the above-mentioned works, two questions arise: why do avian embryos tend to die at certain ages? And what are the causes of these deaths? Then, a number of studies have been done to establish a possible critical time for embryo survival early in incubation. It was suggested that there were respiratory maladjustments during this period before the establishment of special respiratory surfaces (Riddie Citation1930; Romanoff Citation1946). Later on, it was claimed that mortality happens early in embryonic development during vascular formation (Etches Citation1996), although information is lacking in this context.
This study aimed to determine the YSM's vascular density, describe the immunolocalisation of HIF-2α, VEGF-A and its receptor FLK-1 in the YSM, determine the expression of HIF-2α, VEGF-A and FLK-1 in the YSM and establish whether hypobaric hypoxia affects the expression of the above-mentioned genes in the YSM of embryos incubated at 1378 metres above sea level (masl) on days 3 and 4 of incubation with respect to those incubated at 355 masl on the same embryonic ages. During this period, the vascular YSM vascular area starts its function as a respiratory organ (Cirotto & Arangi Citation1989).
Materials and methods
Embryo incubation and tissue samples
A total of 14,580 Cobb fertile eggs from the same breeder flocks were obtained from a local hatchery in Colombia. Standard commercial conditions were employed during incubation, using Chick Master® machines. Half of the eggs were incubated at 355 masl (37.5 °C, 50% humidity, barometric pressure: 730 mmHg, O2 partial pressure (PO2): 153 mmHg and O2 availability of 20%) and the other half, at 1378 masl (37.5 °C, 50% humidity, barometric pressure: 650 mmHg, PO2: 136 mmHg, O2 availability of 17.7%). Fifteen eggs with viable embryos were randomly chosen from each of the above-mentioned groups (355 and 1378 masl) on days 3 and 4 of incubation. The embryos were decapitated. Samples of whole YSM were taken from each specimen and fixed by immersion in a 4% aqueous formalin solution (pH 7.4). Likewise, the YSM were stored in cryotubes and immediately frozen by immersing them in liquid nitrogen and then stored at −80 °C before RNA extraction.
All procedures were approved by the Bioethics Committee of the National University of Colombia in accordance to international normative for the care and use of experimental animals.
Quantification of vascular density
Before collecting samples, the total vascular area in YSM was photographed at a distance of 15 cm from the lens of the camera. Complete vessels maps were traced using the commercial software CorelDRAW® Graphics Suite X4, and the vessels area was quantified using the commercial programme Adobe Photoshop® CS4 Extended by using the tool Image Measurement Panel (Abrams et al. Citation2009). Vascular density was expressed in square millimetres.
Immunohistochemistry (IHC)
Five micrometre paraffin-embedded tissue samples were deparaffinized and a hydrated antigen retrieval solution added (citrate buffer pH 6). After cooling and washing the plates with distilled water, they were immersed in Tris buffer (pH 7). Afterwards, the Universal Blocking Reagent Solution (Biogenex® HK 0.85.5k) was added, as well a dilution of FLIP antibody 1/50. Plates were washed three times with Tris buffer, and then, the stabilizer Super Enhancer Reagent (Biogenex® HK518-YAK) was added, and samples were washed again with Tris buffer. Then, the polymer complex peroxidase-labeled S.S Label Polymer HRP (Biogenex® HK519-YAK) was added, and samples were washed again with Tris buffer, followed by the addition of a chromogen DAB (Biogenex® HK520-YAK) solution. Tissues were then observed under the microscope for the appearance of a brown colour in reactive structures. Harris hematoxylin was employed as a counterstain. Colon adenocarcinoma samples were used as positive controls, and for negative controls, YSM samples without the primary antibody. IHC analyses were carried out using antibodies directed against HIF-2α (Lifespan Biosciences, Seattle, WA, USA. Catalog Number: LS-B4223-50), VEGF-A (Novus Biologicals, Littleton, CO, USA. Catalog Number: NB100-2381) and FLK-1 (Antibodies Online, Atlanta, GA, USA. Catalog Number: ABIN670191). Antibodies dilutions followed the manufacturer's recommendations.
RNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted with the Trizol technique reagent according to brochure instructions (Invitrogen Corp., Carlsbad, CA, USA). Then, mRNA was isolated using a Dynabeads mRNA direct kit (Invitrogen). The concentration of mRNA was quantified by ultraviolet spectrophotometry at an absorbance of 260/280 nm (SpectronicBioMate 3 UV-Vis Spectrophotometer, Thermo Electron Corp., Waltham, MA, USA). cDNA synthesis was performed from 1 µg of mRNA, using 200 U of MMLV-reverse transcriptase (Invitrogen), 20 U ribonuclease inhibitor RNase-Out (Invitrogen) and 1 nM of random hexamer primers (Invitrogen) in a total volume of 30 µl. Reverse transcription (RT) reactions were carried out at 37 °C for 45 minutes, followed by a 15-minutes exposure period at 42 °C and a heating one at 92 °C for 2 minutes. To verify the specificity of the products, RT was performed without adding reverse transcriptase enzyme (negative controls). Polymerase chain reaction (PCR) of the generated cDNA was brought about and the amplified products were resolved in 1.5% agarose gels and visualised with ethidium bromide in order to ensure the size (bp) of obtained products ().
Real-time PCR
From the cDNA obtained by RT, the mRNA expression of HIF2-α, VEGF-A, FLK-1 and 18S ribosomal subunit (S18) was determined in every single sample from 15 individuals, with real-time PCR thermal cycler, using the Roche Light Cycler® and detection methodology of SYBR green PCR Core Reagents (Roche Diagnostics Corp., Indianapolis, IN, USA). The above procedures were performed according to previous reports (Acosta & Hernández Citation2012) in which this technique was validated following manufacturer's instructions. PCR was performed using specific primers for each gene of interest, which were designed with the Primer 3 software (http://frodo.wi.mit.edu/primer3), from the sequences of Gallus gallus genes reported in GenBank. The access number, the initiators and the size of the product are shown in . The final reaction volume was 20 µl. All reactions were carried out under the following conditions: initial denaturation at 95 °C for 3 minutes, 40 cycles of denaturation at 95 °C for 5 seconds, annealing at 60 °C for 15 seconds and extension at 72 °C for 15 seconds. At the end of each run, melting curve profiles were produced (cooling the sample to 68 °C and heating slowly to 95 °C, with continuous measurement of fluorescence) to confirm amplification of specific transcripts. Fluorescence emission readings were quantified using the second derivative maximum method of the Light Cycler software package (Roche). In each sample, mRNA levels were normalised with the expression levels of S18 ribosomal subunit control housekeeping gene (based on the calculation of PCR efficiency). Product purity was confirmed by dissociation curves.
Table 1. Primer pairs sequence used for real-time PCR analysis and for conventional PCR to check the product size.
Statistical analysis
A descriptive analysis of YSM's vascular density values was performed. Measures of central tendency and dispersion were calculated. Significant difference for the variables between both altitudes was determined using Student's unpaired t-test. In the case of mRNA expression of different genes, these were first standardised by the comparative method ΔCT (Livak & Schmittgen Citation2001) before they were compared using the Pfaffl's test (Pfaffl Citation2001), and then with the model 2−ΔΔCT (Livak & Schmittgen Citation2001) and Student's unpaired t-test of theirs ΔCTs. Confidence interval for the mRNAs relative expression was calculated in accord to the reports of Yuan et al. (Citation2006). The data analysis was made using GraphPad® Prism 5 (GraphPad Software Inc., San Diego, CA, USA) and expression analysis software REST® 2009 (Pfaffl et al. Citation2009).
