Abstract
The purpose of the study was to provide a description of normal cross-sectional anatomy of the camel brain and associated structures using computed tomography (CT) and macroscopic cross sections. Transverse images of two isolated camel cadaver heads were obtained by an axial CT equipment. CT scans were processed with a detailed algorithm using bone and soft-tissue windows settings, and compared with the corresponding frozen cross sections of the heads, to assist in the accurate identification of brain and associated structures. CT images provided good differentiation between the bones and the soft tissues of the head. These CT images are intended to be a useful anatomic reference in the interpretation for clinical CT imaging studies of the brain and associated structures in dromedary camels.
1. Introduction
In camels, the exploration of the anatomical structures located in the head and the evaluation of the soft tissues of oral and nasal cavities are difficult due to its complex anatomical organization. Physical exploration and current diagnostic imaging techniques such as radiography and ultrasonography provide limited information for evaluation of soft tissues of the camel head (Osuobeni & Hamidzada Citation1999; Byers et al. Citation2007).
Modern image diagnostic techniques, especially computed tomography (CT), make it possible to obtain body sections from different tomographic planes, achieving images with a good anatomical resolution, high contrast between different structures and excellent tissues differentiation, contributing significantly to the progress of diagnostic imaging in veterinary medicine. In large animals, CT has been used sparingly for descriptive anatomic research (Hathcock et al. Citation1995; Morrow et al. Citation2000; De Zani et al. Citation2010; Zafra et al. Citation2012) and several studies have demonstrated the clinical value of CT in diagnosing diseases of the head (Tietje et al. Citation1996; Warmerdam et al. Citation1997). In camelids, CT anatomy of the nasal cavity (Bai et al. Citation2008; Wang et al. Citation2008), temporomandibular joint (Arencibia et al. Citation2012), tarsus (Hagag et al. Citation2013) and metatarsus and digits (El-Shafey & Kassab Citation2013) has been studied, but to the author's knowledge, there is no published material describing the results of CT anatomy of the brain and associated structures in dromedary camel applying bone and soft-tissue CT windows.
An accurate CT interpretation of the cross-sectional anatomy of the dromedary camel head could be useful in the evaluation and diagnosis of different pathological processes such as fractures, congenital diseases, infections, inflammations, tumours, etc. (Gitao Citation1997; Zayed Citation1998; Byers et al. Citation2007). The objective of this study is to provide an overview of the normal cross-sectional anatomy of the camel brain and associated structures using CT images and gross anatomic sections. These CT images are intended to be a useful anatomic reference for clinical studies of the head in dromedary camels.
2. Materials and methods
Two adult male dromedary camels belonging to a camel farm located in Fataga, Gran Canaria, Canary Islands (Spain) were referred to the Hospital of the Veterinary Faculty of Las Palmas of Gran Canaria, Canary Islands, Spain. One was 8 years old and the other was 10 years old. Animals were subjected to clinical examination including observation and palpation of the head. No external head lesions were seen in the head of the two camels used in this study. Camels were euthanized in the case of severe arthritis. We have used 10 mg/kg intravenous sodium pentobarbital 10% (Braun Medical, Melsungen, Germany). After being anesthetized, they were euthanized using 30–35 ml approximately intravenous T-61 (embutramide, mebezonium iodide, tetracaine hydrochloride) injectable solution (Merck Animal Health; Intervet, Madrid, Spain). This work was approved by the Ethical Committee of the Veterinary Faculty of Las Palmas de Gran Canaria University (Protocol 52/2009). Each head was separated from the body at the atlantoaxial joint level to enable their evaluation in an upright position. Both heads were used for CT study and for obtaining the anatomic sections.
