Abstract
The present investigation studied the effect of short-term cooling around the time of artificial insemination (AI) on body temperature, plasma cortisol and conception rate (CR) in Murrah buffalo heifers. Buffalo heifers (n = 25) were divided into two groups i.e. control group (n = 9) and short-term cooling group (n = 16). In both the groups, estrus synchronization using ovsynch protocol and fixed time AI was carried out. Heifers in short-term cooling group were kept in a specially designed air condition room from 3 h before to 3 h after AI. Blood samples were collected for estimation of cortisol and progesterone and rectal and vaginal temperatures were measured before 3 h of AI, at the time of AI and after 3 h of AI. At the time of AI and after 3 h of AI, both rectal and vaginal temperatures were significantly (P < 0.05) lower in heifers subjected to short-term cooling compared to control group. After 3 h of AI, cortisol levels were significantly (P < 0.05) higher in control group (5.023 ± 0.74 ng/ml) compared to short-term cooling group (1.061 ± 0.99 ng/ml). A trend of higher CR was observed in short-term cooling group compared to control group (68.75 vs. 33.33%, P = 0.087). It may be inferred that short-term cooling reduced the stress at the time of AI and improved the CR in Murrah buffalo heifers during hot-humid season.
1. Introduction
Heat stress is the key factor responsible for lower productive and reproductive performance in Murrah buffaloes during the summer months. Extended periods of high ambient temperature coupled with high relative humidity compromise the ability of the dairy bovines to dissipate excess body heat (Marai et al. Citation2009). It has been reported that thermal stress delays follicle selection and lengthening of the follicular wave which adversely affect the quality of oocytes (Badinga et al. Citation1993) and steroid secretion from follicles (Roth et al. Citation2001) in dairy bovines. Follicles fail to grow up to dominant follicle during summer stress and more number of medium-sized subordinate follicles survive (Roth et al. Citation2000). Thermal stress during oocyte maturation and near ovulation in cows (Putney et al. Citation1988) and mice (Roth et al. Citation2008), may or may not affect the fertilizing capacity of oocyte, however, the resulting embryos develop either slowly or abnormally. Many studies have shown that the body temperature on the day of estrus is extremely critical to the outcome of AI since the embryonic development and viability in cattle and sheep affect more adversely when thermal stress occurred on the day of estrus or three days after estrus (Gwazdauskas et al. Citation1975; Ealy et al. Citation1993). The plasma level of corticosteroid increases (Roman-Ponce et al. Citation1977) and Luteinizing hormone (LH; De Rensis & Scaramuzzi Citation2003) and progesterone (Howell et al. Citation1994) concentrations decreases under heat stress. Altered uterine environment due to reduced blood supply (Gwazdauskas et al. Citation1975; Roman-Ponce et al. Citation1978) and increased uterine temperature (Roman-Ponce et al. Citation1978) drastically reduce the viability of embryo (Ray et al. Citation1992). Such alteration in the biochemistry of uterus results in high early embryonic mortality and reduced proportion of successful inseminations (Thompson et al. Citation1996). It has been reported that embryonic development was impaired in heifers subjected to heat stress for 10 h after the onset of estrus (Putney et al. Citation1988) and on day 1 after breeding (Ealy et al. Citation1993).
Buffaloes are more sensitive to solar radiation and high ambient temperature due to poor thermal tolerance because of dark body colour, relatively less number of sweat glands per unit area of skin and thick epidermal layer of skin which reduce heat loss by evaporative cooling (Marai & Habeeb Citation2010).
There are three basic management approaches for reducing the adverse effect of summer stress in dairy animal, i.e. physical modification of environment, development of genetically thermal resistance breeds and nutritional management (Beede & Collier Citation1986). For ameliorating the effect of thermal stress in buffalo different methods (shade, water splashing, sprinkling, showering, fanning, forced ventilation and wallowing, etc.) have been tried with varied success. Different methods of cooling for reducing the hyperthermia during the final maturation of oocyte and at early development of embryo may be useful in improving the conception rate (CR) in buffaloes during hot-humid season. In this context, a provision of short-term cooling before and after artificial insemination (AI) can serve as a recluse to augment the CR (Moghaddam et al. Citation2009). Thus, the present investigation was conducted with an objective to study the effect of short-term cooling on the CR in the Murrah buffalo heifers during hot-humid season.
