Abstract
The production commensurate with the demand for animal products including meat is increasing due to urbanization and increase in per capita income. However, meat and meat products meant for human consumption should also be safe and free from toxic contaminants. Presence of any xenobiotics in human food web is undesirable and unacceptable particularly in the wake of sanitary and phytosanitary (SPS) measures and strict regulations imposed by many countries on the ground of safety of health of the consumers. In the present study, certain organochlorinated pesticide (OCP) residues in tissues and in blood samples of goat were determined. All samples were analysed for residues of hexachlorocyclohexane (HCH) isomers, dichlorodiphenyltrichloroethane (DDT) isomers and metabolites, endosulfan isomers and metabolite, aldrin, heptachlor and dicofol. The tissue samples were extracted in soxhlet with hexane-acetone (1:1) and cleaned up by florisil column chromatography. The analysis was performed in capillary gas chromatograph equipped with electron capture detector (GC-ECD). The result indicated that 40% tissue samples were contaminated with HCH residues, while DDT and endosulfan were present in 32% samples. In blood samples, no HCH residue could be detected, but DDT and endosulfan were present in 10% and 30% samples, respectively.
1. Introduction
Organochlorinated pesticides (OCPs) are characterized as most persistent due to less biodegradability, chemical inertness, stability and high lipophilicity which allow them to be accumulated in various tissues of the animal body. Normally pesticides enter into the animal body through contaminated feed, fodder, water and also through passive diffusion when used as ectoparasiticides. After entering into the animal body, they come into a steady state and bioconcentrate in tissue lipids according to equilibrium pattern of internal transport and lipid tissue content. The lipid-rich tissues act as depots or reservoirs of persistent organochlorines (OCs) by virtue of their physico-chemical interactions with cellular components and their concentrations decline at a very slow rate, even after sources of contamination are eliminated. Presence of residues of OCs and other pesticides in tissues and in organs of goat, cattle, pig, buffalo, sheep, lamb and chicken have been reported by different investigators both in India and abroad (Battu et al. Citation1984; Al-Omar et al. Citation1985; Frank et al. Citation1990; Hernandez et al. Citation1994; Bedi et al. Citation2005; Vijayan et al. Citation2006; Darko & Acquah Citation2007; Kumar et al. Citation2008; Shrestha et al. Citation2009; Suganthy et al. Citation2009).
Presence of toxic xenobiotics in animal tissues and organs is undesirable as they not only affect the growth of animals by inciting many diseases and affecting immune and reproductive systems but more importantly contaminate human food of animal origin and thus enter human body, thereby posing serious health problems. OCs is having low acute toxicity but possess a greater potential for chronic toxicity which have many repercussion on human health like nervous system disturbances (Kaloyanova & El Batawi Citation1991).
Humans become the final consumer and recipient of the highest concentration of poisonous pesticides by consuming meat which is virtually at the top of the food chain. In fact, meat contains 13 times as much dichlorodiphenyltrichloroethane (DDT) as vegetables, fruits and grass. The IOWA State University once performed experiments which showed that most of the DDT in human bodies comes from meat (www.encognitive.com/node/1589).
Among OCs, DDT is approved and used till date for prevention of vector-borne diseases in India (www.nvbdcp.gov.in). Endosulfan and dicofol are used for control of insect pests and mites in different crops. Lindane (γ-HCH) was used till recently in agriculture and has been banned with effect from 25 March 2013. Therefore, because of present and past use of these OC pesticides, there is enough chance of accumulation of their residues in different environmental compartments which can act as source of contamination of animal body. In this context, the present investigation was carried out to observe the extent and levels of OCP residues in tissues and blood of goat to get an idea about the potential risk to the consumers of goat meat.
2. Materials and methods
2.1. Chemicals and reagents
Certified reference standards of all the pesticides, namely, HCH (α, β, γ and δ), Dichlorodiphenyldichloroethane (DDD) (op1, pp1), Dichlorodiphenyldichloroethylene (DDE) (op1, pp1), DDT (op1, pp1), endosulfan (α, β, and sulphate), aldrin, heptachlor and dicofol were procured from M/s. Sigma Chemicals. All solvents used in the experiments were redistilled and checked for any pesticide contamination. Reagents like florisil and sodium sulphate were properly activated in muffle furnace and oven before use.
2.2. Sampling
2.2.1. Blood
Blood samples of goat were collected from the dairy farm of Indian Grassland and Fodder Research Institute, Jhansi, India. Heparinised residue-free 20 ml glass vials containing 200 USP units of heparin in 0.2 ml solution was used and 10 ml of blood from each animal was taken by sterilized syringes via jugular vein puncture of the animals. Blood samples were transported in dry ice to the laboratory and stored at –20° until analysed.
