Abstract
Cellulase gene recombinant Lactobacillus was constructed in preliminary experiments and 100 one-day-old cocks were randomly divided into four groups. Group 1 was the control group in which cocks were orally drenched with 200 µL of de Man, Rogosa and Sharpe (MRS) broth; cocks in group 2 were orally drenched with 200 µL of fermentation broth containing recombinant Lactobacillus reuteri XNY-Cel15; cocks in group 3 were orally drenched with 200 µL of fermentation broth containing recombinant L. reuteri XNY-Cel73; and cocks in group 4 were orally drenched with 100 µL of broth containing XNY-Cel15 and 100 µL of broth containing XNY-Cel73. The effect of oral drenching with recombinant Lactobacillus on chicken growth and recombinant Lactobacillus colonization of the chicken gut were investigated. Results showed that recombinant L. reuteri XNY-Cel15 and XNY-Cel73 were colonized in the crop, small intestine, and cecum; the reporter gene of recombinant Lactobacillus was detected in 30-day-old chicks, which sequences corresponding with those of the insert gene; the weights of 30-day-old chicks orally drenched with the fermentation broth containing recombinant Lactobacillus were higher than those in the control group; and the weights of chicks in the C1573 group were the most significantly high (P < 0.01).
1. Introduction
Improving the utilization of crude fiber in animal feedstuffs is an area of research interest, and the production and use of feed cellulase has been used to solve this problem. However, the expected effect has not yet been achieved. Previous studies on animal feed with viable bacteria carrying exogenous genes have mainly focused on immunization (Mashkani et al. Citation2013), while studies with viable bacteria carrying the cellulase gene remain limited. Most current methods undertake feed cellulase preparation by bioengineering for use as a feed additive (Song et al. Citation2013), and viable bacterial preparations (i.e. feed probiotics) produced by microbial fermentation technology are used for animal feeding. In preliminary experiments, digestive probiotic Lactobacillus bacteria in animals have been used as hosts to construct the cellulase gene recombinant Lactobacillus (Li et al. Citation2012). As a viable bacterial preparation, this recombinant Lactobacillus has the functions of enzymes and probiotics. Due to its simple gut, relatively few gastrointestinal microflora, and short feeding cycle, chickens are suitable for use as model animals in feeding experiments.
A large number of studies have shown that Lactobacillus has gut colonization capability (Lin Citation2006; Lam et al. Citation2007) most often used in colonization experiments are wild-type Lactobacillus bacteria. Consequently, there are few studies on the colonization capability of recombinant Lactobacillus. We investigated the colonization characteristics of recombinant Lactobacillus and detected the capability of recombinant Lactobacillus to colonize the chicken gut. Moreover, the in vivo expression of target genes of recombinant Lactobacillus and the effect of expression products on animal growth performance were also determined.
2. Material and methods
2.1. Materials
2.1.1. Experimental bacterial strains
Recombinant Lactobacillus reuteri XNY-Cel15 and XNY-Cel73 were preliminarily constructed and used as the strain for colonization (Li et al. Citation2012).
2.1.2. Experimental animals
One hundred one-day-old leghorn chicks were purchased from a hatchery in Yangling District, Shaanxi Province, China, for the feeding experiments and detection of chicken gut colonization.
2.1.3. Experimental diets
Animal feed was reserve laying hen feed (for chicks aged less than six weeks) purchased from the Shaanxi Shiyang Group.
2.1.4. Reagents
The extraction kit for bacterial genomic DNA and Taq enzyme were purchased from the Promega Corporation, various restriction endonucleases from the Takara Corporation, DL2000 Marker from the Xi'an Wolsen Bio-Tech. Co., Ltd., tryptone and yeast extract from the OXOID Corporation, and various antibiotics, glucose, beef extract, dipotassium hydrogen phosphate, ammonium citrate, sodium acetate, magnesium sulfate, manganese sulfate, and Tween-80 from the Sigma Corporation.
2.1.5. Primers
All test primers were synthesized by Shanghai Jierui High-Tech. Co., Ltd. Primers for the detection of target gene Cel15 in recombinant Lactobacillus were Xny15F: 5′-AAA CCG CGG ATG AAA CGG TCA ATC-3′ SacII and Xny15R: 5′-CGC GAG CTC CTA ATT TGG TTC TGT T-3′ SacI. Primers for the detection of target gene Cel73 in recombinant Lactobacillus were Xny73F: 5′-ATA CCG CGG ATG CCT TAT CTG AAA-3′ SacII and Xny73R: 5′-GCC GGA GCT CTT ATT TTT TTG TAT AG-3′ SacI.