2. Materials and methods
2.1. Experimental design and animals
The present investigation was conducted on Murrah buffalo heifers maintained at Livestock Research Centre of National Dairy Research Institute, Karnal, Haryana. A subtropical climate with maximum air temperature during summer about 45–48°C and minimum temperature during winter near to 1–4°C prevailed in the area. In the study area relative humidity ranged between 41% and 85% and annual rainfall between 760 and 960 mm. The day length in the summer varied from 13 to 14 h whereas in winter it was 10–11 h. The duration of the study was two months (hot and humid season; August to September, 2011).
Murrah buffalo heifers (n = 25) that had experienced at least two estrous cycles were taken in the study. All the heifers included in the study were free from anatomical and reproductive disorders and were maintained in loose housing system and group management practice. Buffalo heifers were divided into two groups based on age and body weight, i.e. without cooling group (n = 9, 38.07 ± 3.13 months and 447 ± 20.03 kg) and short-term cooling group (n = 16, 38.04 ± 1.83 months and 445 ± 19.07 kg). All heifers were synchronized using ovsynch protocol (Paul & Prakash Citation2005) where on day 0, 10 µg of a GnRH (Buserelin Acetate, Receptal VET, Intervet India Private Ltd., Pune, Maharashtra, India) was administered to the heifers irrespective of the stage of estrous cycle. On day 7, 25 mg of PGF2α (Dinoprost thromethamine; LutalyseTM, Novartis India Limited, Maharashtra, India) was administered and on day 9 GnRH (10 µg) was administered.
Murrah buffalo heifers in the short-term cooling group (n = 16) were cooled for 3 h before and 3 h after AI by keeping animals inside the specially designed environmental chamber for the study. The environmental chamber was cooled using an air conditioner [Bluestar, Model: 2WAE181YA Capacity: 1.5 Ton, Cooling capacity (w): 4765, Power consumption (w):1870], two wall fans (Power: 50 Watt, rpm: 1500) and one exhaust fan (Power: 50 Watt, rpm: 1500). The wall fans and air conditioner were run continuously while exhaust fan intermittently for 5–10 minutes at hourly interval. The air conditioner was started before 12–14 h of the beginning of experiment to reduce the temperature and relative humidity inside the environmental chamber. At the time of experiment, the temperature and relative humidity inside the chamber was maintained at 26–30°C and 55–60%, respectively. Heifers in without cooling group (n = 9) received no such treatment and maintained in open housing system. Environmental temperature during the study period was 35–40°C and relative humidity was 80–85%.
2.2. Rectal and vaginal temperatures measurement
Rectal and vaginal temperatures were measured by keeping the sensitive point of Digital thermometer (Digital Multi-Stem Thermometer ST-9269B MEXTECHTM China) in close contact with mucus membrane of rectum and vagina, respectively, for 2 minutes. Temperatures were recorded before cooling, i.e. 3.00 h before AI, at the time of AI and 3.00 h after AI in both short-term cooling and without cooling groups.
2.3. Timed AI in synchronized buffalo heifers
Murrah heifers were inseminated at fixed time (i.e. 16–22 h after second GnRH injection) after confirming their estrus stage by per-rectal examination using the semen from a bull of known fertility. All heifers were inseminated by a single Veterinarian. Two inseminations were carried out, first at 9–10 am and second at 4–5 pm on the same day.