2.2.2. Tissues
Fresh goat tissues, namely, muscles, liver, lung, kidney and fat were obtained from different slaughter houses at Jhansi market. Twenty-five samples each weighing about 250 g were randomly collected, wrapped in aluminium foil, placed in an ice-chest containing ice and brought to the laboratory for analysis.
2.3. Analysis
Animal tissue samples were minced to small pieces and mixed thoroughly. The analytical method of Mills et al. (Citation1972) was followed with some modifications. Sub sample of meat (10 g) was grounded with 30 g of anhydrous sodium sulphate in a tissue homoginizer. The free-flowing granular material thus formed was extracted in soxhlet apparatus continuously for 8 h in 200 ml n-hexane and acetone (1:1 v/v) mixture. The extract was then mixed with saturated sodium chloride solution approximately three times the volume and partitioned into hexane. The combined hexane layer was dehydrated in anhydrous sodium sulphate and concentrated. The residue was then transferred to a florisil column pre-saturated with hexane and eluted with 150 ml hexane:dichloromethane (4:1 v/v). The eluate was concentrated to dryness and volume was made-up to the desired level with isooctane for analysis in gas-chromatography (GC).
Blood sample (2 ml) was taken in a centrifuge tube to which 250 mg of NaCl was added and mixed properly followed by the addition of 10 ml hexane and acetone (9:1 v/v). The mixture was then centrifuged at 3000 rpm for 15 min. After centrifugation, the upper solvent layer was removed and passed through anhydrous sodium sulphate. The combined solvent extracts were concentrated and reconstituted with isooctane up to the desired volume for analysis in GC with ECD.
The qualitative and quantitative determination was done in GC on a Varian CP-3800 equipment fitted with Ni63 electron capture detector. The column used was wall-coated open tubular (WCOT)-fused silica capillary having dimension of 30 m × 0.32 mm id × 0.25 μm film thickness (CP-SIL 5CB). The operation conditions of GC were as follows.
Injection port temperature: 260°C (split 1:10), Detector: 300°C, Column: 180°C for 1 min then at the rate of 3°C/min to 250°C for 5min (Programme), Carrier gas: N2: at the rate of 1ml/min, make-up 30ml/min. The OC pesticides were identified and quantified based on the external standard (99.5%) solution injected initially and after every five samples.
2.4. Method of validation and quality control
To check the sensitivity and linearity of the instruments, a five-point external standard calibration using individual and standard mixture at levels from 0.001μg/ml to 0.01μg/ml was performed initially to establish the detector (ECD) linear range.
Linear regression equation was used to quantify the analytes in samples. Calibration of GC was done before sample analysis using the standards of pesticides obtained from authentic sources. Qualitative and quantitative analyses were performed by comparing the retention time and peak area of the samples, respectively, with those of the calibrated reference standards.
The trueness of the method was estimated by calculating the attained recovery from fortification experiments. The mean recoveries were determined from spiked animal tissue and blood samples, previously analysed so as to ensure the absence of pesticide residues, at different levels employing the same extraction and clean-up methods as done in case of actual samples. The levels of spiking were from 0.01 μg/g to 1 μg/g of pesticide standards. The average recovery values were calculated from the calibration curves constructed from the concentration and peak areas of the obtained chromatograms with standards of pesticides. Blank analysis was performed in order to check interference. The average recovery was between 86.2% and 102.5%, and the method limit of detection of different OCPs varied from 0.001 to 0.004 µg/g.
