ABSTRACT
The aim of the present study was to determine the effect of estrus synchronization programmes on parturition time and some reproductive characteristics of Saanen goats. Estrus of Saanen goats was synchronized as follows: by use of intravaginal sponges containing 30 mg flugestone acetate for 11 days following IM injection of 500 IU PMSG (group PP; n = 80) or only intravaginal sponges (group P; n = 80) and natural estrus (control; C, n = 50). Forty-eight hours after removing intravaginal sponges, all goats were introduced to Saanen bucks. Kidding rate and litter size of goats in the PP group were higher than those of goats in the C and P groups (P < .05). Neonatal mortality rate of kids from goats in the PP group was found higher than those of goats in the C and P groups (P < .05). The length of kidding period in goats of group C was longer than goats in the P and PP groups (P < .05). Births showed a unimodal distribution, with maximum parturition at midday and minimum parturition at midnight in all goats. The parturition that occurred during daytime hours in goats of P and PP groups was more than goats in the C group (P < .05). The results of this study may suggest that estrus synchronization shortened the length of the kidding period, concentrated parturition time during daylight hours and increased reproductive performance in Saanen goats.
1. Introduction
Synchronization of estrus is a useful tool for improving and maintaining the production of milk and meat, as well as reducing the labour force or cost, shortening the breeding season, throughout the year in goat farms (Nur et al. Citation2013, Andrabi et al. Citation2015). Additionally, estrus synchronization in goats is practical for optimizing the function of reproduction (Ahmad et al. Citation2014). Therefore, estrus synchronization is extensively applied in the reproductive management of goats.
Intravaginal sponge containing progesterone applications in small ruminants include goat are used worldwide for synchronization and/or the induction of estrus (Kridli et al. Citation2002). Flugestone acetate is a synthetic analogue of progesterone and used for estrus synchronization of goats throughout the breeding and non-breeding periods. Additionally, intramuscular injection of a pregnant mare's serum gonadotropin (PMSG) at withdrawal of progestagen sponge is used for multi-ovulation (Nasr et al. Citation2002, Whitley & Jackson, Citation2004). However, multi-ovulation causes multiple gestations and may increase the mortality rate of kids due to low birth weight or insufficient milk production by the mother for the consumption of each kid in equal amounts or insufficient maternal care.
Previous studies reported that parturition generally occurs at midday times in goats (Romano & Piaggio Citation1999) and periodic checking of the flock throughout the 24 h of the day is essential if kid mortality is to be kept at a minimum during the kidding season. Therefore, determination of possible effects of estrus synchronization on parturition time will be useful for minimum kid mortality and optimal use of labour force during kidding season in goat farms. Additionally, determining of intensive hours of birth during kidding season may decrease total cost in goat farms due to decrease in kid mortality rate and increase in the efficiency of labour force use.
There have been numerous studies investigating the effects of estrus synchronization on reproductive performance such as estrus behaviour, ovulation rate, fertility, gestation rate and kidding rate. However, to our knowledge, no studies determining the effect of estrus synchronization on parturition and kidding characteristics and mortality rates of both goats and kids have been reported. The objective of the present study was therefore to determine the effect of estrus synchronization programmes on the length of kidding period, parturition time, mortality rate of both kids and goat, and some reproductive characteristics of Saanen goats, in the breeding season.
2. Materials and methods
The experimental procedures were approved by the Local Animal Care and Ethics Committee of Ondokuz Mayis University, Samsun, Turkey, ensuring compliance with EC Directive 86/609/EEC for animal experiments. The study was conducted within the normal seasonal breeding cycle of goats in Turkey (September to March). Experimental animals (Saanen goats), ranging from 2 to 3 years of age, were obtained from a private farm in Samsun, Turkey (41°43′N, 35°82′E and 171 m above the sea level). Time of estrus of Saanen goats was synchronized as follows: intravaginal sponges (30 mg flugestone acetate) for 11 days followed by an IM injection of 500 IU PMSG (group PP; n = 80) or intravaginal sponges (group P n = 80) and natural estrus (group control; n = 50, C). At the beginning of the study all goats had similar body weights (47.8 ± 0.7 kg) and body condition scores (2.90 ± 0.20). Forty-eight hours after removing the sponges, all goats including goats in group C were introduced to Saanen bucks (approximately 1 buck for every 20 goats).
During kidding time, goats were monitored at an hourly interval throughout the day. Time of birth was recorded, which was defined as the time when the kid or kids had fully emerged from the vagina. The kidding time was also divided into eight stages; Stage 1; 00:00–03:00, Stage 2; 03:01–06:00, Stage 3; 06:01–09:00, Stage 4; 09:01–12:00, Stage 5; 12:01–15:00, Stage 6; 15:01–18:00, Stage 7; 18:01–21:00, Stage 8; 21:01–00:00.
