Abstract
The aim of present study was to examine the relative mRNA transcription of toll-like receptor 4 (TLR-4), CD14, interleukin-1β (IL-1β), interleukin-8 (IL-8), and tumor necrosis factor alpha (TNF-α) in endometritic buffaloes. Reproductive tracts were collected from 60 buffaloes at postmortem. The genitalia were diagnosed for endometritis based on positive white side test of uterine fluid, presence of >5% polymorphonuclear cells in uterine cytology, and endometrial histopathology. The endometritis-positive genitalia were categorized as luteal (n = 10) and follicular (n = 10) based on the presence of corpus luteum. Further, 20 normal genitalia (luteal, n = 10 and follicular, n = 10) were also included as control. The endometrial tissue scrapings were collected ex vivo from all the samples, total RNA was extracted, complementary DNA was transcribed for each sample, and relative quantification of mRNA was done by real-time polymerase chain reaction. The threshold cycle values of all the samples were normalized with the housekeeping β-actin gene and relative fold change was calculated using 2−ΔΔCt method. Present study revealed a variable upregulation of TLR-4, CD14, IL-1β, IL-8, and TNF-α transcription in endometritic buffaloes either at luteal or follicular stage. Predominantly at luteal stage, the significant upregulation of TLR-4, IL-8, and TNF-α transcripts was 4.66-, 21.19-, and 42.49-fold, respectively, indicated the possibility of cytokine-based diagnostic method for the early indication of endometritis before the onset of cyclic estrus. Nevertheless, further research in respect to threshold level of each cytokine in uterine biopsies involving more number of animals is required to establish a potential marker gene(s) for the diagnosis of endometritis and for devising novel therapeutic intervention.
1. Introduction
Endometritis is one of the most commonly occurring uterine affections in buffaloes both in field and farm conditions. It causes impaired ovarian function and uterine involution, increases days open, precludes fertilization, prevents successful implantation, and infertility at the time of infection but also results in subfertility even after successful clinical resolution of the disease (Semambo et al. Citation1991). Diagnosis of endometritis by rectal examination, vaginoscopic examination (Barlund et al. Citation2008), endometritis clinical score (Williams et al. Citation2005), white side test (Krishnakumar et al. Citation2003), ultrasonography (Barlund et al. Citation2008), and uterine biopsy (Gonzalez et al. Citation1985) has been practiced in cattle under field and farm condition with variable level of success. Most of the techniques are happened to be difficult to apply at the luteal stage of estrous cycle when most of the animals with sub-clinical infection remain undiagnosed. The luteal stage aggravates the endometritic condition thus affecting the fertility of animals in future. However, till date there is no cow-side test available to diagnose the endometritis at luteal stage.
At parturition or during estrus, the physiological barrier of reproductive tract becomes loosened and opportunist pathogens progress up to the uterus. The immune cells in endometrium as well as epithelial cells respond to bacterial components such as endotoxin and peptidoglycan via toll-like receptors (TLRs). The toll-like receptor 4 (TLR-4) and CD14 complex play an important role during infections in the reproductive system. TLRs are transmembrane proteins that exhibit specificity for distinct pathogen-associated molecular patterns (PAMP). Binding of specific PAMP to the TLR initiates a signaling cascades resulting in the production of proinflammatory cytokines, namely IL-1, IL-6, IL-8, tumor necrosis factor alpha (TNF-α), etc. These cytokines stimulates immune cell mobilization into the uterus to clear the infection (Sheldon & Dobson Citation2004).
In recent times, molecular techniques have been employed to diagnose the endometritis in different livestock species. Several researchers have studied the transcription profile of proinflammatory cytokines in peripheral blood or at tissue level in cows, mare, and women and found to have significant correlation in the presence of endometritis (Kim et al. Citation2005; Chapwanya et al. Citation2009; Fischer et al. Citation2010; Galvão et al. Citation2011). The upregulation of mRNA transcription of different proinflammatory cytokines along with the cell surface receptor complex (TLR-4, CD14) at endometrium level might have diagnostic importance for determining the severity of uterine inflammation and would serve as a prognostic indicator in determining the status of reproductive health of the individuals. Few studies have been conducted on endometrial cytokine transcription in dairy cows with endometritis. However, the transcription pattern of these novel proinflammatory cytokines in buffaloes with endometritis has not been explored in greater detail. The present experiment was designed to study the differential transcription pattern of pathogen recognition receptor complex (TLR-4 and CD14) and proinflammatory cytokines, namely IL-1β, IL-8, and TNF-α in endometritic buffaloes at luteal and follicular stages.
