Abstract
A total of 12 sexually mature mixed-breed goat bucks were used in this experiment to study the effects of testosterone application (T; 25 mg IM every three days during three weeks) during the period of sexual inactivity (end of March, 26°N) on libido, odour, Sertoli cell number, seminal characteristics and serum testosterone levels. The experimental design was completely random with two groups with six bucks in each group. Reaction time was shorter (P < 0.05) in T bucks (96 ± 45 sec) than in control bucks (258 ± 44 sec). Testosterone treatment increased semen volume (1.2 ± 0.5 vs. 0.3 ± 0.03 ml for T and control bucks, respectively) and total sperm cells/ejaculate (1.32 ± 0.7 vs. 0.33 ± 0.02 x 109 for T and control bucks, respectively). Buck’s odour (scale 1–5) was more intense (P < 0.05) in T bucks (1.8 ± 0.1) than control bucks (0.6 ± 0.2). Serum testosterone levels were threefold higher in T bucks (8 ng/mL) compared to control bucks (2 ng/mL) after three weeks of exogenous testosterone treatment. It was concluded that testosterone application to sexually inactive goat bucks provoke an increase in serum testosterone which in turn induces an intense sexual behaviour and the improvement of semen quality.
1. Introduction
Mixed-breed goat bucks at 25°N are mildly photosensitive with respect to reproduction (Mellado et al. Citation2014). At this latitude, goats present sexual activity for the most part of the year, but during April and May goats present anestrous (De Santiago-Miramontes et al. Citation2011). As a consequence, very few kids are likely to be born from mid-August through mid-September making it difficult for goat producers to provide a constant supply of goat milk and meat throughout the year.
Goat farmers under extensive conditions in the arid zones of Northern Mexico, seeking to breed does out of season for a year-round supply of milk and meat commonly use the buck effect. In order for this practice to be effective in spring, goat bucks must be sexually active, and this can be brought about through artificial lighting (Pellicer-Rubio Citation2007) or application of testosterone to bucks not in rut previous to the breeding period (Luna-Orozco et al. Citation2012).
The first alternative to induce bucks into sexual activity in spring is costly, cumbersome and unworkable in practice for goat producers under range conditions. Thus, the application of testosterone to sexually inactive buck is a simpler and effective method to bring bucks into sexual activity, which in turn stimulates the doe flock to become sexually active with fertile estrus due to their ‘shallow’ anestrous in spring (Véliz et al. Citation2009; Rivas-Muñoz et al. Citation2010). Testosterone-treated bucks and long-day-treated bucks are equally effective in synchronizing estrus in anovulatory goats and resulted in similar levels of fertility despite the fact that libido in testosterone-treated bucks is lower than that attained by light-treated bucks (Luna-Orozco et al. Citation2012). Additionally, semen quality of bucks is strongly influenced by season independently of the feeding level (Zarazaga et al. Citation2009). The effect of exogenous testosterone on semen quality and testicular germinal epithelium in sexually inactive bucks is unknown. Therefore, the aim of this study was to assess the effect of testosterone application to sexually inactive goat bucks on semen characteristics, sexual drive, germinal epithelium and plasma testosterone levels.
2. Material and methods
2.1. Study site
The present study was carried out in a commercial goat farm under extensive conditions in Northern Mexico (26°23′N Latitude, 104°47′ W Longitude). Average annual precipitation at the study area is 230 mm and altitude is 1124 m. The highest ambient temperature is 41°C in May and in June and the lowest is −3°C in December and January. Relative humidity ranges between 26.14% and 60.59% and day length from 13 h, 41 min during summer solstice (June) to 10 h, 19 min in the winter solstice (December). This arid region is dominated by the shrubs creosote bush (Larrea triedentata), fourwing saltbush (Atriplex canescens) and mesquite (Prosopisglandulosa). Goats were grazed on rangeland, most of the times, and occasionally, on crop residues, mainly corn, sorghum and cotton.