Birth types (quadruplets, triplets, twins and single) of kids were recorded immediately after kidding. Gestation rate; (goats kidding/goats mated) × 100, kidding rate; (kids born/goats mated) × 100 and litter size; (kids born/goats kidding) were calculated for goats in all groups. Following kidding, all kids were kept with their dams in the fold for two weeks and mortality rate of both goats and kids were determined within two weeks after kidding.
To analyse the data, Chi-square, Mann-Whitney U-test and one-way ANOVA were performed according to the structure of the data by use of R software.
3. Results
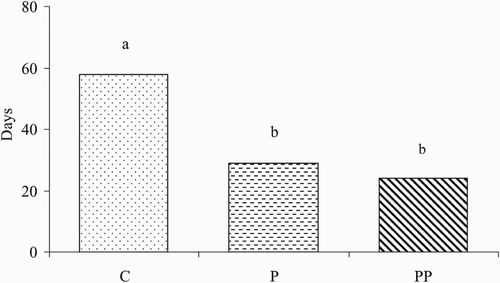
The length of the kidding period of Saanen goats in estrus synchronization programmes is shown in . The kidding period of goats in the C group was longer than those of goats in the C and P groups (P < .05). The kidding period of goats in C, P and PP were 58 days, 29 days and 24 days, respectively.
Figure 1. The length of kidding period of Saanen goats in estrus synchronization programmes. C = control (natural estrus), P = progesterone impregnated sponge, PP = progesterone impregnated sponge + PMSG. a,bP < .05.
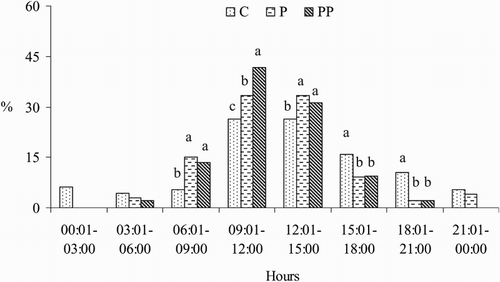
Distribution of parturition in estrus synchronization programmes of Saanen goats throughout the day is shown in . Similar patterns existed throughout the day with maximum parturition frequency occurring in the midday hours and with minimum parturition frequency occurring in the midnight hours in all goats. However, when the parturition occurred during daytime hours in goats, the results of the P and PP groups were found higher than those of goats in the C group (93.8%, 90.9% and 73.7%, respectively; P < .05); the parturition that occurred during nighttime hours in goats in the P and PP groups was lower than that of goats in the C group (6.3%, 9.1% and 26.3%, respectively; P < .05). Moreover, the percentages of parturitions that occurred between 9:01 am and 15 pm in goats in the P and PP groups were 66.7% and 72.75%, respectively (P < .05).
Figure 2. Distribution of parturition in estrus synchronization programmes of Saanen goats throughout the day. C = control (natural estrus), P = progesterone impregnated sponges, PP = progesterone impregnated sponges + PMSG. a,bP < .05.
Gestation, kidding, litter size, multiple and single birth rates and goat and kid mortality rates of Saanen goats in estrus synchronization programmes are presented in . Gestation rates were similar between goats in the C, P and PP groups, but kidding rates, multiple birth rates and litter size of goats in the PP group were higher than those of goats in the C and P groups (P < .05). Moreover, goats in the PP group have higher rates of quadruplets and triplets birth than those of goats in the C and P groups.
Table 1. Gestation, kidding, litter size, multiple and single birth rates and goat and kid mortality rates of Saanen goats in estrus synchronization programmes.
The mortality rate of goats in all groups was similar, but the mortality rate of kids from goats in the PP group was higher than those of goats in the C and P groups (P < .05). Additionally, there was no death in both goats that gave birth to a single kid and kids that were born as a single kid in a litter in all groups.
4. Discussion
The intravaginal progesterone sponge has been the most common choice of treatment for estrus synchronization of small ruminants in the world (Freitas et al. Citation1997). These sponges usually contain about 30–40 mg of synthetic progesterone and are left in place for 9–12 days (Wildeus Citation2000). The most common synthetic progesterone used in sponges is flugestone acetate (Whitley & Jackson Citation2004). In order to improve the success, sponges are widely used with PMSG for tighter synchronization and/or to induce a superovulatory response (Wildeus Citation2000, Whitley & Jackson Citation2004). In the present study, we applied intravaginal sponges containing 30 mg of flugestone acetate for 11 days for estrus synchronization of goats or following intramuscular injection of 500 IU PMSG.