Results
YSM vascular density
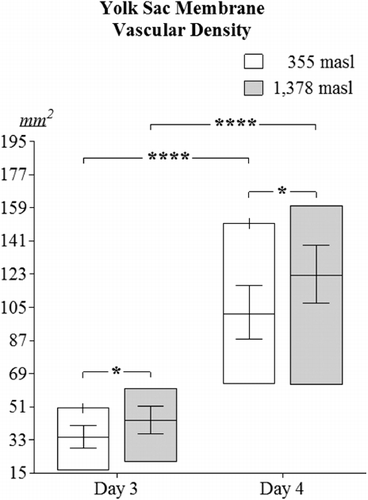
Higher YSM's vascular density was encountered in embryos incubated at 1378 masl (day 3: 43.37 mm2±7.34; day 4: 122.25 mm2±15.59) as compared to those located at 355 masl on days 3 (28.16 mm2±6.21) and 4 of incubation (101.52 mm2±14.51) (p<0.05, ). Significant differences in YSM's vascular densities were found when comparing values obtained for day 3 versus day 4 either at 355 masl or 1378 masl (p<0.0001).
Immunohistochemistry
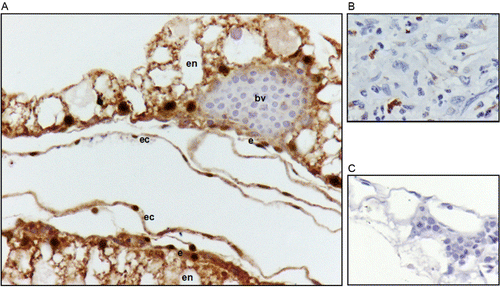
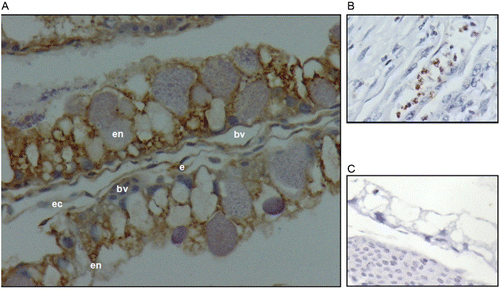
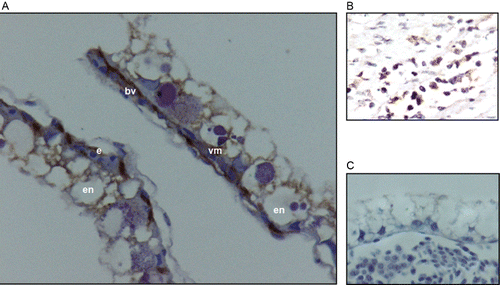
Immunohistochemistry (IHC) staining with the anti-HIF-2α antibody was observed in endodermal, endothelial and ectodermal cells (). IHC staining with the anti-VEGF-A antibody was observed in endodermal, endothelial and ectodermal cells (). IHC staining with the anti-FLK-1 antibody was observed in vascular mesoderm and endothelial cells (). In all cases, the immunolocalisation for each antibody (anti-HIF-2α, anti-VEGF-A, and anti-FLK-1) was the same, either for embryos incubated up to day 3 or embryos incubated until day 4 in both altitudes.