2. Materials and methods
2.1. Animals and sampling
The study was carried out in reproductive tracts of female buffaloes collected from Central Buffalo Slaughter House, Bareilly and transported aseptically to the laboratory in sterilized polythene bags kept on ice. The stage of estrous cycle was determined by gross ovarian morphology following the method described by Ireland et al. (Citation1980). Depending on the presence of corpus luteum in either of the ovary, the genitalia were divided in two categories, namely luteal and follicular. The presence of follicle along with one or more corpus albicans in either of the ovary was considered as follicular stage. Endometritis in buffalo was identified using a combination of diagnostic techniques, i.e., physical examination of uterine mucus (Sheldon et al. Citation2008), uterine cytology (Gilbert et al. Citation2005), positive color reaction of cervico-vaginal mucus to white side test (Krishnakumar et al. Citation2003), and histopathological examination of endometrial tissue section (Bonnet et al. Citation1991). For cytology, endometrial scrappings were aseptically collected using a cytobrush and the smears were prepared and fixed in absolute methanol and stained with Giemsa. A minimum of 200 cells were counted under 400× magnifications in each smear and identified as epithelial, polymorphonuclear (PMNs), and mononuclear cells. Samples with PMNs exceeding the 5% cut-off were taken as positive for endometritis. The PMN cells population was varied at the range of 1–3.13% in samples considered as negative for endometritis. Further, a small piece of endometrial tissue was sectioned from the horn-body junction of uterus and fixed in 10% formalin. Formalin-fixed tissues were paraffin embedded, sectioned in 5–8 µm thickness, stained with haematoxylin and eosin, and observed under microscope at 200×. Based on various microscopic observations, namely the lymphocyte-rich cellular infiltration and the presence of PMN cells as observed in , the samples were considered as positive for endometritis and the inert quiescent uterine section with minimum or no cellular infiltration was indicated as negative for endometritis (Bonnet et al. Citation1991).
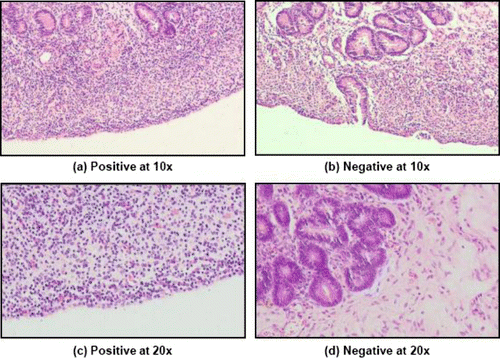
A total of 60 genitalias were screened for the presence of endometritis. The genitalias positive for endometritis at luteal or follicular stages were allocated into two groups, namely Gr. I, (endometritis luteal, n = 10) and Gr. II (endometritis follicular, n = 10). Further, 10 genitalias each at luteal and follicular stages, diagnosed negative for endometritis were considered as healthy control and included in Gr. III (healthy luteal, n = 10) and Gr. IV (healthy follicular, n = 10).
A small piece of endometrial tissue (approximately 100 mg) from the horn-body junction of the uterus using a diethyl pyrocarbonate (DEPC)-treated sterile scalpel was taken in a DEPC-treated storage vial containing 200 µl of RNAlater solution (Ambion) at 4°C for 2–4 hr and later transferred to −20°C till extraction of total RNA. Total RNA was extracted from the collected tissue samples employing Trizol (Invitrogen) method. The quantity and quality of total RNA was checked in a nanodrop spectrophotometer (ND-1000, Thermo-scientific) by measuring the optical density (OD) of the RNA sample solution at 260 and 280 nm. The RNA samples with OD values ranging between 1.8 and 2.0 indicating pure RNA with least protein and DNA contaminants were used for complementary DNA (cDNA) synthesis.
