Abstract
The study was designed to compare the size of dominant follicle (DF) achieved, plasma progesterone decline and plasma estradiol level on the day of estrus and subsequent conception in buffaloes subjected to modified ovsynch-based regimen in buffaloes. In estradiol-based protocol (n = 15) on day 0 (beginning of experiment), buffaloes were administered controlled internal drug release (CIDR) device (1.38 g P4) and concurrently received 1.5 mg estradiol-17β in 1.5 ml canola oil (i.m.). On day 9, CIDR was removed and a PGF2α analogue (500 µg, i.m.) administered. On day 11, buffaloes were administered gonadotropin releasing hormone (GnRH) analogue (20 µg, i.m.) and inseminated on day 11 and day 12. Ovsynch-based group (control, n = 15) received GnRH on day 0 in place of estradiol-17β, CIDR insert for 7 instead of 9 days and the remaining protocol and insemination procedures were same as treatment group. The diameter of DF, plasma progesterone and estradiol levels were not different between the groups. The first service conception rate (FSCR) was higher (p < 0.05) in estradiol-based group than in control (53.33 vs. 33.33%, respectively). In conclusion, replacement of first GnRH with estradiol-17β in ovsynch plus CIDR protocol and increasing CIDR exposure by two days leads to higher FSCR.
1. Introduction
In buffalo, changes in hormone secretion during synchronization are poorly understood and the size of ovary and follicles is less, compared to cattle. Recent protocols for synchronization in buffalo are based on similar studies performed in cattle. In ovsynch regimen, first-gonadotropin releasing hormone (GnRH) usually increases plasma progesterone by inducing ovulation followed by luteinization of a dominant follicle (DF) while the second-GnRH synchronizes pre-ovulatory luteinizing hormone (LH) surge and ovulation. In cattle, administration of a controlled internal drug release (CIDR) device and estradiol (either EB or E17β) plus progesterone treatment effectively synchronized estrus and resulted in acceptable conception rates to fixed-time artificial insemination (FTAI; Martinez et al. Citation2000). Moreover, estradiol-based synchronization protocols induced an estrus more characteristic of a spontaneous ovulation (Martinez et al. Citation2000). The follicles in summer anestrous buffaloes exhibited typical follicular wave pattern and in fact attained ovulatory size (>10 mm diameter) but failed to ovulate followed by their regression (Ghuman et al. Citation2008).
Literature on physiological aspects of the follicular growth, dominance and regression in buffaloes remain sparse (Manik et al. Citation2002). Warriach et al. (Citation2008) reported that the size ovulatory follicle achieved in PGF2α-induced luteolysis and ovsynch protocol in Nili-Ravi buffaloes are 12.4 ± 0.2 mm and 12.1 ± 0.3 mm, respectively. Progesterone assay has been applied in buffalo under practical farming conditions for monitoring the changes in its plasma level and ovarian activity (Banu et al. Citation2012). The concentration of oestradiol-17β in blood during the follicular phase of the cycle also appears to be relatively lower (Kanai et al. Citation1990). This has been suggested as a possible reason for the lower intensity of oestrous signs exhibited by buffalo (Borghese Citation2005). Further the decline in plasma progesterone was followed by increase in the diameter of largest/DFs on the day of CIDR withdrawal during the subsequent 72 h (Ghuman et al. Citation2012) and this might have resulted in elevated levels of estradiol. Moreover, the diameter of largest follicle on day of estrus was larger in buffaloes synchronized with CIDR device. The positive effect of progesterone on the follicular growth rate and diameter of the DF has also been reported in bovines (Sa Filho et al. Citation2010). Hence, the present study was designed with the objectives to determine the size of DF achieved, plasma progesterone decline and plasma estradiol level on the day of estrus and subsequent conception in buffaloes following the treatment with estradiol + CIDR in place of GnRH + CIDR in the conventional protocol.