2.2. Buck management
Twelve sexually experienced mixed-breed 2- to 4 year-old bucks of proven fertility were used from 15 March to 10 April 2011. These animals were housed in a ruffed cement floor pen (6 × 6 m) with free access to water and a mineral mix. Twice daily, bucks were offered alfalfa hay (crude protein content: 171 g/kg dry matter (DM) and energy content: 8.1 MJ ME/kg DM) ad libitum consumption. Additionally, bucks were offered 200 g of concentrate per day (140 g/kg DM and energy content of 10.1 MJ ME/kg DM). This diet satisfied the nutritional requirements of mature phase of goat bucks according to NRC (Citation2007) recommendations.
Bucks were randomly allotted into two groups (six bucks per group): intramuscular injection of 25 mg testosterone (Testosterone 50, Lab Brovel, DF, Mexico) every third day during three weeks. The second group received saline injections every third day for three weeks and served as control.
2.3. Semen collection and sexual drive
Semen was collected twice during the last week of the trial using a restrained doe treated with 2 mg de estradiol cipionate (ECP®, Lab. Pharmacia & Upjohn, Mexico) for mounting by the bucks. A standard ovine artificial vagina at a temperature of 42°C was used to collect the semen from all bucks.
Previously, the artificial vagina was pre-warmed from 30°C up to 41°C, and kept at this temperature until used. Tubes with the freshly collected semen were immediately transported to the laboratory and immersed in a water bath at 38°C. The ejaculate volume (mL) was determined to the nearest 0.1 mL using a glass graduated conical tube. Sperm concentration was determined using 0.025 mL of semen diluted with 0.5 mL of a fixative solution, containing 7% formaldehyde and 0.85% NaCl mixed in a 1:1 (vol/vol) ratio. The diluted semen was placed on a hemocytometer with the sperm counted in five squares of each two chambers, and the concentration of sperm cells was calculated.
Forward progressive motility was scored from 1 to 5, with a score of 5 denoting the greatest forward progressive movement and a score of 1 denoting no motility. This determination was made with a microscope equipped with a heated platform adjusted to 37°C and with phase contrast optics (400×).The total number of sperm cells was determined by multiplying sperm concentration per ml by the volume of the ejaculate. Viable sperm were evaluated by eosin/nigrosin stain exclusion. A drop of stain was mixed with a drop of pure semen and extended on the slide which was observed at a magnification of 1000×. Two hundred spermatozoa were counted, and the unstained spermatozoa were determined as viable sperm cells.
The scrotal circumference was measured with a tape at the broadest part of the scrotum, with the animal being restrained in a lateral recumbent position. On the day of semen collection, animals were weighed and body condition score (BCS, using score system 0–5; Hossamo Citation1984) was recorded.
2.4. Behavioural observations
While the bucks were exposed to their respective estrogenized doe (15 min.), sexual behaviour was measured in terms of reaction time in seconds and was estimated from the time the doe was placed inside the buck’s pen up to the point when the buck started to mount the doe with the penis erected. Approaches, mount attempts in which both forelegs of the buck were raised off the ground but not placed steadily on the doe and mounts in which bucks secured both forelegs on the rump of the doe together with pelvic thrusting movements were recorded.
2.5. Testicular biopsy
Bucks in both groups were subjected to a testicular biopsy using 21-gauge butterfly needles attached to a 20 ml plastic syringe, which served as aspiration device. The butterfly needle was passed through the scrotal skin into the parenquima of the testis. Five different entries were made in each testicle. Before retrieving the needle a small artery forceps was used to clamp the butterfly’s microtubing. The needle was then flushed with Earle’s balanced salt solution (EBSS; Gibco BRL, Life Technologies), with heparin (Sigma Chemical Co., St Louis, MO, USA) into one well of a 4-well plate (Nunc, Copenhagen, Denmark). After centrifugation at 300 g for 10 min cell suspensions were examined under a microscope (Labo America, inc, USA) at ×200 and ×400 magnification to detect the presence of any spermatozoa and germinal tissue.
2.6. Blood sample and testosterone determination
To measure changes in testosterone levels during the study period, jugular blood (5 ml) was collected weekly from each buck by venipuncture during four weeks. After blood samples were allowed to clot, serum was separated by centrifugation at 5000 g for 10 min and stored at –20°C until further analysis. Concentrations of serum testosterone were measured by radioimmunoassay using a commercial assay kit (Siemens, México, D.F). The sensitivity of this assay was 0.4 ng/mL of serum.