2.2. RNA extraction, cDNA synthesis and standardization of polymerase chain reaction (PCR)
The extracted total RNA (2 µg) was reverse-transcribed using RevertAid M-MuLV Reverse Transcriptase enzyme (Fermentas) and oligo (dT)18 primer (Fermentas) to synthesize the cDNA following manufacturers’ instructions using a negative control. The reaction mixture was prepared using RNA template, 1 µl (0.5 µg) Oligo(dT)18 primer, 4 µl of 5× RT buffer, 2 µl of 10 mM dNTPs, 0.5 µl (20 U) RNAse inhibitor, 1 µl (200 U) of RT enzyme, and DEPC water upto 20 µl. Both forward and reverse primers for target genes including housekeeping gene (β-actin) were in house designed using online primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) from the published complete or partial sequences of the respective genes available at the National Centre for Biotechnology Information (). The primer sets of 20 bases were custom synthesized by Bioserve company, India. The PCR of selected target genes (TLR-4, CD14, IL-1β, IL-8, and TNF-α) and housekeeping gene (β-actin) was standardized using different concentrations of mgnesium chloride and annealing temperatures in a thermal cycler (Gene Amp PCR System-9700, Applied Bio System). The reaction mixture comprised 2.5 µl Taq polymerase buffer (10×), 1.5 µl magnesium chloride (15 mM), 0.5 µl each of forward and reverse primer (20 pmol/µl), 0.5 µl dNTPs mixture (10mM) each, 1µl Taq polymerase recombinant, LC (1U/µl, Fermentas), 1µl template cDNA and nuclease-free water up to 25 µl. The initial denaturation was carried out at 94°C for 5 min followed by 30 cycles of cyclic denaturation at 94°C for 30 s, annealing at temperature specified in for 30 s and extension at 72°C for 30 s followed by final extension at 72°C for 5 min. The amplified PCR product was run in 1.5% (w/v) agarose gel using known 100 bp DNA ladder (Fermentas) ().
Table 1. List of primers used for amplification of cytokine genes in endometrial tissues of buffaloes with and without endometritis at luteal and follicular stages.
2.3. Real-time PCR
The target genes (TLR-4, CD14, IL-1β, IL-8, and TNF-α) and housekeeping gene (β-actin) were also amplified in real-time PCR system (iQ5 – Multicolor Real-time PCR Detection System, BioRad) using PCR master mix (2×) supplied with the Maxima SYBR Green qPCR kit (Fermentas). The reaction mixture prepared as per the manufacturer's protocol and the cyclic condition used in real-time PCR was consisted of one cycle of initial denaturation at 95°C for 10 min followed by 40 cycles of cyclic denaturation at 95°C for 15 s, annealing for 30 s at temperature standardized in conventional PCR and cyclic extension at 72°C for 30 s. The fluorescent data acquisition was carried out at the end of each extension step. The dissociation curve underwent a cycle consisted of gradual increase in temperature @1°C at 30 s from 55°C to 95°C. The fluorescent data acquisition was recorded continuously from 55°C to 95°C during the dissociation curve analysis step. The threshold cycle value for each target and housekeeping genes was recorded for evaluation of fold of transcription and the dissociation curve for each amplified product of the target genes was examined to check the specificity of the product and formation of primer–dimer.
The fold of transcription of target genes in endometrial tissues of buffaloes with endometritis at luteal and follicular stages was compared with normal (healthy) buffaloes. The mean fold change (n-fold) for each cytokine gene was determined using the relative quantitation method (2−ΔΔCt) described by Livak and Schmittgen (Citation2001). The threshold cycle value (Ct) of target genes and housekeeping gene were used to determine the fold of transcription of specific genes. The difference of Ct in respect of each target gene (ΔCt) indicated the difference of Ct value of housekeeping gene from the Ct value of target gene. The ΔΔCt of target gene at luteal stage was calculated by deducting the average ΔCt of target gene of healthy animal (normalized calibrator) at luteal stage from the ΔCt of target gene of endometritic animal at luteal stage. Similarly, the ΔΔCt of all the target genes was calculated at follicular stage too. The fold of transcription of target gene in endometritic buffaloes at luteal or follicular stage was finally estimated as 2−ΔΔCt.
2.4. Statistical analysis
Data obtained in this study were analyzed using Statistical software SPSS-16.0 (SPSS Corporation, USA). The Ct values of housekeeping gene (β-actin) were analyzed by Shapiro-Wilk test for normal distribution. Independent t-test was used to test the significance of mean Ct values of β-actin gene between healthy control and endometritis groups. As the data related to fold of transcription (2−ΔΔCt) of cytokines genes were not normally distributed, the statistical analysis was performed on the ΔΔCt values using independent t-test and then converted to 2−ΔΔCt for data presentation. The data obtained from real-time PCR for all target genes have been presented as their relative fold change (n-fold) ± standard error of mean (SEM).