The CT evaluation of head was performed within 12 hours of euthanasia to minimize post-mortem changes. CT imaging was performed at the Radiodiagnostic Service of the Hospital Universitario Insular of Las Palmas de Gran Canaria (Spain), with an axial CT equipment (Toshiba 600 HQ, Toshiba Medical Imaging System®, Tustin, CA, USA). Throughout the procedure, the heads were positioned in ventral recumbency during scanning time. Transverse CT images were obtained using the following parameters: 120 kV, 200 mA, 3 mm slice thickness and 3 mm image spacing. To appreciate the CT appearance of the nasal and oral cavities, two CT windows were applied by adjusting window widths (WWs) and window levels (WLs): a bone window setting (WW = 4000; WL = 335) and a soft-tissue window setting (WW = 328; WL = 75).
At the end of the CT study, the heads were frozen, and sectioned using an electric bandsaw at the positions, which matched the CT images as closely as possible. CT images were compared to the corresponding anatomical sections and with anatomy literature (Smuts & Bezuidenhout Citation1987), to identify the normal CT anatomy of the brain and associated structures of the dromedary camel. In addition, osseous anatomic preparations were used to facilitate accurate anatomic interpretation of the CT images and anatomic sections. According to an internationally accepted veterinary anatomical nomenclature (Schaller Citation2007), several structures were identified.
3. Results
In this study, the results are presented in six figures (–). Each figure consists of three images: (A) bone window CT image, (B) soft-tissue window CT image and (C) anatomic section. Figures are shown from rostral to caudal progression, from the level of the olfactory bulb () to the myelencephalon (). Images are presented with the dorsal aspect of the head at the top of the photograph and the right side of the head on the left side of the photograph. No internal head lesions were seen on CT images. Clinically relevant anatomic structures of the brain and associated structures have been evaluated and labelled according to location and the characteristics of degree of attenuation of the different tissues. No anatomic or degree of attenuation variations were detected in the head of the two camels used in the study because of the similar size of the animals.
All views are rostral. 1, frontal sinus; 2, cribriform plate of ethmoid bone; 3, frontal cortex; 4, olfactory bulb; 5, sphenoidal sinus; 6, extraperiorbital fat; 7, zygomatic process of temporal bone; 8, body of presphenoid bone; 9, vomer bone; 10, tensor and levator palatine muscles; 11, pars nasalis pharyngis; 12, perpendicular plate of palatine bone; 13, upper third molar; 14, soft palate; 15, masseter muscle; 16, oral cavity; 17, lower third molar; 18, tongue and lingual fat; 19, hyoid bone; 20, mandibular canal.
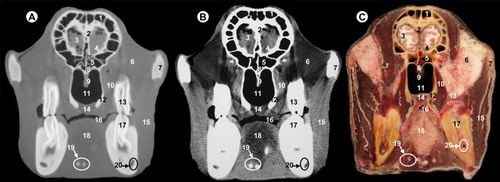
All views are rostral. 1, parietal bone; 2, dorsal sagittal sinus; 3, cerebral longitudinal fissure; 4, temporal muscle; 5, squamous part of temporal bone; 6, cerebral hemisphere; 7, coronoid process of mandible; 8, wing of presphenoid bone; 9, body of presphenoid bone; 10, cavernous sinus; 11, zygomatic process of temporal bone; 12, vomer bone; 13, pars nasalis pharyngis; 14, lateral pterygoideus muscle; 15, perpendicular plate of palatine bone; 16, ramus of mandible; 17, masseter muscle; 18, soft palate; 19, pharyngeal muscles; 20, pars oralis pharyngis; 21, tongue and lingual fat; 22, hyoid bone; 23, mandibular canal; 24, medial pterygoideus muscle.
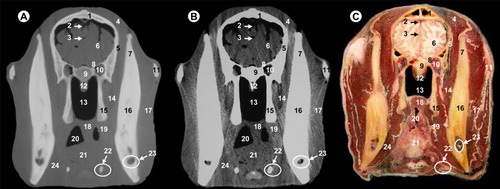
All views are rostral. 1, parietal bone; 2, dorsal sagittal sinus; 3, cerebral longitudinal fissure; 4, lateral ventricle; 5, cerebral hemisphere; 6, temporal muscle; 7, squamous part of temporal bone; 8, third ventricle; 9, thalamus; 10, hypothalamus; 11, hypophysis; 12, cavernous sinus; 13, body of basisphenoid bone; 14, zygomatic process of temporal bone; 15, temporomandibular joint; 16, maxillary vein; 17, lateral pterygoideus muscle; 18, pars nasalis pharyngis; 19, ramus of mandible; 20, mandibular canal; 21, masseter muscle; 22, medial pterygoideus muscle; 23, pharyngeal muscles; 24, pars oralis pharyngis; 25, hyoid bone.