2.2. Methods
2.2.1. Experimental design
The 100 one-day-old leghorn chicks were randomly divided into four groups, with 25 chicks in each group. Group 1 (control) chicks were orally drenched with 200 µL of de Man, Rogosa and Sharpe (MRS) broth; Group 2 (C15 group) chicks were orally drenched with 200 µL of fermentation broth containing recombinant L. reuteri XNY-Cel15; Group 3 (C73 group) chicks were orally drenched with 200 µL of fermentation broth containing recombinant L. reuteri XNY-Cel73; and Group 4 (C1573 group) chicks were orally drenched with 100 µL of broth containing XNY-Cel15 and 100 µL of broth containing XNY-Cel73. Five chick batches in each group were sacrificed at the age of one week, two weeks, three weeks, and one month, respectively, and after Lactobacillus in the crop, small intestine, and cecum were isolated, the existence of recombinant Lactobacillus was determined by polymerase chain reaction (PCR) amplification of the target genes. During feeding, all chicks ate and drank water freely, without immunization. Each batch of chicks was weighed before sacrifice. The changes in chicken body weight during feeding were statistically analyzed.
2.2.2. Detection method for recombinant Lactobacillus colonization of the chicken gut
Chicks at different feeding stages in each experimental group were aseptically sacrificed. An appropriate amount of the crop, small intestine, and cecum content was scraped with a scalpel, placed in a 1.5 ml microcentrifuge tube with 1 ml of liquid MRS medium, and incubated at 37°C for 10 hours. One hundred microliters of the culture were pipetted and then centrifuged at 10,000 rpm/minutes for one minute. After discarding the supernatant and adding 100 µl of sterile water, the suspension was shaken, bathed in boiling water for two minutes, and centrifuged at 10,000 rpm/minutes for one minute. The supernatant was then used as a template, with the upstream and downstream primers (Xny15F and Xny15R as well as Xny73F and Xny73R) of the target genes (Cel15 and Cel73) used as primers, respectively, and PCR was performed. The products of target gene amplification were detected by agarose gel electrophoresis.
3. Results and discussion
3.1. Recombinant Lactobacillus colonization of the chicken gut
After the isolation of culture, the bacterial suspensions from the chicken gut contents were used for PCR amplification. The amplification results are shown in .
Note: The marker in the figure is DL2000; the sample order of PCR detection for each group is the contents of crop, small intestine, and cecum; the lane without labeling is the control group sample; colony growth only occurred in the crop contents of one-week-old chicks and in the crop and small intestine contents of two-week-old chicks in the control group, so there is only one lane and two lanes, respectively, for the two controls.
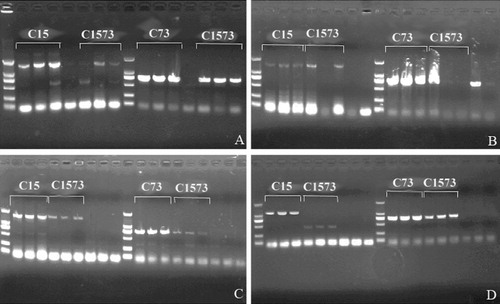
Detection results of samples from seven-day-old chicks are as follows. A 1.5-kb fragment of target gene was detected in the crop, small intestine, and cecum of chicks in the C15 group with recombinant L. reuteri XNY-Cel15, corresponding to the Cel15 gene fragment; a 740-bp target gene fragment was detected in the crop, small intestine, and cecum of chicks in the C73 group with recombinant L. reuteri XNY-Cel73, corresponding to the Cel73 gene fragment; while in the C1573 group with a mixture of two recombinant Lactobacillus, the 1.5-kb fragment corresponding to the Cel15 gene was detected in the small intestine, and cecum but not in the crop of chicks and the 740-bp fragment corresponding to the Cel73 gene was successfully detected in the crop, small intestine, and cecum of chicks. No gene fragments were detected in the control group ().