Relative expression quantification of mRNAs (qPCR)
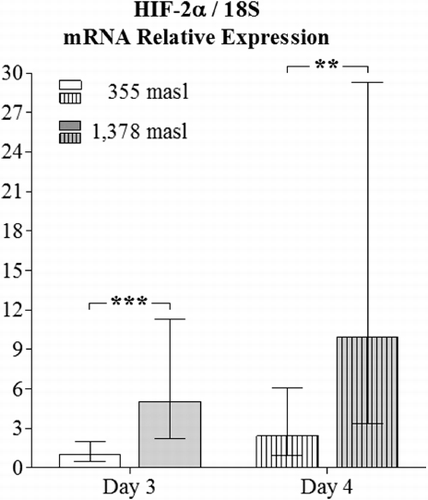
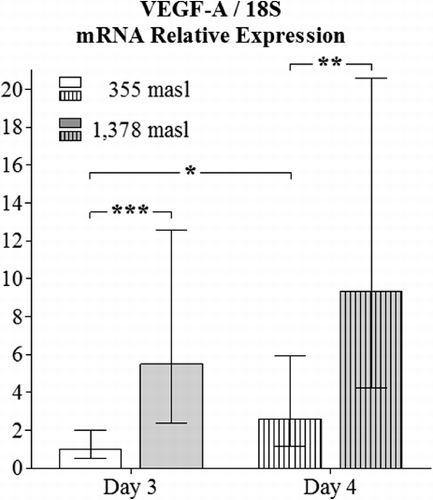
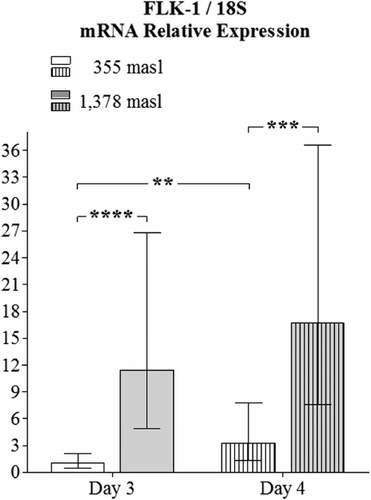
The obtained values for the relative expression of HIF-2α were higher in the YSM of embryos incubated at 1378 masl than those found for embryos incubated at 355 masl on day 3 (p<0.001) and 4 of incubation (p<0.01, ). Additionally, the obtained values for the relative expression of VEGF-A in the YSM had significant differences when comparing specimens incubated at the altitudes and embryo ages presently employed (p<0.001 and p<0.01 respectively, ). Similar findings apply for the relative expression of FLK-1 in the YSM (p<0.0001 and p<0.001, respectively, ).
Discussion
Although it has been established that from 65 to 72 hours for 5–6 days of incubation, the YSM vasculature is the main embryonic respiratory organ (Wangensteen & Rahn Citation1970/1971; Baumann & Meuer Citation1992) studies about vascular development of the YSM are scarce. In 1995, Hoper and Jahn evaluated the effect of hypoxia in the vascularisation of YSM at the fourth day of incubation and found that the vascular density measured as the area occupied by blood vessels in the YSM was higher in embryos exposed to hypoxia. The highest demand for oxygen mainly comes from the YSM (Adair et al. Citation1987); then, when this membrane is subjected to hypoxia an increase in vascular density should be encountered as presently seen. It should be noted that Byerly (Citation1930) observed that the growth of YSM in the avian embryo is only partially dependent on the presence of an embryo. He found that YSM stays alive for several days and grows even after the embryo has died. He had shown that YSM of chicken embryos in the first few days had approximately the same size regardless the size or even the existence of a viable embryo (Byerly Citation1926); however, the opposite has not been determined in the chicken embryos as established in sheep in which the vascular development of the extraembryonic membranes gives cues to identify viable and non-viable embryos (Boshier Citation1968; Rodríguez et al. Citation2005); then, even the results of the present research showed that hypobaric hypoxia affects vascular development in the YSM, it is not feasible to relate the degree of vascularisation of the YSM with embryonic survival in the first 3–4 days of incubation.
Hypoxia leads to the expression of angiogenic factors (Folkman Citation1971). Cells exposed to hypoxic environments express HIF, which controls VEGF's expression and its receptors (Nanka et al. Citation2006). In the present study, it was decided to observe the effect of relative hypobaric hypoxia in the expression of HIF-2α, which had been reported as mainly expressed in extraembryonic tissues (Favier et al. Citation1999), and also, to detect the VEGF-A and its receptor FLK-1 expression in the YSM. The expression of HIF-2α in the YSM observed by IHC with anti HIF-2α was seen in the endoderm, endothelium and ectoderm either on day 3 or 4 at both altitudes studied. This pattern of expression was reported by Elvert et al. (Citation1999) and Favier et al. (Citation1999). It was also found that endodermal cells express HIF-2α and VEGF-A (Flamme et al. Citation1995a, Citation1995b), which might imply that HIF-2α is involved in the normal process of vascularisation (Semenza Citation2001). HIF-2α expression in YSM was presently found in embryos under normoxia and relative hypobaric hypoxia as previously reported by Nanka et al. (Citation2006).