2. Materials and methods
2.1. Experimental animals
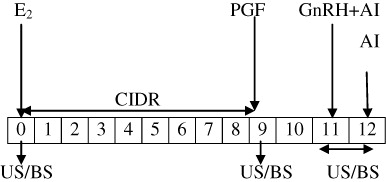
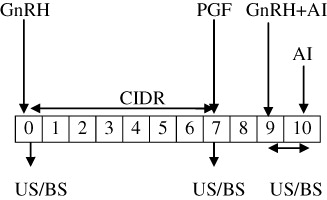
The study was conducted on 30 apparently healthy, lactating Murrah buffaloes in their second to fourth parity. Animals having body weight ranging between 400 and 500 kg and body condition score (BCS) of 4–5 as per the guidelines of BCS chart were selected. The animals had the history of lactational anestrum since last 4–5 months. They were kept under a loose housing system, comprising of concrete sheds and open space to walk around. The animals were fed 10–15 kg green fodder, 8–10 kg wheat straw and 2–3 kg concentrate daily. The buffaloes were having free access to drinking water and wallowing in a pond of water. Buffaloes in estradiol-based group (n = 15; Group I) were inserted CIDR device (1.38 gm progesterone; Pfizer Animal Health Ltd, New York, USA) on day 0 (beginning of the experiment) and concurrently received 1.5 mg of estradiol 17β in 1.5 ml oil (canola oil; i.m.). On day 9, CIDR was removed and each buffalo was given an intramuscular injection of PGF2α analogue (cloprostenol sodium; 500 µg Vetmate TM, Vetcare, Bangalore, India). On day 11, buffaloes were administered GnRH agonist (Buserelin acetate, 20 µg; Receptal®, Intervet, Pune, India) intramuscular. All the buffaloes were inseminated on day 11 and day 12 (). Buffaloes in ovsynch-based group (n = 15; Group I) on day 0 (beginning of the experiment) were administered GnRH agonist (20 µg; i.m.) concurrent with intra-vaginal insertion of CIDR. On day 7, CIDR was removed and each buffalo was administered a PGF2α analogue (500 µg; i.m.). On day 9, buffaloes were again administered GnRH agonist (20 µg; i.m.) and were inseminated on day 9 and day 10 (.
2.2. Ultrasonographic examinations
Trans-rectal ultasonogaphic examinations were made by a single operator with a real-time B-mode diagnostic ultrasound scanner (Agroscan, AL, ECM, Angouleme, France) equipped with interchangeable 5/7.5 MHz linear-array rectal transducer (ALR, 575 probe, ECM, Angouleme, France). The ovaries (ovarian follicles) were monitored on days 0 (beginning of the experiment), 9, 11 and 12 in Group I and on days 0, 7, 9 and 10 in case of Group II. Optical scan images were frozen and the size of antral diameter of largest follicle was determined.
2.3. Blood sampling and hormone analysis
Blood samples were collected on days 0, 9, 11, and 12 in Group I and on days 0, 7, 9 and 10 in case of Group II, by the jugular venipuncture into heparinized vials (1:1000), immediately stored in ice and centrifuged at 1500× g at 4°C for 15 min. Plasma was harvested immediately after blood collection and stored at –200°C until analysed. Plasma concentrations of progesterone were estimated by liquid-phase radioimmunoassay using polyclonal progesterone antiserum raised in our laboratory (Ghuman et al. Citation2009). Sensitivity of the assay was 0.1 ng/ml; intra- and inter-assay variation coefficients were 6.2% and 9.5%, respectively. Plasma estradiol-17β (E2) was estimated using a commercially available Direct Immunoenzymatic Assay kit (Monobind Inc., USA, Accubind estradiol ELISA microwells) and using anti-estradiol IgG-coated wells. Absorbance of each well was taken at 450 nm within 30 minutes. Standard curve was elaborated with the four parameters curve fitting system and the estradiol concentration in samples was calculated.
2.4. Conception rate
Between the groups, first service conception rate (FSCR) was noted by diagnosing pregnancy through trans-rectal ultrasonography on day 60 and rectal palpation on day 90 post-artificial insemination (AI).
2.5. Statistical analysis
The numerical data obtained were expressed as mean ± SEM. Statistical comparison of different parameters in all the sub-groups was carried out by statistical procedures such as analysis of variance (ANOVA), chi-square test and t-test. Differences were considered significant at P < 0.05.
3. Results and discussion
In estradiol-based and ovsynch-based groups, the diameter of the DF at estrus was 12.12 ± 0.38 and 12.54 ± 0.22 mm, respectively (not different, p > 0.05, data shown in ). Moreover, the mean levels of plasma progesterone (P4) and estrogen (E2) on the days of ovulation in estradiol administered group were 0.57 ± 0.07 ng/ml and 0.31 ± 0.20 pg/ml, respectively. In ovsynch-based protocol, the corresponding values were 0.52 ± 0.05 ng/ml and 0.45 ± 0.30 pg/ml, respectively, and the levels were not different between the groups (p > 0.05). The diameter of DF was adequate but not different in estradiol-based group than ovsynch-based group. No such comparisons are available in the literature. Further it was observed that diameter of DF increased significantly (9.5–12 mm in estradiol-based group and 9.8–12.5 mm in ovsynch-based group, p < 0.05) 48 h after progesterone withdrawal and prostaglandin administration. This is in consonance with the conclusion that concomitant with the decline in plasma progesterone, the diameter of largest/DFs observed in different regimens on the day of CIDR withdrawal continuously increased up to the time of ovulation (72 h later). Moreover, the diameter of DF on the day of estrus was larger in buffaloes (Ghuman et al. Citation2012) and cattle (Sa Filho et al. Citation2010) synchronized with CIDR device. The present study thus concluded that administration of PGF may help in development of adequate pre-ovulatory follicle (POF) size and achievement of lowest progesterone levels in buffaloes, 48– 72 h later in both estradiol- and ovsynch-based protocols.
