ABSTRACT
Toona sinensis (TS) leaf is a well-known traditional oriental medicine herb. The purpose of this study was to evaluate in vitro antioxidant capability and performance assessment of White Roman goose supplemented with dried TS leaves (TSL). The TSL extracts contained 22.23 ± 1.13 mg gallic acid equivalent/g dry weight (DW) and 1.38 ± 0.06 mg quercetin equivalent/g DW of total phenolics and flavonoid contents. The scavenging action of 1, 1-diphenyl-2-picrylhydrazyl free radical and superoxide anion was as good as that of the butylated hydroxytoluene (BHT) and ascorbic acid, respectively. Inhibition of lipid peroxidation of TSL extracts at 2.5 mg/mL reached nearly 40% compared with the BHT. One hundred and eight White Roman geese aged 6 weeks were distributed to six pens randomly, and fed a grower diet ad libitum during the growing period, with each pen containing nine males and nine females following completely randomized design. Diets were supplemented with the following levels of dried TSL: 0% (control), 0.1% or 0.2% groups for 6 weeks, respectively. The results revealed no significant effects among treatments in the growth performance, blood biochemical parameters and muscle fibre of the grower geese. Superoxide dismutase (SOD) content of the serum in the 0.2% TSL group was significantly higher than that of the control group. In conclusion, dried TSL powder has antioxidative effects in vitro and could serve as a promising natural feed additive to improve the serum SOD content of grower geese.
1. Introduction
Toona sinensis (syn. Cedrela sinensis A. Juss.; TS) belongs to the Meliaceae family; it is a well-known traditional Chinese medicine herb. The compounds identified from TS possess a wide range of biologic functions, such as antioxidant activities, hypoglycemic effects and anti-low density lipid glycative activity (Chao et al. Citation2014; Chen et al. Citation2014; Liu et al. Citation2014b). Yang et al. (Citation2014b) pointed out five flavonols and three derivatives of gallic acid which have strong antioxidant activity and scavenge superoxide free radicals.
Currently, consumers are increasingly aware of the nutritional quality of the foods they consume. Farm animal products, such as geese, are important sources of nutrients for human; consumption and health demands have changed from quantity to quality. Today's domestic fowl producers are confronted with numerous challenges to prevent diseases and maintain health without the use of sub-therapeutic antibiotics (Webb & O'Neill Citation2008). In Taiwan, according to statistical data from the Council of Agriculture, Executive Yuan (Citation2012), involving 4.93 million geese slaughtered for meat. The major breed of meat geese in Taiwan is the White Roman goose, which represents 97.0% of the market product, the other 3% is provided by Chinese goose. As food/feed safety and animal welfare concerns continue to improve, researchers seek better antibiotic alternatives for domestic geese than those currently used (Yang et al. Citation2014a).
Oxidative rancidity is one of the major causes of deterioration of feed/food for consumption, and it typically causes losses in the production performance. Leeson (Citation2007) showed that the reactive oxygen species could cause lipid peroxidation and oxidative stress of animals, which may lead to metabolic disorders. Improved oxidative status in the living animals and increased oxidative stability of the raw products are considered to be beneficial for consumers. Feed additives have been proven to improve the performance and production quality of poultry and livestock (Ansari et al. Citation2012; Lee & Yu Citation2013; Lee et al. Citation2013). The use of synthetic antioxidants in animals to improve meat quality is being seriously considered. However, because some agents could exert some mutagenic effects at high doses, the innocuousness of these compounds has been questioned. Natural antioxidants such as phytogenics (Chinese medicine herbs) are currently receiving considerable attention in human and animal nutrition fields due to their association with food/meat quality characteristics (Ansari et al. Citation2012; Huang et al. Citation2012).
To our knowledge, little information is available regarding the influence of dietary supplementation of dried TS leaves (TSL) powder in the grower geese's diet. Therefore, the objective of the present study was to investigate the in vitro antioxidant capability and performance assessment of White Roman goose supplemented with dried TSL powder.
2. Materials and methods
2.1. In vitro antioxidant capability
2.1.1 TS sample preparation
TSL used in this study were kindly provided by a local TS producer in Changhua, Taiwan. The fresh TS was dried in a forced air dryer at 40°C for 24 h and then ground to a powder (approximately 1 mm in size) prior to its addition to the feed. The extracts were added to 100% distilled water (1:10, w/v) at 95°C for 2 h after filtering (Advantec NO. 1, Tokyo, Japan). The filtrate was evaporated until dry under vacuum conditions. The lyophilized extracts were rehydrated and the concentration adjusted to 1 mg/mL for subsequent analysis.