Detection results of samples from 14-day-old chicks are as follows. The fragments of corresponding exogenous genes were respectively detected in the crop, small intestine, and cecum of chicks in the C15 and C73 groups; the fragment corresponding to the Cel15 gene was detected in the crop and cecum of chicks in the C1573 group and the fragment corresponding to the Cel73 gene was detected in the crop, without detection of gene fragments in the small intestine. The fragment corresponding to the Cel73 gene was also detected in a sample from the control group, which may relate to possible contamination during sampling ().
The detection results of samples from 21-day-old chicks are as follows. The fragments of corresponding exogenous genes were respectively detected in the crop, small intestine, and cecum of chicks in the C15, C73, and C1573 groups. No gene fragments were detected in the control group ().
The detection results of samples from 30-day-old chicks are as follows. The fragments of corresponding exogenous genes were respectively detected in the crop, small intestine, and cecum of chicks in the C15 and C73 groups; the fragment corresponding to the Cel73 gene was detected in the crop, small intestine, and cecum of chicks in the C1573 group, without the detection of the fragment corresponding to the Cel15 gene. No gene fragments were detected in the control group ().
The nucleotide sequences of gene fragments detected in the above experimental groups were the same as those of the insert genes in recombinant Lactobacillus, suggesting that recombinant Lactobacillus carrying the target gene was successfully colonized in the chicken gut.
Previous studies regarding microbial colonization of the gut mainly focused on heredity and classification (Tierney et al. Citation2004). Fluorescent labeling is commonly used to track the distribution and colonization of microorganisms in the gut (Fortineau et al. Citation2000). In the present study, the distribution and colonization of recombinant Lactobacillus were determined by detecting the reporter genes of recombinant Lactobacillus in the microflora in different parts of the gut and at different stages. The results demonstrated that recombinant Lactobacillus was capable of colonization in all parts of the chicken gut, with a colonization time of at least 30 days. However, the amount of recombinant Lactobacillus colonization in different parts of the chicken gut and at different stages needs to be further investigated.
3.2. Effect of recombinant Lactobacillus on chicken growth performance
Using the SPSS statistics software, one-way analysis of variance was performed for the initial weight of the experimental cocks, weights of cocks aged 7–30 days, and weight gain of experimental cocks during feeding. The results are shown in . There were no differences in initial weights of experimental chicks (P = 0.498), non-significant differences in 14-day-old chick weights, and very significant differences in 30-day-old chick weights (P < 0.01) between the experimental groups during feeding. The average weight of 30-day-old chicks in the C1573 group was significantly higher than that in the control group, and so were those in the C15 and C73 groups, but with non-significant differences. The average weight gains of experimental chicks between the experimental groups showed significant differences (P < 0.01), corresponding with differences in average weight of 30-day-old chicks.
Table 1. The effect to bodyweight of different treatments.
Previous studies regarding Lactobacillus colonization of the chicken gut were mainly concerned with mucosal immunity against pathogenic microorganisms (Dalloul et al. Citation2005). In the present study, with the effects of recombinant Lactobacillus and its secreted cellulase on chicken growth performance as starting points, the results were consistent with those of most studies regarding the addition of exogenous cellulase in animal feed or cellulase expressed by microorganisms (Gao et al. Citation2008). Chicken growth performance was improved by recombinant Lactobacillus colonization. There were very significant differences in the weights of 30-day-old chicks between the experimental groups. The weights of 14- and 21-day-old chicks showed non-significant differences between the experimental groups (P < 0.05), while the weights of 14-day-old chicks in the control group were higher than those in the other experimental groups. The reasons may be that, in the first week of feeding, incandescent heating was provided and an apparent vent-pecking behavior of chicks occurred due to intense light and no debeaking, which influenced eating and water drinking. The vent-pecking behavior was more apparent with more intense light; the chicks in the control group were farthest from the light source and did not exhibit vent-pecking behavior.
The diets used in the present study were basal diets, without control of crude fiber content level. Barley-, wheat- or rye-based diets high in crude fiber and mucopolysaccharides have been used in most previous studies (Liu et al. Citation2007; Yu et al. Citation2008), and the results obtained in those studies showed more significant differences. The weight gains in 30-day-old chicks from the C1573 group with a mixture of two recombinant Lactobacillus bacteria were significantly higher than those in the control group. The main reason is that the synergistic action of the expression products of the two cellulase genes accelerated the decomposition of feed carbohydrates (not just cellulose). This affected chicken growth performance significantly because proteins expressed by the Cel73 gene are not capable of decomposing cellulose directly (Li et al. Citation2009), but are likely to decompose starch polysaccharides other than cellulose, thus increasing the digestibility value of total carbohydrates.