VEGF-A was expressed in the endoderm, endothelium and ectoderm on days 3 or 4 at both altitudes studied. These layers were previously encountered to be positive to the expression of VEGF-A as detected by in situ hybridisation (Flamme et al. Citation1995a), on day 2 of incubation. However, in a subsequent study (Nico et al. Citation2001), it was found that on day 6 of incubation, only endodermal and mesodermal cells were positive to the expression of VEGF-A. As noted above, in the present study, HIF-2α was expressed in endoderm, mesoderm and ectoderm, which support the idea that VEGF and HIF carry out their functions through autocrine and paracrine pathways (Nomura et al. Citation1995, Dias et al. Citation2001) and corroborate the importance of the endoderm for angiogenesis and vasculogenesis in the mesoderm as reported by several authors (Flamme Citation1989; Vokes & Krieg Citation2002; Kelly & Hirschi Citation2009).
The expression of FLK-1 in endothelial and mesodermal cells of YSM coincides with earlier findings (Flamme et al. Citation1995a; Nico et al. Citation2001). The restriction on the expression of FLK-1 to the mesoderm and endothelium in the YSM reinforces the idea that FLK-1 is a marker of endothelial cell line (Yamaguchi et al. Citation1993), although it has been claimed that FLK-1 expression is proper for diverse mesoderm-derived cell lines (Bautch Citation2006).
In the present study, all the three genes (HIF-2α, VEGF-A and FLK-1) had higher expression in the YSM of embryos incubated at high altitude, which might support the long-held theory that an increase in the expression of HIF under hypoxic conditions provokes a higher expression of VEGF-A (Kulshreshtha et al. Citation2007), and both molecules induce FLK-1 transcription (Elvert et al. Citation2003; Hervé et al. Citation2006).
It is known that both VEGF-A and FLK-1 are present in birds blastodisc (early embryo) even before the beginning of incubation, and that they are over-expressed during embryonic gastrulation on day 1 (Flamme et al. Citation1995a). It has been reported that VEGF-A on day 2 of incubation is expressed ubiquitously in layers that make up the YSM, but in the vascularised region. In the case of FLK-1, the expression of mRNA on day 1 initially occurs throughout the mesoderm, and then it is restricted to endothelial cells (Flamme et al. Citation1995a). However, the subsequent expression of VEGF-A and its receptor FLK-1 in the YSM was not investigated until 2001, when Nico et al. reported the pattern of expression for these molecules in the YSM on day 6 of incubation. Therefore, the results of the present study add data for the sequence of expression of VEGF-A and FLK-1 on days 3 and 4 of incubation. Also, it shows that on days 3 and 4, FLK-1 expression is restricted to endothelial and mesodermal cells in the YSM. Later on, FLK-1 will be expressed in ectodermal cells in the YSM as seen by Nico et al. (Citation2001). Nevertheless, it is not known, whether or not the ectoderm has an effect on vascular development through VEGF-A, or if the mesoderm and endoderm mediate a particular effect on the ectoderm through FLK-1.
Favier et al. (Citation1999) found that HIF-2α was expressed in the extraembryonic area where it was located mainly in the ectoderm layer. In contrast, Elvert et al. (Citation1999) saw that HIF-2α mRNA could be detected in all the three germ layers (endoderm, mesoderm and ectoderm) in the 1-day-old avian embryo. It must be noted that although it is known (and confirmed by this research) that HIF-2α is expressed in endothelium of blood vessels in the YSM when the circulation has been established, no expression of this factor has been detected before blood circulatory system development (the blood island cells are devoid of this expression before circulation is established), and even when the embryos are subjected to hypoxia before day 2 of embryonic development, the endothelial cells do not express HIF-2α (Ota et al. Citation2007). Thus, present results provide information on the sequence of expression of HIF-2α, demonstrating that endothelial cells in the YSM on day 3 of incubation already express HIF-2α, but it is not clear whether or not these cells respond to hypoxia by over-regulating the expression of HIF-2α. The endoderm and ectoderm layers would respond to hypoxia through over-expression of VEGF-A, and the former event promotes vascular development, most likely, by binding VEGF-A to FLK-1 in the mesodermal and endothelial cells.