3. Results and discussion
3.1. Pesticide residue in animal tissues
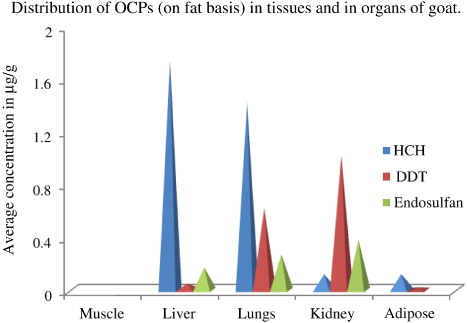
Concentration of residues of OCPs has been calculated on fat basis. The fat content of muscle, liver, lungs, kidney and adipose tissues was 11.4%, 3.14%, 2%, 3% and 70%, respectively. Out of these five different tissues tested, no residues of the targeted OC compounds could be detected in muscle tissue samples. But in other tissues, OC residues were found present at different concentrations (). Two of the five liver samples were positive with HCH and endosulfan at concentrations varying from 1.07 mg/kg to 2.42 mg/kg and 0.09 mg/kg to 0.25 mg/kg, respectively, but DDT was recorded in only one sample at a concentration of 0.05 mg/kg. In lungs, each of HCH, DDT and endosulfan were detected in three samples at varying concentrations. While concentration of total endosulfan was lowest varying from 0.15 mg/kg to 0.4 mg/kg, total HCH and total DDT went up to 2 mg/kg and 1.65 mg/kg, respectively. Three out of five kidney tissues contained DDT and endosulfan residues, but HCH were present in two samples. The concentration of endosulfan residue in kidney was higher (0.1 mg/kg – 0.89 mg/kg) than that in liver or in lungs. Similarly, concentration of total DDT in kidney tissues was also higher (0.05 mg/kg – 2.93 mg/kg) than that in liver or in lungs. However, concentration of total HCH (0.03 mg/kg – 0.2 mg/kg) in kidney was lower than the other two tissues. In adipose tissues (fats), endosulfan was not found to be deposited in any of the five samples analyzed, but HCH in three and DDT in two samples were recorded at levels of 0.084 mg/kg – 0.18 mg/kg and 0.017 mg/kg – 0.023 mg/kg, respectively, which was lower than the levels recorded in other tissues.
When taken all the tissues together, 10 samples out of 25 (40%) were found positive with HCH residues. Total HCH concentration varied from 0.03 mg/kg to 2.42 mg/kg with a mean value of 0.83 mg/kg. Both β-HCH and γ-HCH were detected in similar detection frequency, i.e. seven samples (28%) at concentration ranges of 0.06 mg/kg – 1.91 mg/kg and 0.002 mg/kg – 0.85 mg/kg, respectively. The α and δ-HCH were detected in four (16%) and five (20%) samples at concentrations varying from 0.006 mg/kg – 0.32 mg/kg and 0.016 mg/kg – 0.2 mg/kg, respectively. In terms of average concentration, the decreasing order was β > α > γ > δ (). The mean concentration of β-HCH was significantly higher (p < 0.05) than that of all other isomers, but among other isomers concentration differences were non-significant at that level. When the concentrations of HCH isomers in contaminated samples were compared with Maximum Residue Limit (MRL) as recommended by different agencies (), it was observed that three samples with α-HCH, four with β- and five with γ-HCH exceeded MRL as prescribed by the European Union (EU) for goat meat, fat, liver and kidney, and all the four samples contaminated with α-HCH also exceeded the MRL prescribed by the Codex.
Table 1. HCH residues in different issues of goats (on lipid basis).
Table 2. MRL values of OC pesticides in animal tissues and meat.
DDT residue was present in 8 samples out of a total of 25, and both the isomers of DDT and its metabolites targeted in the study were detected. Each isomer (op1 and pp1) of DDD were detected in two samples (8%), but the concentration of op1 DDD (0.07 mg/kg – 0.3 mg/kg) was higher than the pp1 DDD (0.05 mg/kg – 0.15 mg/kg). Similarly op1 and pp1 DDT was positive in two samples, but the latter had more concentration (0.008 mg/kg) than the former (0.002 mg/kg) unlike DDD. In case of DDE, pp1 isomer was recorded in more number of samples (3, 12%) than its op1 counterpart which was present in two samples only and like DDT, pp1 DDE was having higher concentration (average concentration 0.898 mg/kg) than the op1 DDE (average concentration of 0.552mg/kg; ). The concentrations of total DDT (0.017 mg/kg – 2.93 mg/kg) comprising of all the isomers and metabolites present in a single sample exceeded the MRL of DDT in meat as prescribed by the EU in only two samples.
Table 3. DDT isomers and metabolites in goat tissues (on lipid basis).
Endosulfan residues were found in eight samples (32%) comprising of liver, lungs and kidney. Total endosulfan concentration varied from 0.09 mg/kg to 0.89 mg/kg with an average value of 0.284 mg/kg, and in all the eight samples, concentrations exceeded the MRL of endosulfan in meat (0.05 mg/kg) according to EU, while in four samples, MRL of 0.2 mg/kg as per Codex was violated. Among the isomers, β-endosulfan was present in maximum frequency, which was detected in seven samples (28%) at a concentration range of 0.03 mg/kg – 0.33 mg/kg. Six samples (24%) were positive with α-endosulfan at a concentration range of 0.05 mg/kg– 0.25 mg/kg, while metabolite endosulfan sulphate was found in four (16%) samples at concentration ranged between 0.03 mg/kg and 0.36 mg/kg (). The average concentrations of all these three compounds were around 0.13 mg/kg.