Table 1. Diameter of largest follicle (LF), plasma progesterone (P4) and estrogen (E2) levels in either group (mean ± SE).
In few animals of either group (n = 5 each, data not presented) plasma samples were also collected 72 h post-CIDR removal, the progesterone levels reached to lowest level (0.33 and 0.38 ng/ml in estrogen and ovsynch groups, respectively) and estradiol level further raised (0.41 and 0. 50 pg/ml, respectively). These lowest P4 levels probably coincided with the period around the time of ovulation (observed by ultrasonography). It was recently observed in buffalo that response to a luteolytic dose of PGF2α administered on day of CIDR removal was good and there was decline in plasma progesterone during the subsequent period of 72 h (Ghuman et al. Citation2012). Plasma progesterone has also been found to decline to basal concentrations within 2–3 days after PGF2α and CIDR withdrawal (Ghuman et al. Citation2010a). These findings are in agreement with the present study as the plasma progesterone declined 48–72 h after CIDR withdrawal, the period which coincided with estrus. Hence it could be concluded in the present study that FTAI must be carried out at least 60 h of CIDR removal. Further the decline in plasma progesterone was followed by increase in the diameter of largest/DFs on the during the subsequent 72 h, this is favoured by Ghuman et al. (Citation2012) wherein they observed faster growth of follicles in presence of lower progesterone levels. The positive effect of progesterone on the follicular growth rate and diameter of the DF has also been reported in bovines (Sa Filho et al. Citation2010). Progesterone may decrease the number of hypothalamic estradiol-17β receptors and thereby diminish the potency of estradiol-17β negative feedback and enabling sufficient LH secretion to stimulate pre-ovulatory follicular development (Day & Anderson Citation1998).
In the present study the levels of estradiol were raised on the day of ovulation in both the groups compared to the period before ovulation (data presented in ). The estradiol levels were not different between the groups. The increase in circulating estradiol concentrations was observed in previous studies (Colazo et al. Citation2004). This small increase in circulating estradiol concentrations, in addition to that produced by the pre-ovulatory follicle, had resulted in synchronous ovulation and highly acceptable pregnancy rates following FTAI (Colazo et al. Citation2004). The elevated estradiol level might be attributed to the combined effect of PGF administration and CIDR removal which might have helped in the growth of the DF which subsequently elevated the estradiol level as the diameter of POF and estradiol level were positively correlated.
Duration of ovulation after PGF administration and CIDR withdrawal (61 vs. 58 h) was not different between the animals of either group. This is in contrast with the study in cattle where in estradiol benzoate protocol, interval from CIDR removal to ovulation was shorter and less variable (Martinez et al. Citation2006). The ovulation after CIDR removal was attributed to achievement of plasma LH peak and LH peaked 51.0 ± 5.1 h after CIDR removal and intervals from CIDR removal to ovulation was 78.0 ± 4.1 h (Martinez et al. Citation2006).
FSCR was significantly higher (P < 0.05) in estrogen-based group than ovsynch-based group (8/15 i.e., 53.33% vs. 5/15 i.e., 33.33%, p < 0.05). Similar results in cattle were also observed wherein administration of estradiol cypionate resulted in a small and slow increase in circulating estradiol concentrations due to its slow absorption and hydrolysis of estradiol-17β from the parent molecule (Vynkier et al. Citation1990). Raised circulating estradiol concentrations has been suggested to result in synchronous ovulation which can ultimately result in acceptable pregnancy rates following FTAI (Colazo et al. Citation2004). In the present study the higher FSCR might be attributed to more synchrony of ovulation and this needs comprehensive investigations in buffalo. Estradiol-based protocol, leading to higher conception and also being cost economical might be better indicated to combat losses due to anestrum in buffalo.
Conclusion
Estradiol-based protocol developed an optimal ovulatory follicle around the time of insemination. The size of the POF obtained in estradiol-based protocol was comparable to the size observed in ovsynch-based protocol thereby suggesting its use in the synchronization protocols. In estradiol-based regimen the FSCR was higher as compared to conventional ovsynch-based regimen.