In conclusion, YSM vascular density during days 3 and 4 of incubation was higher in embryos incubated at 1378 masl than those maintained at 355 masl. Hence, hypoxia could have influenced this difference. In accordance to the above-mentioned differences, correspondent relative expression of HIF-2α, VEGF-A and FLK-1 statistical differences were presently encountered. Present results provide additional information on the sequence of expression of HIF-2α, VEGF-A and its receptor FLK-1 in the YSM and demonstrate that endothelial cells in the YSM on day 3 of incubation express HIF-2α, which had not been previously reported.
Acknowledgments
The authors are indebted to Hernán Niño for his assistance to this research and to Eduardo Caminos for his support during the molecular procedures. The financial support for the present study was provided by the División de Investigación de Bogotá and Vicerrectoría de Investigación (Universidad Nacional de Colombia) and doctorate grant from COLCIENCIAS.
References
- Abrams S, Catey S, Moughamian D. 2009. Adobe photoshop CS4 bible. Chapter 28: Working with technical images. Brooklyn (NY): John Wiley and Sons.
- Acosta E, Hernández A. 2012. Vascular density, hypoxia inducible factor, vascular endothelial growth factor, and its receptor expression in the chorioallantois of relatively normoxic and hypoxic chicken embryos, at 6 and 7 days of incubation, and corresponding weight values. Poult Sci. 91:2637–2644.10.3382/ps.2012-02430
- Adair TH, Guyton AC, Montani JP, Lindsay HL, Stanek KA. 1987. Whole body structural vascular adaptation to prolonged hypoxia in chick embryos. Am J Physiol. 252:H1228–H1234.
- Allen WE, Wilson DJ. 1993. Early embryonic angiogenesis in the chick area vasculosa. J Anat. 183:579–585.
- Augustine JM. 1970. Expansion of the area vasculosa of the chick after removal of the ectoderm. J Embryol Exp Morphol. 24:95–108.
- Baumann R, Meuer HJ. 1992. Blood oxygen transport in the early avian embryo. Physiol Rev. 72:941–965.
- Bautch VL. 2006. Flk1 expression: promiscuity revealed. Blood. 107:3–4. 10.1182/blood-2005-10-4061
- Boshier DP. 1968. Histological examination of serosal membranes in studies of early embryonic mortality in the ewe. J Reprod Fertil. 15:81–86. 10.1530/jrf.0.0150081
- Byerly TC. 1926. Local growth in ‘dead’ chick embryos. Anat Rec. 33:319–325. 10.1002/ar.1090330409
- Byerly TC. 1930. Time of occurrence and probable causes of mortality in chick embryos. Conference papers, 4th World's Poultry Congress, Sec. A. London: Editorial; p. 174–181.
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380: 435–439. 10.1038/380435a0
- Christensen VL. 1978. Physiological factors and hatchability in fowl [ dissertation]. Columbia: University of Missouri.
- Christensen VL. 2001. Factors associated with early embryonic mortality. World's Poult Sci J. 57:359–372. 10.1079/WPS20010025
- Cirotto C, Arangi I. 1989. How do avian embryos breathe? Oxygen transport in the blood of early chick embryos. Comp Biochem Physiol. A. 94:607–613. 10.1016/0300-9629(89)90602-6
- Dias S, Hattori K, Heissig B, Zhu Z, Wu Y, Witte L, Hicklin DJ, Tateno M, Bohlen P, Moore MA, Rafii S. 2001. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci USA. 98:10857–10862. 10.1073/pnas.191117498
- Eichmann A, Yuan L, Moyon D, leNoble F, Pardanaud L, Breant C. 2005. Vascular development: from precursor cells to branched arterial and venous networks. Int J Dev Biol. 49:259–267. 10.1387/ijdb.041941ae
- Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. 2003. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1). J Biol Chem. 278:7520–7530. 10.1074/jbc.M211298200
- Elvert G, Lanz S, Kappel A, Flamme I. 1999. mRNA cloning and expression studies of the quail homologue of HIF-2alpha. Mech Dev. 87:193–197. 10.1016/S0925-4773(99)00144-6
- Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. 1997. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1 alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 94:4273. 10.1073/pnas.94.9.4273
- Etches RJ. 1996. Reproduction in poultry. Wallingford: CAB International.