2.7. Odour and sexual behaviour determination
After four weeks of testosterone treatment, both groups of bucks were exposed to estrogenized doe during 15 minutes in order to record sexual behaviour. These behaviours were reaction time, approaches, mounts without ejaculations and mounts and ejaculations upon intromission. Male odour and BCS were determined according to Walkden-Brown et al. (Citation1993, Citation1997).
2.8. Statistical analysis
The procedure MIXED of SAS (SAS Inst. Inc., Cary, NC, USA) was used to investigate the effects on ejaculate volume, sperm concentration, percentage of viable spermatozoa in the ejaculate and total spermatozoa per ejaculate with following the statistical model:
Behavioural data, such as mounts and ejaculations and the odour data, were analysed with the Wilcoxon signed rank test (SAS Citation1996); for comparative purposes, these data are presented as means ± SD. Serum testosterone levels were analysed using a split-plot in time with group as the main plot and time as the subplot factor. This statistical procedure was performed using the Proc Mixed Procedure of SAS. Results were considered statistically significant at P < 0.05.
3. Results and discussion
The mean reaction time was 162 s longer (P < 0.05) in the control bucks compared to T bucks (). The reaction time found in T bucks (96.3 ± 45.0 sec) was very close than the values observed by Chemineau et al. (Citation1986), who reported an average of 98 s for Creole meat bucks. These results indicate that the application of testosterone to sexual inactive bucks provokes clearly defined sexual activity, which suggests that exogenous testosterone in the non-breeding season stimulates cerebral structures mediating male sexual drive.
Table 1. Reaction time and ejaculate characteristics (mean ± SD) in mixed-breed goat bucks either subjected to testosterone treatment or left untreated during the period of sexual inactivity (March) at 26°N.
Semen characteristics of goat bucks collected three weeks after the testosterone treatment is presented in . Group differences (P < 0.05) were observed for the volume of ejaculate, but no treatment differences (P > 0.05) were found for sperm cell concentration per mL, sperm motility and live sperm cells. The lack of differences in these semen traits between traits could be due to the fact that no correlation has been observed between seminal testosterone levels and sperm characteristics (De Oliveira-Souza et al. Citation2011). Another possible reason is that besides the testosterone application, bucks could have been supplemented with Zn and Se for a seminal quality improvement (Kumar et al. Citation2013). On the other hand, testosterone application seemed to stimulate accessory glands so that volume of ejaculated increased six-fold in T bucks compared to control bucks. Other researchers (Sanford et al. Citation1977; Kishk Citation2008) also have observed an increased volume of ejaculates in rams with maximal levels of serum testosterone.
Table 2. Scrotal circumference, Sertoli cells and sperm cells found in testicular biopsies from mixed-breed goat bucks treated or not treated with testosterone during the period of sexual inactivity (March) at 26°N.
T bucks had a higher sperm count per ejaculate than control bucks. The average volume and percent motility were generally close to the estimates reported in the literature for bucks of comparable age and genotype (Mellado et al. Citation2012). On the other hand, values for sperm cells/mL and total number of spermatozoa per ejaculate observed in the present study are much lower than those reported by Roca et al. (Citation1992) with Murciano-Granadina, Barkawi et al. (Citation2006) with Zaraibi and Talebi et al. (Citation2009) and Farshad et al. (Citation2012) with Markhoz goat bucks.
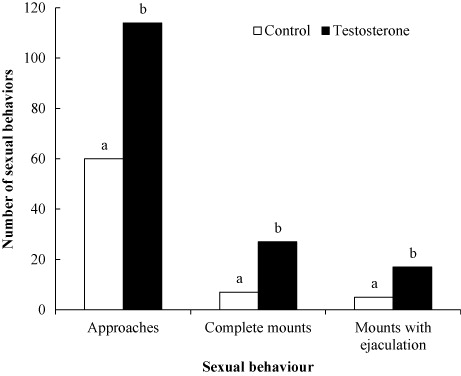
summarizes various sexual behaviours of bucks during the exposure period to estrogenized does. T bucks were more sexually responsive than control bucks, which was reflected in a higher (P < 0.01) approaching with investigatory sniffs, mounts without intromission but with erected penis and mounts with ejaculations. The effectiveness of exogenous testosterone to elicited sexual arousal in sexually inactive bucks was also observed by Luna-Orozco et al. (Citation2012) with mixed-breed bucks at this latitude.