2.4. Estimation of peripheral cortisol and progesterone concentrations
Blood samples were collected twice on the day of AI (before 3 h of AI and after 3 h of AI) and after 21 days of AI at 7.00 am through jugular vein puncture using 9 ml blood collection tubes with Heparin as anticoagulant (Vacuette®, Greiner Bio-one Gmbh, Austria) for the estimation of cortisol and progesterone, respectively. Immediately after the collection, blood samples were centrifuged at 4°C (3000 rpm for 30 minutes) to separate the plasma. The separated plasma samples were stored in cryovials at –20°C till the assay for the hormone was conducted. For assessing the stress level in buffaloes plasma cortisol was measured before and after cooling (3 h before and after AI) using Bovine Cortisol ELISA test Kit (Endocrine Technologies, INC. USA), as per the instructions provided by the manufacturer. Progesterone hormone was estimated by using Bovine Progesterone ELISA test Kit (Endocrine Technologies, Inc. Newark, CA) for early pregnancy diagnosis.
2.5. Pregnancy diagnosis and CR
Pregnancy diagnosis was performed by two methods, i.e. measuring the plasma progesterone concentration and by per-rectal examination of reproductive tract. For early pregnancy diagnosis, estimation of plasma progesterone level was carried out after 21 days of AI. The animals those found with lower plasma progesterone value (<1 ng/ml) were considered as non-pregnant. In second method of pregnancy diagnosis experimental heifers were examined using ultrasound (Prosound 2, Aloka Ltd., Japan) after 60 days of AI. For calculation of CR following formula was used:
2.6. Statistical analysis
In the present study descriptive statistics were calculated for plasma progesterone level with all means is represented as Mean ± SEM. Rectal and vaginal temperatures before 3.00 h of AI, at the time of AI and after 3.00 h of AI in short-term cooling and without cooling group were compared by two-way analysis of variance (ANOVA) with interaction using general linear model. The comparison of plasma cortisol concentrations before 3.00 h of AI and after 3.00 h of AI in short-term cooling group and without cooling group was also done by two-way ANOVA with interaction using general linear model. Chi-square test was performed to determine the difference between the CR in short-term cooling group and without cooling group. Power analysis was conducted at a power of 0.8. In the two-way ANOVA means were separated on the basis of least square mean and all pair wise multiple comparisons was performed using Tukey test as post hoc test. When the P value was <0.05, then all analysis were considered to be significantly differing, while a trend was reported if P value ≥0.05 and ≤0.1. All the analyses were performed using Sigma Plot 11® software (Systat software Inc., USA).
3. Results
3.1. Rectal and vaginal temperatures
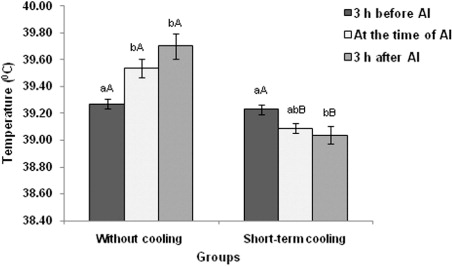
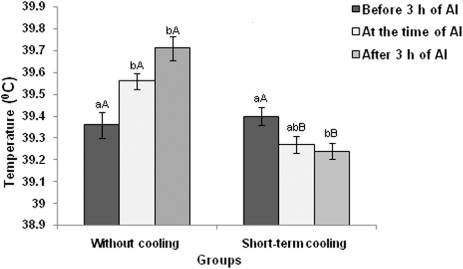
The rectal temperature was significantly (P < 0.05) lower at the time of AI (39.09 ± 0.03°C) as well as after 3 h of AI (39.04 ± 0.06°C) in short-term cooled group compared to control (without cooling) group (). In control group significantly (P < 0.05) higher rectal temperature was observed at the time of AI (39.54 ± 0.07) and after 3.00 h of AI (39.70 ± 0.09°C) as compared to before 3.00 h of AI (39.27 ± 0.03°C). But in treatment group, significantly (P < 0.05) lower rectal temperature was observed at 3.00 h after AI (39.04 ± 0.06°C) compared to 3.00 h before AI (39.23 ± 0.03°C). The same trend was observed in vaginal temperature also () indicating the positive effects of short-term cooling on rectal temperature.