2.1.2 Bioactive components assay
Total phenolic contents were determined using a Folin-Ciocalteu reagent according to the method as described previously (Kujala et al. Citation2000). The Folin-Ciocalteu reagent was mixed evenly with TSL extracts before adding the Na2CO3 solution, and was measured with a spectrophotometer (HITACHI U-2900, Tokyo, Japan) at 730 nm. Then, with the contents of the phenolic compounds of the extracts, a microgram of the gallic acid equivalent (GAE) was determined using an equation that was obtained from the standard gallic acid graph. The flavonoid content of TSL extracts was determined by following the colorimetric method. Briefly, 0.5 mL of TSL extracts in methanol were mixed with methanol, 10% aluminium chloride and 1 M potassium acetate, and left for 20 min at room temperature. The absorbance of the reaction mixture was measured at 415 nm with a spectrophotometer, and the calibration curve was obtained by preparing quercetin solutions (Chang et al. Citation2002).
2.1.3 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) free radicals and superoxide anion radicals scavenging capacity assay
The free radical scavenging activity of TSL extracts was measured by DPPH using the method of Blois (Citation1958). Briefly, 0.1 mM solution of DPPH in ethanol was prepared and phosphate buffered saline extracts were added at different concentrations. After 20 min, absorbance was measured at 517 nm and was again used as a control butylated hydroxytoluene (BHT). Lower absorbance of the reaction mixture indicated higher free radical scavenging activity, and was calculated using the following equation:where A0 was the absorbance of the control reaction and A1 was the absorbance in the presence of TSL extracts.
Superoxide anion scavenging activity of TSL extracts was based on the method described by Nishimki et al. (Citation1972). The superoxide radicals were generated in Tris-HCl buffer (16 mM, pH 8.0) containing 600 µM of nitroblue tetrazolium; 1872 µM of dihydromicotineamidadenibe dinucleotide and sample solution of TSL extracts were mixed. The reaction had a further 240 µM of phenazine methosulphate added and left to rest at room temperature for 5 min. The absorbance was measured at 560 nm in a spectrophotometer with ascorbic acid used as a control. Decreased absorbance of the reaction mixture indicated increased superoxide anion scavenging capacity. The percentage inhibition of superoxide anion generation was calculated using the following formula:where A0 was the absorbance of the control, and A1 was the absorbance of TSL extracts.
2.1.4 Lipid peroxidation assay
The lipid peroxidation method described was modified slightly by Duh et al. (Citation1999). Lecithin (300 mg) was sonicated in an ultrasonic cleaner (BRANSON 5210, Danbury, USA) in 30 mL of a 50 mM phosphate buffer (pH 7.4) for 4 h. To the sonicated solution (0.5 mL, 10 mg lecithin/mL), FeCl3 (0.5 mL, 1 mM), ascorbic acid (0.5 mL, 400 mM) and TSL extracts were added. The mixture was incubated for 1 h at 37°C, and oxidation was measured using the 2-thiobarbituric acid (TBA) method. The absorbance of the sample was read at 532 nm against a negative control, which contained all reagents except lecithin; BHT served as the positive control.
The inhibition of lipid peroxidation percentage equalled [1–(A1/A0)] × 100 where A0 was the absorbance of the negative control reaction, and A1 was the absorbance in the presence of TSL extracts/BHT.
2.2 Animals and experiment design
The experimental protocols for all geese were approved by the Animal Care and Use Committee according to the Regulations of Laboratory Animals, Council of Agriculture in Taiwan. The geese were reared in Changhua Animal Propagation Station, Livestock Research Institute (CAPS-LRI, located at 23°51'N and 120°33'E), Changhua, Taiwan. One hundred and eight White Roman geese aged 6 weeks were distributed to six pens randomly. Before the 6 weeks of age, the rations provided were starter containing apparent metabolizable energy (AME) 2900 kcal/kg and crude protein (CP) 20% for birds during 0–6 weeks old and grower containing AME 2800 kcal/kg and CP 15% for birds during 7–14 weeks old. The rations and drinking water were freely accessible for the birds at all time. Each pen contained nine males and nine females following completely randomized design. They were fed diets supplemented with the following levels of dried TS: 0% (control group), 0.1% (A group) or 0.2% (B group) groups for 6 weeks, respectively. During the experimental period, the geese experienced a natural photoperiod and day length of 11.0–12.0 h. The controlled-environment finishing house was 1.26 m2 per goose. The pen of the finishing house was 11.9 × 3.8 m (45.2 m2). It contained a wire floor and one tank and two water dispensers. In the experimental period, the geese were fed a grower diet () formulated to meet the nutrient requirements according to the National Research Council (Citation1994); drinking water was provided ad libitum.