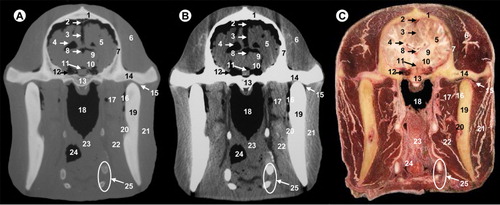
All views are rostral. 1, parietal bone; 2, dorsal sagittal sinus; 3, temporal muscle; 4, cerebral longitudinal fissure; 5, cerebral hemisphere; 6, third ventricle; 7, mesencephalic tectum; 8, mesencephalic aqueduct; 9, temporal sinus; 10, cerebral peduncle; 11, subarachnoid space; 12, body of basisphenoid bone; 13, ophthalmic, abducens, trochlear and oculomotor nerves; 14, zygomatic process of temporal bone; 15, temporomandibular joint; 16, condylar process of mandible; 17, lateral pterygoideus muscle; 18, maxillary vein; 19, pars nasalis pharyngis; 20, medial pterygoideus muscle; 21, masseter muscle; 22, hyoid bone; 23, pharyngeal muscles; 24, pars oralis pharyngis; 25, epiglottis.
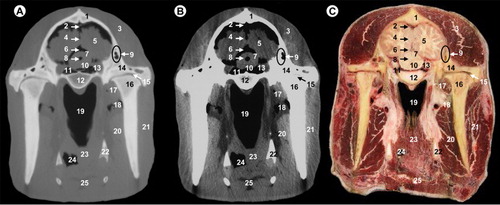
All views are rostral. 1, parietal bone; 2, transverse sinus; 3, temporal muscle; 4, cerebellar hemisphere; 5, cerebellar vermis; 6, fourth ventricle; 7, pons; 8, petrous part of temporal bone; 9, basilar part of occipital bone; 10, tympanic part of temporal bone; 11, pars nasalis pharyngis; 12, pars laryngea pharyngis; 13, medial pterygoideus muscle; 14, ramus of mandible; 15, parotid gland and masseter muscle; 16, pharyngeal muscles; 17, vestibule of larynx; 18, epiglottis; 19, hyoid bone; 20, mandibular gland.
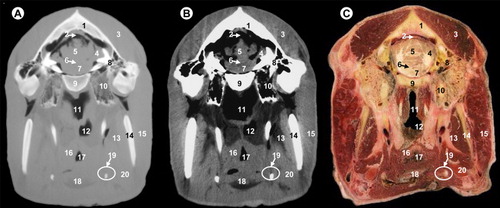
All views are rostral. 1, parietal bone; 2, transverse sinus; 3, temporal muscle; 4, cerebellar vermis; 5, cerebellar hemisphere; 6, temporal sinus; 7, fourth ventricle; 8, pons; 9, basilar part of occipital bone; 10, petrous part of temporal bone; 11, rectus ventralis and longus capitis muscles; 12, pars laryngea pharyngis; 13, hyoid bone; 14, ramus of mandible; 15, parotid gland and masseter muscle; 16, medial pterygoideus muscle; 17, vestibule of larynx; 18, pharyngeal muscles; 19, mandibular gland.