3.2. Plasma cortisol concentrations
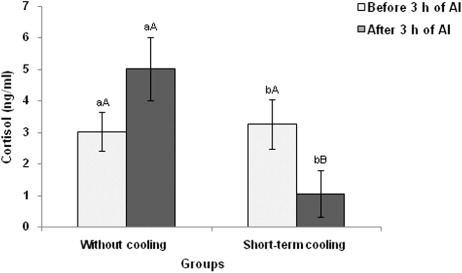
At 3 h before AI, there was no difference in plasma cortisol concentrations in both control as well as treatment group (). However, significantly (P < 0.05) lower plasma cortisol concentrations were observed in short-term cooling group (1.061 ± 0.993 ng/ml) compared to control group (5.023 ± 0.740 ng/ml) after 3.00 h of AI. In treatment group, although a decreasing trend was observed in cortisol concentration from 3.00 h before AI to 3.00 h after AI, the difference was statistically non-significant. In the control group, the cortisol concentrations 3 h after AI increased to 5.023 ± 0.74 ng/ml from 3.031 ± 0.78 ng/ml at 3h before AI, however, the difference was not significant.
3.3. Plasma progesterone concentration at 21st day after AI
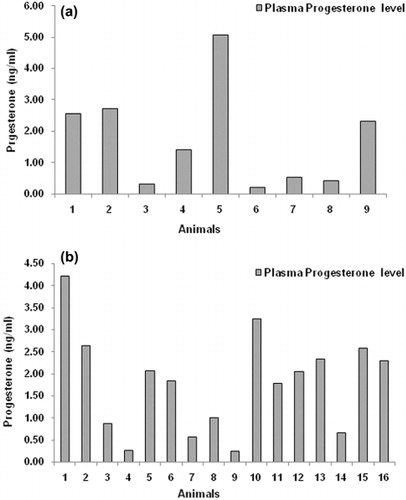
In control group, the mean plasma progesterone concentrations at 21st day after AI in nonpregnant and pregnant Murrah heifers were 0.37 ± 0.06 ng/ml and 2.82 ± 0.31 ng/ml, respectively. The corresponding concentrations in the treatment group were 0.52 ± 0.11 ng/ml and 2.37 ± 0.25 ng/ml in non-pregnant and pregnant heifers, respectively. Although non-significant, the progesterone levels were higher in control group compared to the treatment group. Two heifers in control group had high plasma progesterone concentrations (3.81 ± 1.24 ng/ml) on 21st day after AI () but during pregnancy examination after 60 days post-AI these animals were confirmed as non-pregnant.
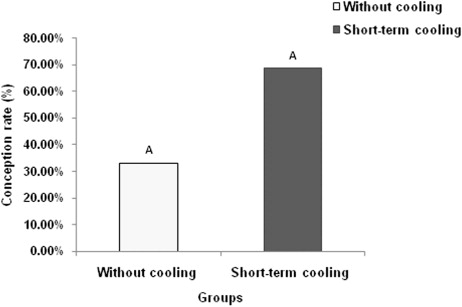
3.4. Effect of short-term cooling on CR
The study revealed a trend of higher CR (P = 0.087) in short-term cooling group compared to control group (). Out of nine buffalo heifers in the control group, three conceived leading to a CR of 33.33%. In treatment group, out of 16 heifers 11 conceived and the CR in this group was 68.75%. Retrospective power analysis based on CR observed in the present study between short-term cooling and control group revealed that total sample size required for 80% power with α = 0.05 was 65.
4. Discussion
Rise in body temperature is known to affect several physiological functions of the body including reproduction. High temperature induces stress to the animals resulting in increased production and release of anti-stress factors and hormones, which may impair reproduction (Badinga et al. Citation1993; Roth et al. Citation2001). The present study reports the beneficial effects of short-term cooling (before and after AI) on CR in buffaloes, which are more susceptible to heat stress owing to several reasons including black colour and reduced sweat glands.