3. Results and discussion
The status of endometritis whether clinical or subclinical can be elucidated by determining the level of mRNA transcription of pathogen recognition receptor (TLR-4), cell surface marker (CD14), and major proinflammatory cytokines playing a pivotal role in uterine immune defense mechanisms. The relative mRNA transcription of TLR-4, CD14, and proinflammatory cytokines, i.e. IL-1β, IL-8, and TNF-α in endometrial tissues of buffaloes with and without endometritis at luteal and follicular stage was determined by real-time PCR. The mRNA transcription of housekeeping gene (β-actin) was consistent (P >0.05) in endometrial tissues of buffaloes with and without endometritis, irrespective of luteal and follicular phase. The average Ct values of β-actin gene in endometritic and healthy buffaloes were 27.52 ±1.61 and 25.90 ± 0.92 (P = 0.35) at luteal and 25.90 ± 1.52 and 24.10 ± 1.43 (P = 0.42) at follicular stage, respectively. The non-significant transcription of β-actin gene confirms its suitability as an endogenous control (reference gene).
The present study depicts the differential transcription profile of cell surface marker for pathogen recognition and induction of inflammatory process (TLR-4 and CD14) in endometrial tissue of buffaloes with endometritis during luteal and follicular stages (). TLR-4 mRNA transcription was upregulated during endometritis both at luteal (4.66, P < 0.01) and follicular stage (3.11-fold, P = 0.061) relative to healthy control. Although the cell surface marker (CD14) showed a higher tendency to transcribe at luteal stage (1.14, P = 0.095), it was highly significant in endometritis of follicular stage (3.25-fold, P < 0.01). A higher endometrial mRNA transcription of TLR-4 and CD14 both at luteal and follicular stages in endometritic buffaloes indicates pathogen-associated induction of immune response. It is in accordance with the findings of Herath et al. (Citation2006) and Chapwanya et al. (Citation2009) who reported that the bovine endometrium cells expressed transcripts for TLR-4 − CD14 receptor complex are necessary for bacterial lipopolysaccharide (LPS) recognition. It was also explained that following pathogen recognition cell surface receptor complex triggers a signaling cascade that activates transcription factor NFkappa-B which controls the induction of a proinflammatory immune response through activation of genes encoding cytokines (IL-1, IL-8, TNF-α, etc.). A significantly higher fold change in TLR-4 mRNA at luteal stage possibly indicated steroidal regulation on its transcription in endometrial tissue. Similarly, Herath et al. (Citation2006) also indicated that the response of TLR-4 − CD14 complex to bacterial LPS is dependent on steroid hormone milieu, which has important implications for the establishment of uterine infection.
Table 2. Fold change in cytokine genes expression in endometrial tissues of buffaloes with endometritis at luteal and follicular stages relative to non-endometritis.
The differential transcription profile of three key proinflammatory cytokines, namely IL-1β, IL-8, and TNF-α in endometrial tissue of buffaloes with endometritis was also presented in the present study (). Upregulation in transcription of all three proinflammatory cytokines (IL-1β, IL-8, and TNF-α) was observed in buffaloes with endometritis either at luteal or follicular stages. The mRNA transcription of IL-1β in endometritis at luteal stage was found to be higher (1.43, P < 0.10) though it was significantly upregulated during follicular stage (13.96-fold) relative to control. The higher IL-1β transcription during follicular stage is corroborated with the findings of Fischer et al. (Citation2010) who also observed a higher level of IL-1β mRNA in endometrial tissues during the pre and post-ovulatory phases of estrous cycle. A significant upregulation of IL-1β in endometritic cows was also reported by Chapwanya et al. (Citation2009). This upregulation in IL-1β was also found in mare susceptible to post-breeding endometritis compared to mare resistant to endometritis (Fumoso et al. Citation2003). At endometrium, IL-1β is mainly produced by mononuclear phagocytes, T and B lymphocytes cells that are infiltrated and elicit the release of histamine and prostaglandin E2 (PGE2) which triggers vasodilatation and increase permeability. IL-1β stimulates the production of IL-8 responsible for chemoattraction of neutrophils and monocytes to clear the pathogen (Roach et al. Citation2002) and enhances synthesis of several other cytokines and inducible nitric oxide synthase to potentiate the immune response.