Minoia and Taranto (Citation1975) reported high fertility following synchronization using synthetic progesterone in goats. On the contrary, Greyling and Van der Nest (Citation2000) have identified gestation rates to be lower in estrus synchronized goats than non-synchronized (natural cycle) goats. Moreover, the application methods and/or amount of hormones used for synchronization of estrus can lead to impairment in the fertility of goats (Greyling & Van der Nest Citation2000). The results in the present study indicate that application of intravaginal sponges containing flugestone acetate and following PMSG increased kidding rates, multiple birth rates and litter size of goats. Additionally, as we expect, estrus synchronization of goats shortened the length of the kidding period (approximately 32 days). This situation may be explained by the shortening of the onset time of estrus by synchronization. These findings were in agreement with Chao et al. (Citation2008), who reported that flugestone acetate treatment shortens the time to onset of estrus and shortens the breeding season in Korean native goats.
Increasing goat productivity by increasing kidding frequency and litter size has high importance for goat production. On the other hand, increasing litter size in goats offers the best opportunity to increase the efficiency of kid meat production. It is known that IM injection of PMSG following progesterone treatment enhances and improves ovarian activity and ovulation, resulting in high conception rate and litter size (Wildeus Citation2000). Therefore, PMSG administration makes it possible to increase the litter size. Hence, in this study to increase fertility rate and induce follicular growth, PMSG was administered. However, PMSG application may cause variations in ovulation response because of the genetic differences between breeds (Quintero-Elisea et al. Citation2011). For example, Zarkawi et al. (Citation1999) reported that 200 IU PMSG application at sponge withdrawal showed 1.95% litter size in Damaskus goats while Ahmed et al. (Citation1998) reported that 300 IU application at sponge withdrawal showed 1.60% litter size in Nubian goats. In the present study, although IM injection of PMSG after progesterone treatment was more (500 IU) than in the above studies, the litter size only increased by about 30% in Saanen goats. This difference may be explained by genetic differences between goat breeds.
There are two types of parturition timing patterns in animals. The first is uniformly distributed throughout the day and the second is concentrated during specific periods of the day, such as during daytime hours or nighttime hours (Romano & Piaggio Citation1999). Goats, like most animals, tend to give birth anytime from late evening to early morning, but occasionally in the middle of the day. Also, parturition is most likely to occur during the day at a time when goats are generally inactive (Houpt, Citation2010). Lickliter (Citation1985) reported that parturition in Toggenburg goat breed generally occurs during daytime. Similarly, Bosc et al. (Citation1988) and Romano and Piaggio (Citation1999) showed that Alpine and Nubian goat breeds mostly give birth at midday. The diurnal distribution pattern of kidding established in our study is similar to previous studies (Lickliter Citation1985, Bosc et al. Citation1988, Romano & Piaggio Citation1999). In the present study, there seems to be a remarkable relationship between daylight and kidding. It is show a clear regulation of the parturition mechanism, powerfully influenced by the day cycle. The distribution of the parturition time of all goats was concentrated during daytime hours, from 9 am to 18 pm, in this study. However, parturition occurred predominantly during daytime hours in goats in the P and PP groups. These results may suggest that intensive use of labour force during the kidding season occurs during daytime hours in goat farms. Thus the efficiency of labour force use may increase in goat farms.
Neonatal loss rates of kids until weaning is one of the crucial elements in the economic process of goat breeding systems (Snyman Citation2010). Al-Najjar et al. (Citation2010) reported that the mortality rate of kids from birth to weaning ranges from 32% to 40% in different goat breeds. Numerous researches have stated that kid birth weight, litter size, sex and birth season affect the pre-weaning survival of kids (Awemu et al. Citation1999, Lehloenya et al. Citation2005, Al-Najjar et al. Citation2010, Snyman Citation2010). Also, environmental factors such as temperature, disease and malnutrition are mostly considered to contribute to kid mortality (Awemu et al. Citation1999). In the present study, PMSG treatment increased multiple births and litter size of goats and also caused increase in neonatal loss of kids. Unfortunately kid birth weight and maternal interest were not investigated in the present study, but high multiple birth rates and litter size of goats might have reduced maternal interest and kid birth weight. Hence, high neonatal loss of kids in PP goats may be caused by PMSG-induced multiple births.
In conclusion, the results of the present study confirmed that synchronization of estrus shortened the length of the kidding period, concentrated time of birth during daylight hours and increased reproductive performance in Saanen goats. Also, the results suggested that estrus synchronization may reduce labour cost throughout the year due to the shortening of the length of the kidding period in goat flock. Because, concentration of kidding at certain times of the day may increases both works satisfaction and the efficiency of labour use of farmers in goat farms. The results of this study also suggest that the use of PMSG in synchronization programmes may cause high mortality rate of kids due to more multiple births if newborn multiple birth kid maintenance conditions do not improve in goat farms.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahmad N, Javed K, Abdullah M, Hashmi AS, Ali A. 2014. Estrus induction in beetal goats during low breeding season. J Anim Plant Sci. 24(5):1283–1287.
