ABSTRACT
The objective of this experiment was to evaluate the effect of bee glue (BG) on the performance, relative visceral weights, some blood parameters, and immune status of broilers. A total of 200 male Ross 308 broiler chicks were randomly assigned to 5 dietary treatments: basal diet (BD), and basal diet supplemented with 600, 700, 800, and 900 mg kg−1 of BG. Each of the 5 diets was fed to 4 replicates of 10 chicks each from 0 to 42 days of age. The results indicated that the highest body weight, average daily gain, average daily feed intake, carcass weight, and carcass yield were observed in broilers offered 800 mg kg−1 of BG (P < .05). Inclusion of BG improved the feed conversion ratio compared with the control group (P < .01). The calculated European broiler index and crop percentage were greater for the birds received 800 and 900 mg kg−1 of BG compared with that of the control birds (P < .05). Inclusion of 900 mg kg−1 of BG significantly increased the relative weight of spleen and bursa, but reduced total triglycerides, cholesterol, LDL, and LDL: HDL ratio than the control group (P < .05). The serum IgG and IgM levels were increased for the birds received 700 to 900 mg kg−1 BG (P < .01), and addition of BG to the basal diet significantly increased antibody response against sheep red blood cell at 35 days of age (P < .05). It was concluded that an addition of 800 mg kg−1 BG to diet improves economic efficiency possibly by creating miniscule improvement in FCR and promoting immune response of broilers.
1. Introduction
Bee glue (BG) is a brownish resinous material collected by worker honeybees (Apis mellifera L.) from leaf buds of numerous tree species, sap flows, or other botanical sources, and mixed with their wax and salivary enzymes. It has strong antibacterial (Bankova et al. Citation2000), antioxidant (Banskota et al. Citation2000), antiviral (Vynograd et al. Citation2000), anti-inflammatory (Sforcin Citation2007), antifungal and immunostimulatory properties (Bankova et al. Citation2000), and cytostatic and hepatoprotective activities (Banskota et al. Citation2000). Due to the presence of important compounds, including flavonoids, terpenoid, and phenolic constituents, the use of BG in broiler diets has been recommended as a way to improve the immune responses (Sforcin & Bankova Citation2011). It seems that these compounds are responsible for the biological and pharmacological activities of BG samples (Banskota et al. Citation2000). It also has been well documented that, flavonoids have antioxidant properties against the superoxide anion radical in the cell membrane (Sforcin Citation2007). In recent years, attention has been focused on the use of BG as a health supplement suited to consumers in developed countries (Bankova et al. Citation2000). It is well established that BG contains most of the essential nutritional elements necessary for growth and development in poultry and humans (Bell et al. Citation1983). Several studies indicated that BG can be used as a natural growth promoter and stimulation of immune system in broilers (Attia et al. Citation2014; Hegazi et al. Citation2012) and Japanese quails (Canogullari et al. Citation2009). Immunostimulation through natural compounds may be considered an alternative for the prevention and cure of infectious diseases. Stimulation of the immune system by natural substances has already been reported (Kong et al. Citation2004).
There is a little information about the effect of BG on the immunological parameters in broilers. The most studies have been performed on young broiler chicks; therefore, the long-term effect of BG on the biochemical and physiological variables of broilers has not yet been studied. Considering the possibility of future antibiotic restrictions and potential benefits of BG, the effect of BG on the immune system should be investigated in broilers chickens. In addition, the effect of high levels of BG on immunological and haematological parameters has not been studied previously. Therefore, the aim of the present study was to investigate the supplemental effects of BG on productive performance, digestive tract development, and immune responses of broilers.
2. Materials and methods
2.1. Birds management and diets
A total of 200 one-day-old male broiler chickens (Ross 308) with an initial BW of 45.4 ± 0.4 g were obtained from commercial hatchery (Broiler Breeder of South Khorasan Complex Productive Co., Iran). The chicks were weighed individually and distributed randomly in 20 floor pens in an environmentally controlled house up to day 42 of age. The room temperature was maintained at 34°C for the first 3 days and then gradually reduced to 24°C by 21 days. All chicks were continuously provided with uniform light for 24 h and had free access to feed in mash form and water throughout the experiment.