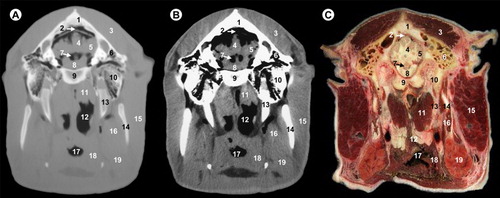
In this study, the frontal bone (orbital part and squama), parietal, temporal (with their squamous, petrous and tympanic parts), the presphenoid bone (body and wing), basisphenoid bone (body and wing) and the occipital bone (with their basilar part, lateral parts and squama) could be identified. In our study, the use of the bone window (–) produced excellent images with high bone details of the cranial bones, mandible and hyoid bones, so that clear differentiations could be made between the cortical (high attenuating line) and medulla (homogeneous low attenuating structure), compared to other structures such as the muscles or salivary glands observed with intermediate degree of attenuation. However, with the soft-tissue window CT image (–), cortical and medulla cannot be differentiated.
The surrounding soft-tissue structures such as the muscles, salivary glands and associated mucosal surfaces of the pharynx and larynx cavities were visible in both anatomic sections and CT images, and are better defined with the soft-tissue window compared to the bone window. Thus, the soft palate, the tongue and masticatory muscles such as the temporal muscle, the masseter muscle, lateral and medial pterygoideus muscles appear with intermediate degree of attenuation, compared with the cranial bones observed with high degree of attenuation.
The relationship between various air cavities was easily visualized in CT images and anatomic sections. Thus, frontal and sphenoidal sinuses, pars nasalis pharyngis, pars oralis pharyngis, pars laryngea pharyngis and cavity of the larynx were also visible. These cavities appear with low degree of attenuation on both CT images compared to associated mucosal surfaces observed with intermediate degree of attenuation.
Several parts of the encephalon could be identified on CT images: the olfactory bulb, olfactory peduncle, corpus callosum, fornix, internal capsule, optic chiasm, thalamus, hypothalamus, hypophysis, cavernous sinus, mesencephalon (including the mesencephalic tectum and cerebral peduncle), pons, myelencephalon, cerebral hemispheres and cerebellum (with their vermis and hemispheres). The lateral ventricle, the third ventricle, the mesencephalic aqueduct and the fourth ventricle were also visible.
Other anatomic structures such as the inferior alveolar nerve in their respective mandibular canal appear with intermediate attenuation with both CT windows settings. In the bone window, fat appears with an intermediate attenuation, and could not differentiate of the muscles. In the soft-tissue window lingual fat is observed with a low degree of attenuation, compared to intermediate degree of attenuation observed in the masticatory and pharyngeal muscles. Vascular structures such as the inferior alveolar artery and vein in their mandibular canal of the ramus of mandible appeared with intermediate degree of attenuation in both CT windows, and could be differentiated through their lower attenuation compared to the surrounding structures.
4. Discussion
In camels, the exploration of the anatomical structures of the brain and associated structures is difficult due to its complex anatomical organization. CT images provided excellent details of clinically relevant anatomy and correlated well with corresponding gross specimens of the brain and associated structures of the dromedary camel. Knowledge of normal cross-sectional anatomy of the camel head is essential for the evaluation of CT scans. This study could serve as an initial reference to aid in the diagnosis by CT of disorders of the camel head.
CT imaging is a diagnostic technique that offers considerable advantages over traditional radiography: a lack of superimposition of the tissues and a higher differentiation of tissue densities (Solano & Brawer Citation2004; De Zani et al. Citation2010). CT provides excellent spatial resolution and good discrimination between bone and soft tissue of the head (Hathcock et al. Citation1995; Morrow et al. Citation2000; De Zani et al. Citation2010). CT is more sensitive in detecting diseases, and distinguishes normal and abnormal structures accurately (Tietje et al. Citation1996; Warmerdam et al. Citation1997; Byers et al. Citation2007). In camels, the principal disadvantages of CT imaging are related to the availability of the equipment and the need for general anaesthesia. With developing technology, CT imaging may soon become more readily available for large animals. Recent studies of other anatomical regions, such as the tarsus (Hagag et al. Citation2013) and metatarsus and digits (El-Shafey & Kassab Citation2013), described the potential of CT in camel medicine. Because only two cadaver heads were included in our study, further studies concerning the CT appearance of the camel head in juvenile and adults live animals would be necessary to compare the normal relative positions and sizes of anatomic structures.