We observed a higher CR, although non-significant, in the short-term cooling group as compared to without cooling group. Reducing the effects of heat stress around the time of AI has been shown to improve CR by facilitating proper hormonal milieu at the time after conception (Gwazdauskas et al. Citation1975; Ealy et al. Citation1993). It has been reported that altered LH secretion in heatstressed cows, resulted in impaired ovulation and formation of inferior quality corpus luteum (Sartori et al. Citation2002) that ultimately reduce the CR. Thermal stress associated conception failure has already been reported (Sprott et al. Citation2001; Al-Katanani et al. Citation2002). Similarly it has been shown that hyperthermia due to heat stress leads to embryonic absorption and abortion in female buffaloes. We also observed that two animals in control group () had high plasma progesterone concentrations on day 21 post-AI, however, they turn out to be non-pregnant upon ultrasound examination at 60 days post-AI indicating that these animals might had embryonic mortality. However, we did not find such incidences in short-term cooled group indicating that there was no embryonic mortality in these animals (). The heifers in short-term cooled group had lower body temperature compared to those in control group indicating that this method is efficient in maintaining normal body temperature around the time of AI. Earlier reports also suggest that cooling methods like wallowing, intermittent splashing of water (Singh et al. Citation2005), evaporative cooling (Khongdee et al. Citation2006; Moghaddam et al. Citation2009) and direct watering with forced ventilation (Flamenbaum & Galon Citation2010) had ameliorative effect on rectal and body temperature. It has been reported that under thermal stress the blood flow in female animals increased in the proximity of the superficial vessels in detriment of the deep vessels. Such changes in the deep circulatory flow affect the circulation and nutritive supply at the uterus and ovarian level causing impairment in its normal physiology and the magnitude of the depression in the CR is proportional to the degree of hyperthermia (Grunert et al. Citation2005). It is possible that avoiding elevations in body temperature around the time of AI by short-term cooling in the present study might be the reason for higher CR obtained in this group.
Our findings on CR (68.75% and 33.33% in short-term cooled and control groups, respectively) are in consonance with the results (71.43% and 14.29% in evaporately cooled and non-cooled cows, respectively) reported by Khongdee et al. (Citation2006). Similar findings were also reported by Moghaddam et al. (Citation2009), who observed higher CRs (40–56.7%) in cooled compared to non-cooled (23.3%) Holstein heifers. In buffaloes, Ealy et al. (Citation1994) also observed that the CR in cooled cows (sprinkler + forced ventilation) was higher compared to those animals kept under shed only. Recently Flamenbaum and Galon (Citation2010) also reported that the CR was 59% in buffaloes subjected to cooling while it was only 17% in buffaloes that were not cooled. Although the earlier reports and the results of the present study agrees that cooling increases the CR in cattle and buffaloes, the wised variation in the CR obtained in different studies might be due to the method of cooling and duration of cooling.
Since it has been reported that cortisol concentrations in peripheral circulation are elevated during stress conditions, we estimated the peripheral cortisol concentrations in cooled and non-cooled heifers. We observed that the cortisol levels were significantly (P < 0.05) higher at the time of AI and after 3 h of AI in non-cooled heifers compared to those heifers subjected to short-term cooling. Increased levels of cortisol in without cooling group might be due to the stress associated with increased body temperature (Loypetjra et al. Citation1987). Heat stress stimulates the hypothalamo pituitary–adrenal axis and the sympathoadrenal system and activates the corticotropin-releasing factor neurones in the para-ventricular nucleus, and secretion of these neuropeptides into the hypophyseal portal system to stimulate the corticotrophs of the anterior pituitary gland (Tilbrook et al. Citation2000). Under the influence of heat stress, Adrenocorticotropic hormone acts on the cortex of adrenal glands to stimulate the synthesis and secretion of glucocorticoids like cortisol (Abilay et al. Citation1975). These observations are further supported by Dhami et al. (Citation2006), who observed a rising trend of cortisol concentration with increase in heat stress from May to July along with increase in Temperature Humidity Index. Effect of cooling on reduced cortisol concentration was also reported by several workers in buffaloes (Loypetjra et al. Citation1987; Aggarwal & Singh Citation2010).