Acknowledgements
Authors are highly thankful to support by National Fund for Basic and Strategic Research in Agriculture (NFBSRA) and Indian Council of Agriculture Research (ICAR), New Delhi, India.
References
- Banu TA, Shamsuddin M, Bhattacharjee J, Islam MF, Khan SI, Ahmed JU. 2012. Milk progesterone enzyme-linked immunosorbent assay as a tool to investigate ovarian cyclicity of water buffaloes in relation to body condition score and milk production. Acta Veterinaria Scandinavia. 54:30. 10.1017/S002202990002567X
- Borghese A. 2005. Buffalo production and research. FAO regional office for Europe, technical series 67. Rome: Food and Agriculture Organization.
- Colazo MG, Kastelic JP, Martinez MF, Whittaker PR, Wilde R, Ambrose JD, Corbett R, Mapletoft RJ. 2004. Fertility following fixed-time AI in CIDR-treated beef heifers given GnRH or estradiol cypionate and fed diets supplemented with flax seed or sunflower seed. Theriogenology. 61:1115–1124. 10.1016/j.theriogenology.2003.06.005
- Day ML, Anderson LH. 1998. Current concepts on the control of puberty in cattle. J Anim Sci. 76:1–15
- Ghuman SPS, Dadarwal D, Honparkhe M, Singh J, Dhaliwal GS. 2009. Production of polyclonal antiserum against progesterone for radioimmunoassay. Indian Vet J. 86:909–911.
- Ghuman SPS, Honparkhe M, Singh J. 2010a. Optimizing ovarian and reproductive events of anestrous buffaloes using intravaginal progesterone, GnRH, and PGF2α. Paper presented at: Proceedings of International Buffalo Conference held at National Agricultural Science Centre Complex; New Delhi, India.
- Ghuman SPS, Honparkhe M, Singh J, Dhami DS Ajeet Kumar, Nazir G, Ahuja C 2012. Fertility response using three estrus synchronization regimens in lactating anestrous buffaloes. Indian J Anim Sci. 82:162–166.
- Ghuman SPS, Singh J, Dhaliwal GS. 2008. Recent concepts in ovarian activity of buffaloes (Bubalus bubalis) with prepubertal anestrus and its alleviation through endocrine interventions. Paper presented at: Proceedings of XXIV Annual Convention and National Symposium of ISSAR: Bangalore, India.
- Kanai Y, Abdul-Latief T, Ishikawa N, Shimizu H. 1990. Behavioural and hormonal aspects of the oestrous cycle in swamp buffaloes reared under temperate conditions. In: Domestic buffalo production in Asia. Vienna: International Atomic Energy Agency; p. 113–120.
- Manik RS, Palta P, Singla SK, Sharma V. 2002. Folliculogenesis in buffalo (Bubalus bubalis): a review. Reprod Fertil Dev. 14:315–325.10.1071/RD01126
- Martinez MF, Adams GP, Kastelic JP, Bergfelt D, Mapletoft RJ. 2000. Induction of follicular wave emergence for estrus synchronization and artificial insemination in heifers. Theriogenology. 54:757–769.10.1016/S0093-691X(00)00388-5
- Martínez MF, Kastelic JP, Colazo MG, Mapletoft RJ. 2006. Effects of estradiol on gonadotrophin release, estrus and ovulation in CIDR-treated beef cattle. Domest Anim Endocrinol. 33:77–90.
- Sa Filho MF, Ayres H, Ferreira AM, Marques MO, Reis EL, Silva RCP, Rodrigues CA, Madureira EH, Bo GA, Baruselli PS. 2010. Equine chorionic gonadotropin and gonadotropin-releasing hormone enhance fertility in a norgestomet-based, timed artificial insemination protocol in suckled Nelore (Bos indicus) cows. Theriogenology. 73:651–658.10.1016/j.theriogenology.2009.11.004
- Vynkier L, Debakere M, De Kruif A, Coryn M. 1990. Plasma estradiol-17β concentrations in the cow during induced estrus and after injecting of estradiol-17β benzoate and estradiol-17β cypionate – a preliminary study. J Vet Pharmacol Ther. 13:36–42. 10.1111/j.1365-2885.1990.tb00745.x
- Warriach HM, Channa AA, Ahmad N. 2008. Effect of estrus synchronization methods on estrus behaviour, timing of ovulation and pregnancy rate during the breeding and low breeding seasons in Nili-Ravi buffaloes. Anim Reprod Sci. 107:62–67. 10.1016/j.anireprosci.2007.06.007