- Eyal-Giladi H, Kochav S. 1976. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol. 49:321–337. 10.1016/0012-1606(76)90178-0
- Favier J, Kempf H, Corvol P, Gasc J. 1999. Cloning and expression pattern of EPAS1 in the chicken embryo colocalization with tyrosine hydroxylase. FEBS Lett. 462:19–24. 10.1016/S0014-5793(99)01476-3
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 380:439–442. 10.1038/380439a0
- Flamme I. 1989. Is extraembryonic angiogenesis in the chick embryo controlled by the endoderm? Anat Embryol. 180:259–272. 10.1007/BF00315884
- Flamme I, Breier G, Risau W. 1995a. Vascular endotelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 169:699–712. 10.1006/dbio.1995.1180
- Flamme I, von Reutern M, Drexler HCA, Syed-Ali S, Risau W. 1995b. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increased vascular permeability without alterations of embryonic pattern formation. Dev Biol. 171:399–414. 10.1006/dbio.1995.1291
- Folkman J. 1971. Tumor angiogenesis: therapeutic implications. New Engl J Med. 285:1182–1186. 10.1056/NEJM197108122850711
- Hamilton HL. 1952. Sensitive periods during development. Ann NY Acad Sci. 55:177–187. 10.1111/j.1749-6632.1952.tb26533.x
- Hara S, Hamada J, Kobayashi C, Kondo Y, Imura N. 2001. Expression and characterization of hypoxiainducible factor (HIF)-3a in human kidney: Suppression of HIF-mediated gene expression by HIF-3a. Biochem Biophys Res Commun. 287:808–813. 10.1006/bbrc.2001.5659
- Hervé MAJ, Geduri G, Petit FG, Domet TS, Lazennec G, Mourah S, Perrot-Applanat M. 2006. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J Endocrinol. 188:91–99. 10.1677/joe.1.06184
- Hoper J, Jahn H. 1995. Influence of environmental oxygen concentration on growth and vascular density of the area vasculosa in chick embryos. Int J Microcirc: Clin Exp. 15:18–192.
- Kappel A, Roènicke V, Damert A, Flamme I, Risau W, Breier G. 1999. Identification of VEGF receptor-2 (Flk-1) promoter/enhancer sequences driving endothelium-specific transcription in transgenic mice. Blood. 93:4284–4292.
- Kelly MA, Hirschi KK. 2009. Signaling hierarchy regulating human endothelial cell development. Arterioscler Thromb Vasc Biol. 29:718–724. 10.1161/ATVBAHA.109.184200
- Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu C, Croce CM, Negrini M, et al. 2007. A microRNA signature of hypoxia. Mol Cell Biol. 27:1859–1867. 10.1128/MCB.01395-06
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 25:402–408. 10.1006/meth.2001.1262
- Mayer BW, Packard JR. 1978. A study of the expansion of the chick area vasculosa. Dev Biol. 63:335–351. 10.1016/0012-1606(78)90138-0
- Mortola JP. 2009. Gas exchange in avian embryos and hatchlings. Comp Biochem Physiol. A. 153:359–377.