No effect of treatment on Sertoli cells numbers in testicular biopsy samples was observed (). This was expected because these cells become fixed and unmodifiable around puberty, although recent data have challenged this view (Tarulli et al. Citation2012). On the other hand, treatment affected the number of sperm cells in testicular biopsies, with higher numbers in control bucks compared to T bucks. These results were unexpected, and it is believed that this difference may be due to sampling errors (selection, preparation or technique) or processing of sample tissue.
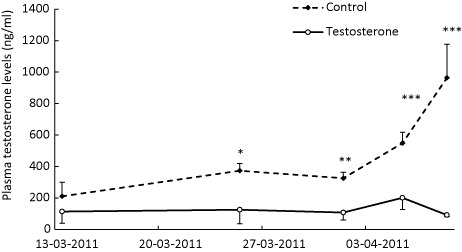
Progressive increase in the profile of serum testosterone occurred in bucks as the testosterone application period progressed (). Concentration of T increased nearly threefold three weeks after the beginning of testosterone application. This marked increases in mean serum levels of testosterone apparently triggered the increase of volatile odours in T bucks (). It has been well established that the male goat odours: 6-trans nonenal (Smith et al. Citation1984) a pheromone responsible for the male effect is produced in the sebaceous gland under the control of testosterone (Iwata et al. Citation2000).
4. Conclusions
The study reaffirmed that the application of testosterone to sexually inactive mature mixed-breed bucks at 26°N elicited a marked sexual arousal and increased total sperm cells per ejaculation. The practical implications are that during the non-breeding season, testosterone-treated bucks could be used to stimulate the sexual activity of anestrous does and to fecundate these animals in spring.
References
- Barkawi AH, Elsayed EH, Ashour G, Shehata E. 2006. Seasonal changes in semen characteristics, hormonal profiles and testicular activity in Zaraibi goats. Small Rumin Res. 66:209–213. 10.1016/j.smallrumres.2005.09.007
- Chemineau P, Varo H, Grudé A. 1986. Sexual behaviour and gonadal activity during the year in the tropical Creole meat goat. II. Male mating behaviour, testis diameter, ejaculate characteristics and fertility. Reprod Nutr Devel. 26:453–460. 10.1051/rnd:19860306
- De Oliveira-Souza LW, Andrade AFC, Carvalho-Celeghini EC, Negrão JA, Paes de Arruda R. 2011. Correlation between sperm characteristics and testosterone in bovine seminal plasma by direct radioimmunoassay. R Bras Zootec. 40:2721–2724.
- De Santiago-Miramontes MA, Luna-Orozco JR, Meza-Herrera CA, Rivas-Muñoz R, Carrillo E, Véliz-Deras FG, Mellado M. 2011. The effect of flushing and stimulus of estrogenized does on reproductive performance of anovulatory-range goats. Trop Anim Health Prod. 43:1595–1600. 10.1007/s11250-011-9849-6
- Farshad A, Yousefi A, Moghaddam A, Khalili B. 2012. Seasonal changes in serum testosterone, LDH concentration and semen characteristics in Markhoz goats. Asian-Aust J Anim Sci. 25:189–193. 10.5713/ajas.2011.11179
- Hassomo HL. 1984. Body condition scoring of fat-tail sheep and effects of spring degree on productivity of ewes. Damascus (Syria): The Arab center for the studies of arid zones and dry lands (ACSAD).
- Iwata E, Wakabayashi Y, Kakuma Y, Kikusui T, Takeuchi Y, Mori Y. 2000.Testosterone-dependent primer pheromone production in the sebaceous gland of male goat. Biol Reprod. 62:806–810. 10.1095/biolreprod62.3.806
- Kishk WH. 2008. Interrelationship between ram plasma testosterone level and some semen characteristics. Slovak J Anim Sci. 41:67–71.