Table 4. Endosulfan residues in goat tissues (on lipid basis).
3.2. Pesticide residues in blood
Out of 20 blood samples of farm animal (goat) tested, 8 (40%) were contaminated. No HCH residue was found in any of the samples. But DDT could be detected in two (10%) of them at an average concentration of 0.007 mg/kg. The op1 and pp1 isomers of DDD and op1 DDT contributed for the total DDT. Endosulfan was also found in six samples (30%) at a concentration range of 0.006 mg/kg – 0.044 mg/kg with an average value of 0.019 mg/kg (). Among the six samples containing endosulfan, five samples recorded only endosulfan sulphate and one sample recorded the presence of only β-endosulfan, while α-endosulfan was not found in any of the samples.
Table 5. OC pesticide residues in blood samples of goat.
Residues of different OC compounds like DDT, HCH, dieldrin, heptachlor, trichlorophenol (TCP), pentachlorobenzene (PCB), etc., have been reported to be occurring in pork, chicken, sheep, lamb, goat and beef from many places. All the four isomers of HCH, i.e., α, β , γ and δ were detected in the goat liver, gizzard and fat samples by Suganthy et al. (Citation2009) in Tamilnadu. The maximum level of HCH detected by them was 0.681 mg/kg. The average levels of HCH in adipose tissues, muscle, liver and kidney of goat reported from Punjab were 0.441, 0.137, 0.126 and 0.092 mg/kg according to Bedi et al. (Citation2005). The γ-isomer was most predominant among the four isomers of HCH. All the four isomers of HCH were also detected in goat tissue under present study, but at levels lower than those detected earlier, as found in the earlier reports. Similarly, the levels of lindane reported in the present study (0.002 mg/kg–0.85 mg/kg) were below the level (0.004 mg/kg –1.611 mg/kg) reported by Al-Omar et al. (Citation1985) in lamb from Baghdad and several other workers including Kannan et al. (Citation1992), who found lindane in the range of 0.005 mg/kg –1.1 mg/kg from different locations in India.
Battu et al. (Citation1984) observed that ppl DDE constituted a major share (0.047 mg/kg) in goat tissue. This may be due to metabolic conversion of ppl DDT to ppl DDE in animals which are more stable and resistant to degradation. In the present study, along with ppl DDE and ppl DDD other components of total DDT were also recorded. Bedi et al. (Citation2005) detected DDT residues in adipose tissues, muscle, liver and kidney of goats in Punjab. DDT was the most abundant pesticide, and pp1 DDE was most reigning. They reported average concentration of total DDT in various tissues varying from 0.06 mg/kg to 0.57 mg/kg, while in the present study, higher concentrations (0.02 mg/kg–2.93 mg/kg) calculated on lipid weight basis was obtained.
In case of endosulfan, occurrence of only β-endosulfan was reported in mutton samples (Suganthy et al. Citation2009). They could find β-endosulfan up to 0.417 mg/kg in gizzard of goat. In the present investigation, α-endosulfan and endosulfan sulphate were also recorded along with β-endosulfan which was present up to at a maximum concentration was 0.33 mg/kg recorded in kidney of goat.
Regarding occurrence of OCP residues in blood, a number of studies have been reported for human beings (Walker et al. Citation2003; Bhatnagar et al. Citation2004; Waliszewski et al. Citation2004; Mishra et al. Citation2011) and the correlation between OCP residues and development of cancer, particularly breast cancer. Also, reports are also available about OC residues in blood of different animals and birds (Bustnes et al. Citation2005) which indicate the exposure of living beings to the toxic compounds leading to their accumulation in blood. In the present investigation, blood of farm animals (goat) was found to contain DDT residues in minute concentration and also endosulfan at a relatively higher level with no presence of HCH residues.
Residues of different OC compounds along with their isomers and metabolites were detected in goat meat and tissues, but their concentrations are declining as observed after comparing the results of the present paper with various reports from both India and abroad. It was also observed that in almost all the cases, the concentration of OCPs in the positive samples were much below the threshold levels, i.e. MRLs prescribed by different agencies/countries.
4. Conclusion
Therefore, it can be concluded that animal tissues and blood collected from selected sites and farm of Jhansi, India, were found to contain residues of different OCs to some extent. But the levels at which they were detected were very less and below the permissible level in case of tissues.