- Nanka O, Valasek P, Dvorakova M, Grim M. 2006. Experimental hypoxia and embryonic angiogenesis. Dev Dyn. 235:723–733. 10.1002/dvdy.20689
- Nico E, Vacca A, De Giorgios M, Roncali L, Ribatti D. 2001. Vascular endotelial growth factor and vascular endotelial growth factor receptor-2 expression in the chick embryo area vasculosa. Histochem J. 33:283–286. 10.1023/A:1017977007479
- Nomura M, Yamagishi S, Harada S. 1995. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 270:28316–28324. 10.1074/jbc.270.47.28316
- Ota K, Nagai H, Sheng G. 2007. Expression and hypoxic regulation of hif1α and hif2α during early blood and endothelial cell differentiation in chick. Gene Expr Patterns. 7:761–766. 10.1016/j.modgep.2007.05.007
- Pardanaud L, Yassine F, Dieterlen-Lievre F. 1989. Relationship between vasculogenesis, angiogenesis and hematopoiesis during avian ontongeny. Development. 105:473–485.
- Payne LF. 1919. Distribution of mortality during the period of incubation. J Am Assoc Instructors Investigators Poult Husbandry. 6:9–12.
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res. 29:2002–2007. 10.1093/nar/29.9.e45
- Pfaffl MW, Vandesompele J, Kubista M. 2009. Data analysis software. In: Logan J, Edwards K, Saunders N, editors. Real-time PCR: current technology and applications. Norwich, UK: Caister Academic Press; p. 65–83.
- Poole TJ, Coffin JD. 1989. Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool. 251:224–231. 10.1002/jez.1402510210
- Risau W. 1997. Mechanisms of angiogenesis. Nature. 368:671–674.
- Ribatti D. 1995. A morphometric study of the expansion of the chick area vasculosa in shell-less culture. J Anat. 186:639–644.
- Riddie O. 1930. The age distribution of mortality in bird embryos and its probable significance. Am J Physiol. 94:535–547.
- Rodríguez J, Hernández A, Hidalgo G. 2005. Área vascular del alantocorion ovino: un posible indicador post-mortem de sobrevivencia embrionaria [Ovine allantochorionic vascular area: a possible post-mortem indicator of embryonic survival]. Rev Cient. FCV-LUZ 15(1):14–19.
- Romanoff AL. 1946. Critical periods and causes of death in avian embryonic development. The Auk. 66:264–270. 10.2307/4080357
- Romanoff AL. 1967. Biochemistry of the avian embryo. New York (NY): John Wiley and Sons.
- Semenza GL. 2001. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 10.1016/S0955-0674(00)00194-0
- Taylor LW, Kreutziger GO, Abercrombie GL. 1971. The gaseous environment of the chick embryo in relation to its development and hatchability. 5. Effect of carbón dioxide and oxygen levels during the terminal days of incubation. Poult Sci. 50:66–78. 10.3382/ps.0500066
- Tengerdy RP, Fitch JW, Moreng RE. 1970. Hatchability of chicken embryos under simulated high altitude conditions. Poult Sci. 48:751–753. 10.3382/ps.0480751
- Tian H, McKnight SL, Russell DW. 1997. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 11:72. 10.1101/gad.11.1.72
- Veikkola T, Alitalo K. 1999. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 9:211–220. 10.1006/scbi.1998.0091
- Visschedijk AHJ. 1985. Gas exchange and hatchability of chicken eggs incubated at simulated high altitude. J Appl Physiol. 58:416–418.
- Vokes SA, Krieg PA. 2002. Endoderm is required for vascular endothelial tube formation, but for angioblast specification. Development. 129:775–785.
- Wang H, Lu C, Wang X, Bao Y, Meng X, Wu Y, Li Y. 2007. Chick yolk sac membrane assay: a novel angiogenesis model. J Biol Res. 7:93–97.
- Wang GL, Semenza GL. 1995. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 270:1230–1237. 10.1074/jbc.270.3.1230
- Wangensteen D, Rahn H. 1970/1971. Respiratory gas exchange by the avian embryo. Respir Physiol. 11:31–45. 10.1016/0034-5687(70)90100-3
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. 1993. Flk-1, an Flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 118:489–498.
- Yuan JS, Reed A, Chen F, Stewart CN. 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics. 7:85–96. 10.1186/1471-2105-7-85