Reports of CT imaging of the head in llama (Hathcock et al. Citation1995) and horse (Morrow et al. Citation2000; Smallwood et al. Citation2002; Solano & Brawer Citation2004; De Zani et al. Citation2010) applied similar technical parameters (kV, mA). Nevertheless, in most cases the slice thickness and image spacing were 5–10 mm, while we have used 3 mm in our study. In this respect, we believe that a smaller slice thickness and image spacing allowed us to obtain a major degree of identification of anatomical structures. In our study, we have used two modalities of CT windows: the bone window and the soft-tissue window. Both CT windows were selected according to a protocol used in human medicine. Other authors used different CT windows. In this respect, Hathcock et al. (Citation1995) used the bone and soft-tissue CT window; Morrow et al. (Citation2000) and Smallwood et al. (Citation2002) do not specify the CT window used on the head in the horse; only Morrow et al. (Citation2000) indicated that realized adjustments of the WW and WL for the differentiation of the different cephalic tissues; Solano and Brawer (Citation2004) used the bone, the nasal and the soft-tissue CT windows; and De Zani et al. (Citation2010) used the bone window.
The sectional anatomy allows a correct morphologic and topographic evaluation of the anatomic structures, and it is a helpful tool for the identification of the CT images (Smallwood et al. Citation2002; De Zani et al. Citation2010). Another important aspect to consider is the fact of used isolated heads instead of alive animals. Several reports of CT imaging of the head use alive animals (Hathcock et al. Citation1995; Probst et al. Citation2005), whereas Morrow et al. (Citation2000); Smallwood et al. (Citation2002); Solano and Brawer (Citation2004); and De Zani et al. (Citation2010) use isolated heads. In the present study, to minimize soft-tissue findings associated with post-mortem changes, the CT study was performed within 12 hours of euthanasia; however, several venous vessels were air-filled in the images. The air accumulation was attributed to post-mortem changes as described Morrow et al. (Citation2000). In this respect, due to processes of coagulation or to the absence of blood flow, some vascular structures do not correctly appreciate observed in CT images and anatomic sections.
5. Conclusion
CT is an excellent method for the detailed assessment of the anatomical structures of the brain and associated structures of camels. The bone CT window provides more anatomical details to identify the cranial bones and mandible, whereas the soft-tissue CT window is recommended to identify encephalic structures, muscles or salivary glands. CT images should provide initial anatomic reference for further anatomical and clinical CT imaging studies of the brain and associated structures of the camel and for interpreting lesions of these anatomic regions.
Acknowledgements
The authors would like to thank to Radio Diagnostic Service of the Hospital Insular of Las Palmas de Gran Canaria (Spain), especially, Dra. Alemán for her technical assistance.
Additional information
Funding
References
- Arencibia A, Blanco D, Gonzalez N, Rivero MA. 2012. Computed tomography and magnetic resonance imaging features of the temporomandibular joint in two normal camels. Anat Res Int. 2012:1–6.
- Bai ZT, Wang HJ, Chen JC, Yuan GQ, He JB, Wang JL. 2008. The computed tomography and gross anatomies of nasal cavity and sinuses in the Bactrian camel (Camelus bactrianus). Sciencepaper Online 1–9 [cited 2013 Nov 15]. Available from: http://www.paper.edu.cn/index.php/default/en_releasepaper/downPaper/200812-184
- Byers SR, Parish SM, Holmes SP, Donahoe SL, Barrington GM. 2007. A fungal granuloma of the frontal sinus in a llama. Can Vet J. 48:939–941.