The IL-8 was also significantly upregulated both at luteal (21.19-fold) and follicular (14.38-fold) stages in buffaloes with endometritis. In the present study, a significant upregulation in IL-8 mRNA transcription in endometritic buffaloes reflecting an inflammatory status was prevailed at the endometrium level irrespective of stages of estrous cycle and corroborated with the reports of Fumoso et al. (Citation2003) who also reported a consistently higher IL-8 mRNA expression throughout the estrous cycle in mares with post-breeding endometritis. Fischer et al. (Citation2010) also reported a significant upregulation in IL-8 mRNA transcription in cows with endometritis during postpartum period. The IL-8 is known to be produced by many cell types, including mononuclear phagocytes, antigen-activated T cells, endothelial and epithelial cells, and even neutrophils (Miller and Krangel Citation1992). The profound impact of IL-8 lies in its chemotactic effects on neutrophils and its ability to stimulate granulocyte activity by upregulating cell surface adhesion molecule expressions, thereby mediates recruitment and activation of neutrophils in inflamed tissue (Warren Citation1990). Similarly, Zerbe et al. (Citation2003) also showed that infusing recombinant human IL-8 (rhIL-8) into the uterus of cows could attract PMNs into the uterus while anti-IL-8 monoclonal antibody treatment prevented PMN-dependent tissue damage as well as PMN infiltration into the uterus. These observations confirm that IL-8 is an important chemoattractant for PMNs and may be considered as an important indicator to determine the presence of inflammatory status.
The level of TNF-α was congruent with IL-8 and the highest level of transcription was observed in endometritis at luteal (42.49, P < 0.01) and also found to be significant (P < 0.01) during follicular (11.04-fold) stage. This finding receives support from several workers (Gabler et al. Citation2009; Fischer et al. Citation2010) who also observed a relatively higher transcription of TNF-α mRNA in cows with sub-clinical and clinical endometritis compared with healthy ones. In contrary, others did not find any alteration in TNF-α mRNA transcription in endometritis cows (Chapwanya et al. Citation2009; Galvão et al. Citation2011). TNF-α is produced by activated macrophages, monocytes, mast cells, and some T and natural killer cells and exerts secondary inflammatory effects by stimulating IL-6 synthesis in several cells including mononuclear phagocytes, T cells, and fibroblasts (Warren Citation1990). IL-6 then mediates its own effects and those of TNF-α and IL-1β in inducing acute phase response, and thereby perpetuating inflammatory response through a cascade of cytokines with overlapping properties (Warren Citation1990).
It has been established that a high level of TNF-α stimulates progesterone and PGE2 production that lengthens the luteal phase of estrous cycle further, and the interaction of TNF-α, PGE2, and NO at endometrium level participates in luteoprotective mechanism by modifying the ratio of PGE2 and PGF2α (Skarzynski et al. Citation2003). Furthermore, the increase in basal level of prostaglandins fails to initiate luteolysis during uterine infection as the proinflammatory cytokines along with progesterone suppress the oxytocin receptor expression (Herath et al. Citation2006). Thus, it is reasonable to suggest that in bacterial-contaminated uterine environment the luteoprotective mechanisms of TNF-α may further augment the prevailing inflammatory status and result in extended luteal phase and thereby affect the reproductive performance.
4. Conclusions
From this study, it is concluded that the endometrial transcripts of TLR-4, CD14, and proinflammatory cytokines upregulated differentially at luteal and follicular stages in endometritic buffaloes compared to the healthy one. Moreover, a higher degree of upregulation in mRNA transcription of TLR-4, IL-8, and TNF-α in endometritic buffaloes at luteal stage might represent a potential marker for the detection of endometritis, prior to clinical exhibition of endometritis at follicular stage and would be beneficial for devising novel diagnostic and therapeutic intervention.
Acknowledgment
The authors are grateful to the Director, Indian Veterinary Research Institute (Indian Council of Agri. Research, New Delhi) for providing the facility to carry out the research work. The study was partly funded by the Institute plan project.
References
- Barlund CS, Carruthers TD, Waldner CL, Palmer CW. 2008. A comparison of diagnostic techniques for postpartum endometritis in dairy cattle. Theriogenology. 69:714–723. 10.1016/j.theriogenology.2007.12.005
- Bonnet BN, Martin SW, Gannon VPJ, Miller RB, Etherington WG. 1991. Endometrial biopsy in Holstein-Friesian dairy cows I. technique, histological criteria and results. Can J Vet Res. 55:155–161.