Acknowledgements
The authors are thankful to the Head of Plant Animal Relationship Division and also the Director of Indian Grassland and Fodder Research Institute, Jhansi, India, for extending their help, encouragement and necessary facilities for the work.
References
- Al-Omar M, Al-Bassomy NS, Al-Ogaily N, Al-Din D. 1985. Residue levels of organochlorine insecticides in lamb and beef from Baghdad. Bull Environ Contam Toxicol. 34: 509–512. 10.1007/BF01609768
- Battu RS, Gupta SC, Chawala RP, Kalra RL. 1984. Residues of DDT and BHC in market samples of meat of various animals. Indian J Ecol. 2:177–182.
- Bedi JS, Gill JPS, Aulakh RS, Joia BS, Sharma JK. 2005. Contamination levels of DDT and HCH residues in different goat tissues. Indian J Anim Sci. 75:11–13.
- Bhatnagar VK, Kashyap R, Zaidi SSA, Kulkarni PK, Saiyed HN. 2004. Levels of DDT, HCH and HCB residues in human blood in Ahmedabad, India. Bull Environ Contam Toxicol. 72:261–265. 10.1007/s00128-003-9049-9
- Bustnes JO, Skaare JE, Berg V, Tvera T. 2005. Interseasonal variation in blood concentrations of organochlorines in great black-backed gulls (Larus marinus). Environ Toxicol Chem. 24:1801–1806. 10.1897/04-203R.1
- Darko G, Acquah SO. 2007. Levels of organochlorine pesticide residues in meat. Food Addit Contam. 13:231–235.
- Frank R, Braun HE, Stonefield KI, Rasper J, Luyken H. 1990. Organochlorine and organophosphorus residues in the fat of domestic farm animal species, Ontario, Canada 1986–1988. Food Addit Contam. 7:629–636. 10.1080/02652039009373928
- Hernandez LM, Fernandez MA, Jimenez B, Gonzalez MJ, Garcia JF. 1994. Organochlorine pollutants in meats and cow's milk from Madrid (Spain). Bulletin Environ Contam Toxicol. 52:246–253. 10.1007/BF00198495
- Kaloyanova FP, El Batawi MP. 1991. In: Human toxicology of pesticides, CRC Press.
- Kannan K, Tanabe S, Ramesh A, Subramanian A, Tatsukawa R. 1992. Persistent organochlorine residues in foodstuffs from India and their implications on human dietary exposure. J Agric Food Chem. 40:518–524. 10.1021/jf00015a032
- Kumar P, Singh SP, Ahmad AH, Rao VDP. 2008. Determination of chlorpyriphos residues in buffalo meat samples using highi performance liquid chromatography. Int J Food Saf Nutr Pub Health. 1:189–199. 10.1504/IJFSNPH.2008.023019
- Mills PA, Bong BA, Kamps LR, Burke JA. 1972. Elution solvent system for florisil column clean up in organochlorine pesticide residue analysis. J Assoc Off Anal Chem. 55:39–43.
- Mishra K, Sharma RC, Kumar S. 2011. Organochlorine pollutants in human blood and their relation with age, gender and habitat from North-East India. Chemosphere. 85:454–464.
- Shrestha SK, Aulakh RS, Bedi JS, Gill JPS. 2009. Organochlorine pesticide residues in buffalo meat in Punjab, India. Pak J Zool. 9:135–137.
- Suganthy M, Kuttalam S, Chandrasekharan S. 2009. Residues of chlorinated hydrocarbon insecticides and chlorpyriphos in market samples of mutton. Madras Agric J. 96:221–224.
- Vijayan R, George T, Naseema Beevi S, Kurien MO. 2006. Residues of organochlorine insecticides in meat of slaughtered animals in Kerala. Pestic Res J. 18:228–230.
- Waliszewski SM, Carvajal O, Infanzon RM, Trujillo P, Hart MM. 2004. Copartition ratios of persistent organochlorine pesticides between human adipose tissue and blood serum lipids. Bull Environ Contam Toxicol. 73:732–738. 10.1007/s00128-004-0487-9
- Walker JB, Seddon L, McMullen Ed, Houseman J, Tofflemire K, Corriveau A, Weber JP, Mills C, Smith S, Oostdam JV. 2003. Organochlorine levels in maternal and umbilical cord blood plasma in Arctic Canada. Sci Total Environ. 302:27–52. 10.1016/S0048-9697(02)00319-4
- www.encognitive.com/node/1589: One man's meat is another man's poison.
- www.nvbdcp.gov.in: National vector borne disease control program, Director General of Health Services, Ministry of Health & Family Welfare, Govt. of India.