Table 1. Ingredients and nutrient compositions of the experimental diet.
2.2.1 Growth performance and blood sampling
At the end of 12 weeks, the performance of the grower geese was assessed by measuring the feed intake and body weight (BW); the BW gain and feed conversion ratio were recorded. On the same day, 12 geese (6 males and 6 females) from each treatment (6 geese per pen) were randomly selected according similar weight and blood samples were obtained by jugular venipuncture from each goose. Blood samples were centrifuged at 3000 × g for 30 min at 4°C; samples were then stored at –20°C until analysed.
2.2.2 Serum biochemical determinations
Serum biochemical values of geese were measured by automatic biochemical analyzer (Hitochi, 7150 auto-analyzer, Hitachi, Tokyo, Japan). A spectrophotometer was used to colorimetrically assay the activities of superoxide dismutase (SOD) and Trolox equivalent antioxidant capacity (TEAC) (Wheeler et al. Citation1990; Lee et al. Citation2012). The procedures were conducted with assay kits that were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). All of the samples were measured in triplicate and at appropriate dilutions to allow the enzymatic activities to achieve the linear range of standard curves. Antioxidative enzyme activities were expressed as unit (U) per millilitre of serum.
2.2.3 Muscle fibre tissue analysis
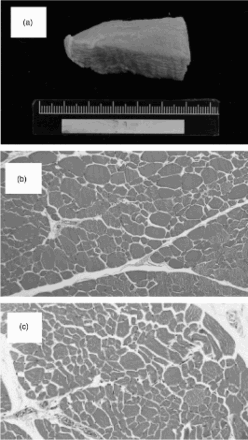
Before geese slaughter, electrical stunning and bleeding carotid artery were done. Right breast meat tissue were further processed, embedded in paraffin, cut at 2 μm by microtone (Leica RM 2145, Nussloch, Germany), stained with Hematoxylin & Eosin (H&E) staining and evaluated under light microscope (BX-51, Olympus, Tokyo, Japan) and image Pro-plus processing software (Image Pro-Plus, CA, USA) for muscle fibre evaluation.
2.3 Statistical analyses
The data of the variables collected were statistically analysed using the general liner models procedure of SAS software (Citation2004) following a random arrangement.
The mathematic model was:where
is the observed response of bird in a pen; µ theoverall mean;
the fixed effect of TS supplementation and
the residual error when pen was regarded as experimental unit,
.
The mean values were compared between three TS supplementations using the least square means with the significant level at p < .05.
3. Results
3.1 Bioactive components assay
The analysis of the active ingredients of TS is given in . The TSL extracts contained 22.23 ± 1.13 mg GAE/g dry weight (DW) and 1.38 ± 0.06 mg quercetin equivalent/g DW of total phenolics and flavonoid contents.
Table 2. Analysis of the active ingredients of TS.
3.2 DPPH free radicals and superoxide anion radicals scavenging capacity and lipid peroxidation assay
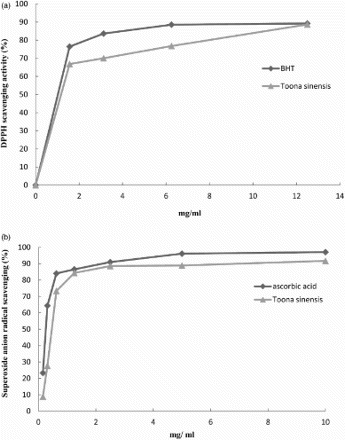
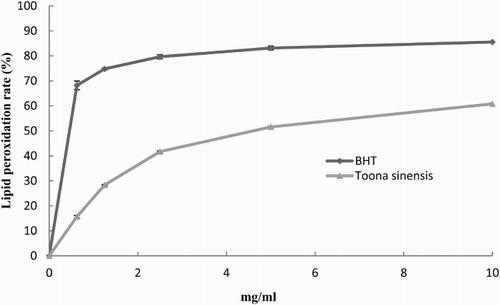
The DPPH free radicals and superoxide anion radicals scavenging activity of TS are shown in . The scavenging capacity of DPPH free radical ((a)) and superoxide anion ((b)) was as good as that of the BHT and ascorbic acid, respectively. The liposome oxidation assay of TS is presented in . The results shows that the inhibition of lipid peroxidation of TSL extracts at 2.5 mg/mL reached nearly 40%.