4. Conclusions
Recombinant L. reuteri XNY-Cel15 and XNY-Cel73 were colonized in the crop, small intestine, and cecum of experimental chicks; and the reporter gene of recombinant Lactobacillus were detected in 30-day-old chicks, with its sequences corresponding with those of the insert gene. The weights of 30-day-old chicks orally drenched with the fermentation broth containing recombinant Lactobacillus were higher than those in the control group, and the weights of chicks in the C1573 group were the most significantly high (P < 0.01).
Funding
This study was under the auspices of the National Natural Science Foundation of China [No. 31101744].
Additional information
Funding
References
- Dalloul RA, Lillehoj HS, Tamim NM, Shellem TA, Doerr JA. 2005. Induction of local protective immunity to Eimeria acervulina by a lactobacillus-based probiotic. Comp Immunol Microbiol Infect Dis. 28:351–361. 10.1016/j.cimid.2005.09.001
- Fortineau N, Trieu-Cuot P, Gaillot O, Pellegrini E, Berche P, Gaillard J-L. 2000. Optimization of green fluorescent protein expression vectors for in vitro and in vivo detection of Listeria monocytogenes. Res Microbiol. 151:353–360. 10.1016/S0923-2508(00)00158-3
- Gao F, Jiang Y, Zhou GH, Han ZK. 2008. The effects of xylanase supplementation on performance, characteristics of the gastrointestinal tract, blood parameters and gut microflora in broilers fed on wheat-based diets. Anim Feed Sci Technol. 142(1–2):173–184. 10.1016/j.anifeedsci.2007.07.008
- Lam EKY, Yu L, Wong HPS, Wu WKK, Shin VY, Tai EKK, So WHL, Woo PCY, Cho CH. 2007. Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur J Pharmacol. 565:171–179. 10.1016/j.ejphar.2007.02.050
- Li W, Huan X, Zhou Y, Ma Q, Chen Y. 2009. Simultaneous cloning and expression of two cellulase genes from Bacillus subtilis newly isolated from Golden Takin (Budorcas taxicolor Bedfordi). Biochem Biophys Res Commun. 383:397–400. 10.1016/j.bbrc.2009.04.027
- Li W, Yang M-M, Zhang G-Q, He W-L, Li Y-X, Chen Y-L. 2012. Electrotransformation and expression of cellulase genes in wild-type Lactobacillus reuteri. J Mol Microbiol Biotechnol. 22:228–234. 10.1159/000341906
- Lin X-Y. 2006. The research of adhesion of Lactobacillus in chicken gut. Tai'an: Shandong Agricultural University.
- Liu JR, Lai SF, Yu B. 2007. Evaluation of an intestinal Lactobacillus reuteri strain expressing rumen fungal xylanase as a probiotic for broiler chickens fed on a wheat-based diet. Br Poult Sci. 48:507–514. 10.1080/00071660701485034
- Mashkani B, Odell AF, Byrnes EM, Griffith R, Ashman LK. 2013. Expression of biologically active human colony stimulating factor-1 in Pichia pastoris. Protein Expr Purif. 88:93–97. 10.1016/j.pep.2012.11.017
- Song Y, Lee YG, Choi IS, Lee KH, Cho EJ, Bae H-J. 2013. Heterologous expression of endo-1,4-β-xylanase a from Schizophyllum commune in Pichia pastoris and functional characterization of the recombinant enzyme. Enzyme Microb Technol. 52:170–176. 10.1016/j.enzmictec.2012.12.012
- Tierney J, Gowing H, Van Sinderen D, Flynn S, Stanley L, McHardy N, Hallahan S, Mulcahy G. 2004. In vitro inhibition of Eimeria tenella invasion by indigenous chicken Lactobacillus species. Vet Parasitol. 122:171–182. 10.1016/j.vetpar.2004.05.001
- Yu B, Liu JR, Hsiao FS, Chiou PWS. 2008. Evaluation of Lactobacillus reuteri Pg4 strain expressing heterologous β-glucanase as a probiotic in poultry diets based on barley. Anim Feed Sci Technol. 141:82–91. 10.1016/j.anifeedsci.2007.04.010