- Ahmed MMM., Makawi SE., Jubara AS. 1998. Synchronization of oestrus in Nubian goats. Small Rumin Res. 30(2):113–120. doi: 10.1016/S0921-4488(98)00104-7
- Al-Najjar K, Salhab S, Al-Merestani R, Kasem R, Al-Azzawi W, Dawa M, Omed H, Saatci M. 2010. Environmental factors affecting kid mortality in Shami goats. Kafkas Univ J Fac Vet Med. 6:431–435.
- Andrabi SMH, Anwar M, Mehmood A. 2015. Efficacy of short-term estrus synchronization protocols and timed artificial insemination in subtropical goats. J Anim Plant Sci 25(1):298–300.
- Awemu EM, Nwakalor LN, Abubakar BY. 1999. Environmental influence on preweaning mortality and reproductive performance of Red Sokoto does. Small Rumin Res. 34(2):161–165. doi: 10.1016/S0921-4488(99)00058-9
- Bosc M, Guillimin P, Bourgy G, Pignon P. 1988. Hourly distribution of time of parturition in the domestic goats. Theriogenology. 30(1):23–33. doi: 10.1016/0093-691X(88)90260-9
- Chao LM, Takayama K, Nakanishi Y, Hamana K, Takagi M, Kubota C, Kojima T. 2008. Luteal lifespan and fertility after estrus synchronization in goats. J Vet Sci. 9(1):95–101. doi: 10.4142/jvs.2008.9.1.95
- Freitas VJF, Baril G, Saumande J. 1997. Estrus synchronization in dairy goats: use of fluorogestone acetate vaginal sponges or norgestomet ear implants. Anim Reprod Sci. 46(3–4):237–244. doi: 10.1016/S0378-4320(96)01614-4
- Greyling JPC, Van der Nest M. 2000. Synchronization of oestrus in goats: dose effect of progestagen. Small Rumin Res. 36(2):201–207. doi: 10.1016/S0921-4488(99)00165-0
- Houpt KA. 2010. Domestic animal behavior for veterinarians and animal scientists. 5th ed. Ames, USA: Wiley-Blackwell. p. 576.
- Kridli RT, Abdullah AYM, Husein Q. 2002. Protocols for estrus synchronization in awassi ewes under arid environmental conditions. Asian Australas J Anim Sci. 15(7):957–962. doi: 10.5713/ajas.2002.957
- Lehloenya KC, Greyling JPC, Schwalbach LMJ. 2005. Reproductive performance of South African indigenous goats following oestrous synchronisation and AI. Small Rumin Res. 57(2-3):115–120. doi: 10.1016/j.smallrumres.2004.05.004
- Lickliter RE. 1985. Behavior associated with parturition in the domestic goats. Appl Anim Behav Sci. 13(4):335–345. doi: 10.1016/0168-1591(85)90013-9
- Minoia P, Taranto FD. 1975. Preliminary attempts at estrus synchronization in Gargana goats. Acta Med Vet. 21:209–213.
- Nasr RE, Haddad SG, Al-Karablieh EK. 2002. Economic assessments of hormonal and nutritional treatments for improvement of Awassi sheep production in Jordan. Asian Australas J Anim Sci. 15(8):1110–1114. doi: 10.5713/ajas.2002.1110
- Nur Z, Nak Y, Nak D, Ustuner B, Tuna B, Simsek G, Sagirkaya H. 2013. The use of progesterone-supplemented co-synch and Ovsynch for estrus synchronization and fixed-time insemination in nulliparous Saanen goat. Turkish J Vet Anim Sci. 37:183–188.
- Quintero-Elisea JA, Macías-Cruz U, Álvarez-Valenzuela FD, Correa-Calderón A, González-Reyna A, Lucero-Magaña FA, Soto-Navarro SA, Avendaño-Reyes L. 2011. The effects of time and dose of pregnant mare serum gonadotropin (PMSG) on reproductive efficiency in hair sheep ewes. Trop Anim Health Prod. 43(8):1567–1573. doi: 10.1007/s11250-011-9843-z
- Romano JE, Piaggio J. 1999. Time of parturition in nubian goats. Small Rumin Res. 33(3):285–288. doi: 10.1016/S0921-4488(99)00023-1
- Snyman MA. 2010. Factors affecting pre-weaning kid mortality in South African Angora goats. South Afr J Anim Sci. 40:54–64.
- Whitley NC, Jackson DJ. 2004. An update on estrus synchronization in goats: A minor species. J Anim Sci. 82:270–276.
- Wildeus S. 2000. Current concepts in synchronization of estrus: Sheep and goats. J Anim Sci. 77:1–14.
- Zarkawi M, Al-Merestani MR, Wardeh MF. 1999. Technical Note Induction of synchronized oestrous in indigenous Damascus goats outside the breeding season. Small Rumin Res. 33(2):193–197. doi: 10.1016/S0921-4488(99)00012-7