3.3 Growth performances and serum biochemical determinations
The effect of TS on growth performance in grower geese at 7–12 weeks is given in . The results reveal no significant affects among the treatments in the growth performances of grower geese (p > .05). Otherwise, it indicates that the BW before 6 weeks and BW gain during 7–12 weeks in male was significantly heavier than that of female (p < .05). The effect of TS on blood biochemical parameter in grower geese at week 12 is given in . The results present no significant effects among treatments in the blood biochemical parameter of grower geese (p > .05). The effect of TS on serum antioxidant enzyme activities in grower geese at week 12 is given in . It indicates that the SOD content of the serum in the 0.2% TS group was significantly higher than that of control group (p < .05). Moreover, the results reveal no significant affects among the sex treatments in the SOD and TEAC contents of grower geese (p > .05).
Table 3. Effect of TS on growth performance in grower geese at 7–12 weeks.1
Table 4. Effect of TS on blood biochemical parameter in grower geese at week 12.a
Table 5. Effect of TS on serum antioxidant enzyme activities in grower geese at week 12.1
3.4 Muscle fibre tissue analysis
The effect of TS on muscle fibre in grower geese at week 12 is shown in . The results reveal no significant effects between the control and 0.2% TS groups by H&E stain for muscle fibre of grower geese (p > .05).
4. Discussion
Herbal remedies or botanicals were added to the animal diets to stimulate the performance and/or to enhance the antioxidant system (Ansari et al. Citation2012; Lee et al. Citation2013; Chao et al. Citation2014). The TS are usually used as a vegetable and traditional Chinese medicine herb in China. Chao et al. (Citation2014) and Hseu et al. (Citation2008) showed that the TSL extracts and gallic acid possess effective antioxidant capacity against various oxidative systems in vitro, including the scavenging of free radicals and superoxide anion radicals, metal chelation and reducing power. Chen et al. (Citation2012) presented that the chelating capacities of ferrous ion in the TSL extracts obtained from different solvent concentrations were above 80% and mentioned its extracts could be used as biopreservatives of food product. Huang et al. (Citation2014) also pointed out that dried TSL could reduce the lipid oxidation as represented by TBA reactive substances in toona barbecue sauce, and the main antioxidative constituents are gallic acid and its derivatives, and flavonol glycosides might play an important role in its antioxidant activity in TS. Wang et al. (Citation2007) showed that the main antioxidative constituents in the TS are gallic acid and its derivatives; gallotannins and flavonol glycosides might play an important role in the antioxidant activity of this tree vegetable. Otherwise, total phenolic contents’ main function is the redox reaction, which plays an important role in neutralizing/adsorbing those free radicals such as superoxide anion (O2·–) radicals, hydroxyl (·OH) radicals, and hydrogen peroxide (H2O2, as oxygen in non-free radical state) for minimizing the damage to cells and inhibiting lipid oxidation (Parr & Bolwell Citation2000). Gulcin (Citation2005) analysed the superoxide anion scavenging capacities of black pepper seeds and used the ascorbic acid as a control. The results pointed out that the scavenging abilities of pepper seeds, upon being extracted by water and ethanol, were 64.2% and 22.6%, respectively, for ascorbic acid when the concentration of extracts was 0.075 mg/mL. In comparing TSL extracts, superoxide anion was as good as that of the ascorbic acid, indicating that TSL extracts had a better superoxide anion scavenging abilities than the black pepper seeds extracts. Therefore, in this study, we demonstrated that dried TS powder has high total phenolic contents and antioxidative capacities by in vitro evaluation.
Liao et al. (Citation2006) pointed out that there was no significant difference in BW, feed intake and organ weights with 1.0% TSL root or bark supplemented in the mice's diet. Wang et al. (Citation2014) demonstrated that TSL could inhibit microglia-mediated neuroinflammation, but not cause cytotoxicity. Liao et al. (Citation2009) indicated that TS extracts have no significant toxic effect in regard to the parameters of body and organ weight in rats, as well as hematological, biochemical and urinary effects. In this study, the results also reveal no significant effects on growth performance with 0.1% and 0.2% dried TS powder supplemented in the grower geese. Tilki et al. (Citation2005) indicated that the difference in live weight between the sexes became significant statistically at 6, 8, 10, 12, 14 and 16 weeks of age. Çelik and Bozkurt (Citation2009) presented that the BW of geese, abdominal fat, hot and cold carcass and mean values of hot and cold carcass were higher in males than females. It is displayed by the male and female geese weight difference; therefore, in this study, the treatment with sex show no interaction effect. Meanwhile, the muscle fibre pattern results reveal no significant effects between control and 0.2% TS group in grower geese.