- Kumar P, Yadav B, Yadav, S. 2013. Effect of zinc and selenium supplementation on antioxidative status of seminal plasma and testosterone, T4 and T3 level in goat blood serum. J Appl Anim Res. 41:382–386. 10.1080/09712119.2013.783482
- Luna-Orozco JR, Guillen-Muñoz JM, De Santiago-Miramontes MA, García JE, Rodríguez-Martínez R, Meza-Herrera CA, Mellado M, Véliz FG 2012. Influence of sexually inactive bucks subjected to long photoperiod or testosterone on the induction of estrus in anovulatory goats. Trop Anim Health Prod. 44:71–75. 10.1007/s11250-011-9889-y
- Mellado J, Veliz FG, De Santiago-Miramontes MA, Meza-Herrera CA, Mellado M. 2014. Buck-induced estrus in grazing goats during increasing photoperiod and under cold stress at 25° N. Vet Med Zoot. 88:40–45.
- Mellado M, Meza-Herrera CA, Arévalo JR, García JE, Veliz FG. 2012. Effect of dietary energy intake and somatotropin administration after weaning on growth rate and semen characteristics of Granadina goat bucks. Turk J Vet Anim Sci. 36:338–345.
- NRC. 2007. Nutrient requirements of small ruminants – sheep, goats, cervids, and new world camelids. Washington (DC): National Research Council. The National Academic Press.
- Pellicer-Rubio M-T, Leboeuf B, Bernelas D, Forgerit Y, Pougnard JL, Bonné JL, Senty E, Chemineau P. 2007. Highly synchronous and fertile reproductive activity induced by the male effect during deep anoestrus in lactating goats subjected to treatment with artificially long days followed by a natural photoperiod. Anim Reprod Sci. 98:241–258. 10.1016/j.anireprosci.2006.03.002
- Rivas-Muñoz R, Carrillo E, Rodriguez-Martinez R, Leyva C, Mellado M, Véliz FG. 2010. Effect of body condition score of does and use of bucks subjected to added artificial light on estrus response of Alpine goats. Trop Anim Health Prod. 42:1285–1289.
- Roca J, Martinez E, Vazquez JM, Coy P. 1992. Characteristics and seasonal variations in the semen of Murciano-Granadina goats in the Mediterranean area. Anim Reprod Sci. 29:255–262. 10.1016/0378-4320(92)90038-F
- Sanford LM, Palmer WM, Howland BE. 1977. Changes in the profiles of serum LH, FSH and testosterone, and in mating performance and ejaculate volume in the ram during the ovine breeding season. J Anim Sci. 45:1382–1391.
- SAS. 1996. SAS/STAT statistical analysis systems, version 6.12. Cary (NC): SAS Institute Inc.
- Smith PW, Parks OW, Schwartz DP. 1984. Characterization of male goat odors: 6-trans nonenal. J Dairy Sci. 67:794–801. 10.3168/jds.S0022-0302(84)81369-7
- Talebi J, Souria M, Moghaddam A, Karimi I, Mirmahmoodi M. 2009. Characteristics and seasonal variation in the semen of Markhoz bucks in western Iran. Small Rum Res. 85:18–22.
- Tarulli GA, Stanton PG, Meachem SJ. 2012. Is the adult sertoli cell terminally differentiated?. Biol Reprod. 87:13. 10.1095/biolreprod.111.095091
- Véliz FG, Meza-Herrera CA, De Santiago-Miramontes MA, Arellano-Rodriguez G, Leyva C, Rivas-Muñoz R, Mellado M. 2009. Effect of parity and progesterone priming on induction of reproductive function in Saanen goats by buck exposure. Livest Sci. 125:261–265.
- Walkden-Brown SW, Restall BJ, Henniawati 1993. The male effect in the Australian Cashmere goat. 3. Enhancement with buck nutrition and use of estrous females. Anim Reprod Sci. 32:69–84.
- Walkden-Brown SW, Restall BJ, Scaramuzzi RJ, Martin GB, Blackberry MA. 1997. Seasonality in male Australian cashmere goats: long term effects of castration and testosterone or oestradiol treatment on changes in LH, FSH, and prolactin concentrations, and body growth. Small Rumin Res. 26:239–252. 10.1016/S0921-4488(97)00017-5
- Zarazaga LA, Guzmán JL, Domínguez C, Pérez MC, Prieto R. 2009. Effects of season and feeding level on reproductive activity and semen quality in Payoya buck goats. Theriogenology. 71:1316–1325. 10.1016/j.theriogenology.2009.01.007