- Chapwanya A, Meade KG, Doherty ML, Callanan JJ, Mee JF, O'Farrelly C. 2009. Histopathological and molecular evaluation of Holstein-Friesian cows postpartum: toward an improved understanding of uterine innate immunity. Theriogenology. 71:1396–1407. 10.1016/j.theriogenology.2009.01.006
- Fischer C, Drillich M, Odau S, Heuwieser W, Einspanier R, Gabler C. 2010. Selected proinflammatory factor transcripts in bovine endometrial epithelial cells are regulated during the oestrous cycle and elevated in case of sub-clinical or clinical endometritis. Reprod Fertil Dev. 22:818–829. 10.1071/RD09120
- Fumoso E, Giguere S, Wade J, Rogan D, Videla-Dorna I, Bowden R. 2003. Endometrial IL-1β, IL-6 and TNF-α, mRNA expression in mares resistant or susceptible to post breeding endometritis; effect of estrous cycle, artificial insemination and immunomodulation. Vet Immunol Immunopathol. 96:23–41.
- Gabler C, Drillich M, Fischer C, Holder C, Heuwieser W, Einspanier R. 2009. Endometrial expression of selected transcripts involved in prostaglandin synthesis in cows with endometritis. Theriogenology. 71:993–1004. 10.1016/j.theriogenology.2008.11.009
- Galvão KN, Santos NR, Galvão JS, Gilbert RO. 2011. Association between endometritis and endometrial cytokine expression in postpartum Holstein cows. Theriogenology. 76:290–299. 10.1016/j.theriogenology.2011.02.006
- Gilbert RO, Shin, Sang T, Guard CL, Hollis EN, Frajblat M. 2005. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology. 64:1879–1888. 10.1016/j.theriogenology.2005.04.022
- Gonzalez HE, Crowell WA, Caudle AB, Thompson FN. 1985. Morphometric studies of the bovine uterus: microscopic lesions and retrospective reproductive history. Am J Vet Res. 46:2588.
- Herath S, Fischer DP, Werling D, Williams EJ, Lilly ST, Dobson H, Bryant CE, Sheldon MI. 2006. Expression and function of toll-like receptor-4 in the endometrial cells of the uterus. Endocrinology. 147:562–570. 10.1210/en.2005-1113
- Ireland JJ, Murphee RL, Coulson PB. 1980. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci. 63:155–160. 10.3168/jds.S0022-0302(80)82901-8
- Kim IH, Na KJ, Yang MP. 2005. Immune response during the postpartum period in dairy cows with postpartum endometritis. J Reprod Dev. 51:757–764. 10.1262/jrd.17036
- Krishnakumar K, Amarnath M, Rajasundaram RC, Chandrahasan C. 2003. Efficacy of white side test for subclinical endometritis in crossbred cows. Indian J Dairy Sci. 56:119–120.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 10.1006/meth.2001.1262
- Miller MD, Krangel MS. 1992. Biology and biochemistry of the chemokines, a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 12:17–46.
- Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 168:4620–4627.
- Semambo DK, Ayliffe TR, Boyd JS, Taylor DJ. 1991. Early abortion in cattle induced by experimental intrauterine infection with pure cultures of Actinomyces pyogenes. Vet Rec. 129:12–16. 10.1136/vr.129.1.12
- Sheldon IM, Dobson H. 2004. Postpartum uterine health in cattle. Anim Reprod Sci. 82–83:295–306. 10.1016/j.anireprosci.2004.04.006
- Sheldon IM, Williams EJ, Aleisha NA, Deborah MN, Herath SI. 2008. Uterine disease in cattle after parturition. Vet J. 176:115–121. 10.1016/j.tvjl.2007.12.031
- Skarzynski DJ, Bah NM, Deptula KM, Woclawek-potocka I, Korzekwa A, Shibaya M, Pilawski W, Okuda K. 2003. Roles of tumor necrosis factor α of the estrous cycle in cattle, an in vivo study. Biol Reprod. 69:1907–1913. 10.1095/biolreprod.103.016212
- Warren JS. 1990. Interleukins and tumor necrosis factor in inflammation. Crit Rev Clin Lab Sci. 28:37–59. 10.3109/10408369009105897
- Williams EJ, Fischer DP, England GCW, Dobson H, Pfeiffer DU, Sheldon IM. 2005. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the inflammatory response to endometritis in cattle. Theriogenology. 63:102–117. 10.1016/j.theriogenology.2004.03.017
- Zerbe H, Schuberth HJ, Engelke F, Frank J, Klug E, Leibold W. 2003. Development and comparison of in vivo and in vitro models for endometritis in cows and mares. Theriogenology. 60:209–223. 10.1016/S0093-691X(02)01376-6