Antioxidant enzymes are synthesized and regulated endogenously (Giannenas et al. Citation2010). One of them, SOD which is regulated and synthesized endogenously antioxidant mechanisms, are an important index of antioxidant capacity of animal/human tissue (Jiang et al. Citation2007); moreover, SOD catalyses the dismutation of the superoxide anion (O2–) into hydrogen peroxide, and prevents the generation of free radicals. TEAC measures the capacity of a compound to capture 2,2'-azinobis- (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), a radical cation. Botanical extracts such as isoflavone from soybean have been shown to increase the TEAC and SOD content of the plasma of chickens, and decrease lipid peroxidation (Jiang et al. Citation2007). Brenes et al. (Citation2008) demonstrated that the inclusion of 3.0% of grape pomace concentrate increased antioxidant activity (using ABTS method) in broiler serum and excreta. Moreover, Liu et al. (Citation2014a) found that supplementation with quercetin, which is well known for rich phenolic components and high antioxidant effects, could increase chickens’ SOD activity. Yang et al. (Citation2014a) showed that aqueous extracts of TS improved functions of sperm and testes for male rats under oxidative stress. In this study, high antioxidant activity of TS could better increase the serum SOD content than the control group in the 0.2% TS group; hence, it may suggest that natural secondary metabolites found in the aforementioned extracts affect gene expression of antioxidant enzymes (Puiggross et al. Citation2005).
5. Conclusion
Based on the results, it may be concluded that dried TS powder has antioxidative effects in vitro and could serve as a promising natural feed additive to improve the serum SOD content of grower geese.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ansari J, Khan SH, Haq Au, Yousaf M. 2012. Effect of the levels of Azadirachta indica dried leaf meal as phytogenic feed additive on the growth performance and haemato-biochemical parameters in broiler chicks. J Appl Anim Res. 40:336–345.
- Blois MS. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 26:1199–1200.
- Brenes A, Viveros A, Goñi I, Centeno C, Sáyago-Ayerdy SG, Arija I, Saura-Calixto F. 2008. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poultry Sci. 87:307–316.
- Çelik B, Bozkurt Z. 2009. Slaughter and carcass traits of native geese reared in muş province. Int Symp Anim Breeding in the Context of Modern Agriculture From Science to Practice. 42:423–428.
- Chang C, Yang M, Wen H, Chern J. 2002. Estimated of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 10:178–182.
- Chao PY, Lin SY, Lin KH, Liu YF, Hsu JI, Yang CM, Lai JY. 2014. Antioxidant activity in extracts of 27 indigenous Taiwanese vegetables. Nutrients. 6:2115–2130.
- Chen C, Tong Z, Liao D, Li Y, Yang G, Li M. 2014. Chemical composition and antimicrobial and DPPH scavenging activity of essential oil of Toona sinensis (A. Juss.) Roem from China. Bioresources. 9:5262–5278.
- Chen CM, Lin CY, Lin LC, Wan TC. 2012. Antioxidation activity and total phenolic contents of various Toona sinensis extracts. Afr J Biotechnol. 11:13831–13837.
- Council of Agriculture. (2012) Livestock Production, in: 2010 Agricultural Statistics Yearbook. Taipei: Council of Agriculture; p. 124–125.
- Duh PD, Tu YY, Yen GC. 1999. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). Lebensmittel-Wissenschaft and – Technologie. 32:269–277.
- Giannenas I, Pappas IS, Mavridis S, Kontopidis G, Skoufos J, Kyriazakis I. 2010. Performance and antioxidant status of broiler chickens supplemented with dried mushrooms (Agaricus bisporus) in their diet. Poultry Sci. 89:303–311.
- Gulcin I. 2005. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nut. 56:491–499.
- Hseu YC, Chang WH, Chen CS, Liao JW, Huang CJ, Lu FJ, Chia YC, Hsu HK, Wu JJ, Yang HL. 2008. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 46:105–114.
- Huang CW, Lee TT, Shih YC, Yu B. 2012. Effects of dietary supplementation of Chinese medicine herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. J Anim Physiol Anim Nutr. 96:285–294.