Taken together the findings of the present study suggest short-term cooling around the time of AI maintained low body temperature and cortisol concentrations which in turn might help in improving the CR in Murrah buffalo heifers during hot and humid season. Although the sample size in the present study is small, it provides information about the association of short-term cooling with CR. Furthermore, detailed study need to be conducted with optimum sample size to find the effect of short-term cooling on the CR in Murrah buffalo heifers during hot-humid season.
5. Conclusion
From the findings of present investigation it may be concluded that short-term cooling around the time of insemination improved the CR in Murrah buffalo heifers. This method can be used as a management tool to improve the reproductive performance in Murrah buffalo heifers during hot-humid season.
Acknowledgements
The authors express their sincere sense of gratitude to Director and Vice Chancellor, National Dairy Research Institute, Karnal, for providing all research facilities for the successful completion of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abilay TA, Johnson HD, Madan M. 1975. Influence of environmental heat on peripheral plasma progesterone and cortisol during the bovine estrous cycle. J Dairy Sci. 58:1836–1840. 10.3168/jds.S0022-0302(75)84795-3
- Aggarwal A, Singh M. 2010. Changes in skin and rectal temperature in lactating buffaloes provided with showers and wallowing during hot-dry season. Trop Anim Health Prod. 40:223–228.
- Al-Katanani YM, Paula-Lopes FF, Hansen PJ. 2002. Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J Dairy Sci. 85:390–396. 10.3168/jds.S0022-0302(02)74086-1
- Badinga L, Thatcher WW, Diaz T, Drost M, Wolfenson D. 1993. Effects of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology. 39:797–810. 10.1016/0093-691X(93)90419-6
- Beede DK, Collier RJ. 1986. Potential nutritional strategies for intensively managed cattle during thermal stress. J Anim Sci. 62:543–554.
- De Rensis F, Scaramuzzi RJ. 2003. Heat stress and seasonal effects on reproduction in the dairy cow: a review. Theriogenology. 60:1139–1151. 10.1016/S0093-691X(03)00126-2
- Dhami AJ, Parmar BC, Raval R, Gupta RS, Trivedi MM. 2006. Effect of challenge feeding during summer on the productive and reproductive performance and blood biochemical, metabolic and hormonal profile of crossbred lactating cows. Int J Cow Sci. 2:973–2241.
- Ealy AD, Arechiga CF, Bray DR, Risco CA, Hansen PJ. 1994. Effectiveness of short-term cooling and vitamin E for alleviation of infertility induced by heat stress in dairy cows. J Dairy Sci. 77:3601–3607. 10.3168/jds.S0022-0302(94)77304-5
- Ealy AD, Drost M, Hansen PJ. 1993. Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. J Dairy Sci. 76:2899–2905. 10.3168/jds.S0022-0302(93)77629-8
- Flamenbaum I, Galon N. 2010. Management of heat stress to improve fertility in dairy cows in Israel. J Reprod Dev. 56:S36–S41.
- Singh G, Kamboj ML, Patil NV. 2005. Effect of thermal protective measures during hot humid season on productive and reproductive performance of Nili-Ravi buffaloes. Indian Buffalo J. 3:101–104.
- Grunert E, Birgel EH, Vale WG. 2005. Patologia e clínica da reprodução dos animais mamíferos domésticos –Ginecologia [Pathology and Clinic reproduction of domestic mammals –Ginecologia]. Ed. Varela, São Paulo; p. 551.
- Gwazdauskas FC, Wilcx CJ, Thatcher WW. 1975. Environmental and managerial factors affecting conception rate in a subtropical climate. J Dairy Sci. 58:88–92. 10.3168/jds.S0022-0302(75)84523-1
- Howell JL, Fuquay JW, Smith AE. 1994. Corpus luteum growth and function in lactating Holstein cows during spring and summer. J Dairy Sci. 77:735–739. 10.3168/jds.S0022-0302(94)77007-7
- Khongdee S, Chaiyabutr N, Hinch G, Markvichitr K, Vajrabukka C. 2006. Effects of evaporative cooling on reproductive performance and milk production of dairy cows in hot wet conditions. Int J Biometeorol. 50:253–257. 10.1007/s00484-006-0030-2
- Loypetjra P, Chaiyabutr N, Chanpongsang S, Pichaicharnarong A, Katti P, Johnson HD. 1987. Effect of high ambient temperature on serum cortisol, triiodothyronine, prolactin and growth hormone in swamp buffaloes. Thai J Vet Medi. 17:287–305.