- De Zani D, Borgonovo S, Biggi M, Vignati S, Scandella M, Lazzaretti S, Modina S, Zani D. 2010. Topographic comparative study of paranasal sinuses in adult horses by computed tomography, sinuscopy, and sectional anatomy. Vet Res Commun. 34:13–16. 10.1007/s11259-010-9381-6
- El-Shafey A, Kassab A. 2013. Computed tomography and cross-sectional anatomy of the metatarsus and digits of the one-humped camel (Camelus dromedarius) and buffalo (Bos bubalis). Anat Histol Embryol. 42:130–137. 10.1111/j.1439-0264.2012.01174.x
- Gitao CG. 1997. An investigation of camelpox outbreaks in two principal camel (Camelus dromedarius) rearing areas of Kenya. Rev Sci Tec. 16:841–847.10.1016/S0277-3791(96)00115-1
- Hagag U, Brehm W, Ramadan RO, Al Mubarak A, El Vahas A, Gerlach K. 2013. Computed tomography and cross-sectional anatomy of the normal dromedary camel tarsus (one humped camel). Anat Histol Embryol. 42:266–274. 10.1111/ahe.12011
- Hathcock JT, Pugh DG, Cartee RE, Hammond L. 1995. Computed tomography of the llama head: technique and normal anatomy. Vet Radiol Ultrasound. 36:290–296. 10.1111/j.1740-8261.1995.tb00265.x
- Morrow KL, Park RD, Spurgeon TL, Stashak TS, Arceneaux B. 2000. Computed tomographic imaging of the equine head. Vet Radiol Ultrasound. 41:491–497. 10.1111/j.1740-8261.2000.tb01876.x
- Osuobeni EP, Hamidzada WA. 1999. Ultrasonographic determination of the dimensions of ocular components in enucleated eyes of the one-humped camel (Camelus dromedarius). Res Vet Sci. 67:125–129. 10.1053/rvsc.1998.0288
- Probst A, Henninger W, Willmann M. 2005. Communications of normal nasal and paranasal cavities in computed tomography of horses. Vet Radiol Ultrasound. 46:44–48. 10.1111/j.1740-8261.2005.00008.x
- Schaller O. 2007. Illustrated veterinary anatomical nomenclature. 2nd ed. Stuttgart: Enke.
- Smallwood JE, Wood BC, Taylor E, Tate Jr LP. 2002. Anatomic reference for computed tomography of the head of the foal. Vet Radiol Ultrasound. 43:99–117. 10.1111/j.1740-8261.2002.tb01657.x
- Smuts MM, Bezuidenhout AJ. 1987. Anatomy of the dromedary. Oxford: Clarendon Press.
- Solano M, Brawer RS. 2004. CT of the equine head: technical considerations, anatomical guide, and selected diseases. Clin Tech Equine Pract. 3:374–388. 10.1053/j.ctep.2005.02.016
- Tietje S, Becker M, Bockenhoff G, 1996. Computed tomographic evaluation of head diseases in the horse. 15 cases. Equine Vet J. 28:98–105. 10.1111/j.2042-3306.1996.tb01599.x
- Wang H, Bai Z, Gao C, Wang J, 2008. Numerical simulation of inspiratory airway in nasal cavity of Bactrian camels (Camelus bactrianus). Sciencepaper Online 1–8 [cited 2013 Nov 15]. Available from: http://www.paper.edu.cn/index.php/default/en_releasepaper/downPaper/200809-784
- Warmerdam EPL, Klein WR, Van-Herpen BP. 1997. Infectious temporomandibular joint disease in the horse: computed tomographic diagnosis and treatment of two cases. Vet Rec. 141:172–174. 10.1136/vr.141.7.172
- Zafra R, Carrascosa C, Rivero M, Peña S, Fernández T, Suárez-Bonnet A, Jaber JR. 2012. Analysis of equine cervical spine using three-dimensional computed tomographic reconstruction. J Appl Anim Res. 40:108–111. 10.1080/09712119.2011.621532
- Zayed AA. 1998. Localization and migration route of Cephalopina titillator (Diptera: Oestridae) larvae in the head of infested camels (Camelus dromedarius). Vet Parasitol. 80:65–70. 10.1016/S0304-4017(98)00182-4