- Huang GL, Wang BJ, Weng YM. 2014. Physicochemical properties and activities of Toona sinensis Roem leaves as affected by drying methods. J Food Process Preserv. 38:364–372.
- Jiang ZY, Jiang SQ, Lin YC, Xi PB, Yu DQ, Wu TX. 2007. Effects of soybean isoflavone on growth performance, meat quality, and antioxidation in male broilers. Poultry Sci. 86:1356–1362.
- Kujala TS, Loponen JM, Klika KD, Pihlaja K. 2000. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agri Food Chem. 48:5338–5342.
- Lee TT, Ciou JY, Chen CL, Yu B. 2013. Effect of Echinacea purpurea L. on oxidative status and meat quality in Arbor Acres broilers. J Sci Food Agri. 93:166–172.
- Lee TT, Ciou JY, Chiang CJ, Chao YP, Yu B. 2012. Effect of Pleurotus eryngii stalk residue on the oxidative status and meat quality of broiler chickens. J Agri Food Chem. 60:11157–11163.
- Lee TT, Yu B. 2013. Application of biologics to feedstuff. Afr J Biotechnol. 12:526–530.
- Leeson S. 2007. Metabolic challenges: past, present, and future. J Appl Poultry Res. 16:121–125.
- Liao JW, Hsu CK, Wang MF, Hsu WM, Chan YC. 2006. Beneficial effect of Toona sinensis Roemor on improving cognitive performance and brain degeneration in senescence-accelerated mice. Br J Nutr. 96:400–407.
- Liao JW, Yeh JY, Lin YC, Wei MM, Chung YC. 2009. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis Roemor leaves. J Food Sci. 74:T7–T13.
- Liu HN, Liu Y, Hu LL, Suo YL, Zhang L, Jin F, Feng XA, Teng N, Li Y. 2014a. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poultry Sci. 93:347–353.
- Liu HW, Tsai YT, Chang SJ. 2014b. Toona sinensis leaf extract inhibits lipid accumulation through up-regulation of genes involved in lipolysis and fatty acid oxidation in adipocytes. J Agri Food Chem. 62:5887–5896.
- Natioanl Research Council. 1994. Nutrient requirements of poultry. 9th rev. ed. Washington (DC): National Academy Press.
- Nishimki M, Rao NA, Yagi K. 1972. The occurrence of super-oxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 46:849–853.
- Parr A, Bolwell GP. 2000. Phenols in the plant and in man: the potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agri. 80:985–1012.
- Puiggros F, Llópiz N, Ardévol A, Bladé C, Arola L, Salvadó MJ. 2005. Grape seed procyanidins prevent oxidative injury by modulating the expression of antioxidant enzyme systems. J Agri Food Chem. 53:6080–6086.
- SAS Institute. 2004. SAS® user's guide version 8.1. Cary (NC): Edition SAS Institute Inc.
- Tilki M, Saatci M, Kirmizibayrak T, Aksoy A. 2005. Effect of age on growth and carcass composition of Native Turkish Geese. Archiv für Geflügelkunde. 69:S77–S83.
- Wang CC, Tsai YJ, Hsieh YC, Lin RJ, Lin CL. 2014. The aqueous extract from Toona sinensis leaves inhibits microglia-mediated neuroinflammation. Kaohsiung J Med Sci. 30:73–81.
- Wang KJ, Yang CR, Zhang YJ. 2007. Phenolic antioxidants from Chinese toon (fresh young leaves and shoots of Toona sinensis). Food Chem. 101:365–371.
- Webb EC, O'Neill HA. 2008. The animal fat paradox and meat quality. Meat Sci. 80:28–36.
- Wheeler CR, Salzman JA, Elsayed NM, Omaye STJr. Korte DW. 1990. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem. 184:193–199.
- Yang H, Gu Q, Gao T, Wang X, Chue P, Wu Q, Jia X. 2014b. Flavonols and derivatives of gallic acid from young leaves of Toona sinensis (A. Juss.) Roemer and evaluation of their anti-oxidant capacity by chemical methods. Pharmacognosy Mag. 10:185–190.
- Yang HL, Huang PJ, Liu YR, Kumar KJ, Hsu LS, Lu TL, Chia YC, Takajo T, Kazunori A, Hseu YC. 2014a. Toona sinensis inhibits LPS-induced inflammation and migration in vascular smooth muscle cells via suppression of reactive oxygen species and NF-kB signaling pathway. Oxidative Medicine and Cellular Longevity Article ID 901315. p.16.