- Marai IFM, Daader AH, Soliman AM, El-Menshawy SMS. 2009. Non-genetic factors affecting growth and reproduction traits of buffaloes under dry management housing (in sub-tropical environment) in Egypt. Livest Res Rural Dev. 21:1–13.
- Marai IFM, Habeeb AAM. 2010. Buffalo’s biological functions as affected by heat stress: a review. Livest Sci. 127:89–109.
- Moghaddam A, Karimi I, Pooyanmehr M. 2009. Effect of short-term cooling on pregnancy rate of dairy heifers under summer heat stress. Vet Res Commun. 33:567–575.
- Paul V, Prakash BS. 2005. Efficacy of the Ovsynch protocol for synchronisation of ovulation and fixed–time artificial insemination in Murrah buffaloes (Bubalus bubalis). Theriogenology. 64:1049–1060. 10.1016/j.theriogenology.2005.02.004
- Putney DJ, Malayer JR, Gross TS, Thatcher WW, Hansen PJ, Drost M. 1988. Heat-stress induced alterations in the synthesis and secretion of proteins and prostaglandins by cultured bovine conceptuses and uterine endometrium. Biol Reprod. 39:717–728. 10.1095/biolreprod39.3.717
- Ray DE, Halback TJ, Armstrong DV. 1992. Season and lactation number effects on milk production and reproduction and reproduction of dairy cattle in Arizona. J Dairy Sci. 75:2976–2983. 10.3168/jds.S0022-0302(92)78061-8
- Roman-Ponce H, Thatcher WW, Buffington DE, Wilcox CJ, Van Horn HH. 1977. Physiological and production responses of dairy cattle to a shade structure in a subtropical environment. J Dairy Sci. 60:424–430. 10.3168/jds.S0022-0302(77)83882-4
- Roman-Ponce H, Thatcher WW, Canton D, Barron DH, Wilcox CJ. 1978. Thermal stress effects on uterine blood flow in dairy cattle. J Anim Sci. 46:175–180.
- Roth Z, Aroyo A, Yavin S, Arav A. 2008. The antioxidant epigallocatechin gallate (EGCG) moderates the deleterious effects of maternal hyperthermia on follicle-enclosed oocytes in mice. Theriogenology. 70:887–897. 10.1016/j.theriogenology.2008.05.053
- Roth Z, Meidan R, Braw-Tal R, Wolfenson D. 2000. Immediate and delayed effect of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J Reprod Fertil. 120:83–90. 10.1530/jrf.0.1200083
- Roth Z, Meweidan R, Shaham-Albalancy A, Braw-Tal R, Wolfenson D. 2001. Delayed effect of heat stress on steroid production in medium-size and preovulatory bovine follicles. Reproduction. 121:745–751. 10.1530/rep.0.1210745
- Sartori R, Rosa GJ, Wiltbank MC. 2002. Ovarian structures and circulating steroids in heifers and lactating cows in summer and lactating and dry cows in winter. J Dairy Sci. 85:2813–2822. 10.3168/jds.S0022-0302(02)74368-3
- Sprott LR, Selk GE, Adams DC. 2001. Review: factors affecting decisions on when to calve beef females. Prof Anim Scientist. 17:238–246.
- Thompson JA, Magee DD, Tomaszewski MA, Wilks DL, Fourdraine RH. 1996. Management of summer infertility in Texas Holstein dairy cattle. Theriogenology. 46:547–558. 10.1016/0093-691X(96)00176-8
- Tilbrook AJ, Turner AI, Clarke IJ. 2000. Effects of heat stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod. 5:105–113. 10.1530/ror.0.0050105