The dietary treatments were (1) basal diet (BD), (2) BD + 600 mg kg−1 BG, (3) BD + 700 mg kg−1 BG, (4) BD + 800 mg kg−1 BG, (5) BD + 900 mg kg−1 BG. The feeding regimen consisted of a starter (1–21 days) and finisher diet (22–42 days). The experimental diets were formulated to meet or exceed the NRC (Citation1994) nutrient requirements for broiler chickens (). All experimental procedures used were approved by the Animal Welfare Committee of the Department of Animal Science, Islamic Azad University, Birjand Branch.
Table 1. The ingredients and chemical composition of the basal diets (g/kg, as fresh matter).
2.2. Data collection
In this experiment, pen was the experimental unit and data on body weight and feed intake were measured weekly, and their data were used to calculate data on average daily gain (ADG), average daily feed intake (ADFI), feed conversion ratio (FCR), and European broiler index (EBI) based on the following equation (Euribrid Citation1994);
Mortality was recorded up on occurrence.
At 42 days of age, two randomly chosen birds per replicate (8 birds per treatment) slaughtered through cutting of jugular veins and carotid arteries, and processed manually and collections were made following a 4-h fast. After evisceration, the carcasses were individually weighed (carcass weight [CW]) and the data were presented as a percentage of live weight (carcass yield [CY]), and the ratio of abdominal fat (AF) weight to CW was calculated. The weight of crop, proventriculus, gizzard, gall bladder, liver, pancreas, spleen, and bursa were also expressed proportional to live BW. Different segments of the small intestine including the duodenum (from gizzard to the end of pancreatic loop), jejunum (part between pancreatic loop and Meckel’s diverticulum), and ileum (part between Meckel’s diverticulum and ileocaecal junction) were removed and their lengths were recorded. The length of each organ was expressed relative to the total BW.
Blood samples were obtained from same killed birds and drawn into vacuumed capillary tubes in order to determine the blood total cholesterol, total triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels. After coagulation, blood samples were centrifuged at 2500 × g for 10 min. The samples were stored at −20°C in 3-mL eppendorf tubes for subsequent analyses. Blood cholesterol, triglyceride, HDL, and LDL levels were determined spectrophotometrically by using commercial kits (IL Test Cholesterol and IL Test Triglycerides kits, Instrumentation Laboratory, Braunschweig, Germany). Blood parameter values were expressed as milligrams per dL.
At 28 days of age, two birds from each pen were inoculated with a volume of 0.2 mL of a 7.5% suspension of sheep red blood cell (SRBC). At 35 days of age (seven days after each injection), blood samples were collected in non-heparinized tubes by puncturing the brachial vein. Serum was separated by centrifugation at 2500 × g for 10 min at 4°C, and stored at −20°C until assayed. Individual serum samples were analysed for antibody responses against SRBC by the ELISA technique using commercial kits, and the plates were read at 405 nm on an ELISA reader (Van der Zijpp Citation1983). Total IgG and IgM concentrations in same individual serum samples were measured automatically by the BNII nephelometric immunoassay of Beckman Coulter (Brea, CA).
2.3. Statistical analysis
The collected data were subjected to analysis of variance using the general linear model of SAS (Citation2006). Treatment means were separated using Tukey-Kramer test. For the different statistical tests, significance was declared at P < .05
3. Results
Inclusion of 800 mg kg−1 of BG significantly increased BW at days 21 and 42 of age (). In the finisher (22–42 days of age) and overall (1–42 days of age) periods, ADG and ADFI responded markedly to 800 mg kg−1 of BG supplementation of the control diet. There was also a significant (P < .05) ADG response in the starter period (1–21 days of age) at the same level of BG. Compared to the control group inclusion of BG improved FCR (P < .01). The mean mortality rate was reduced () and the calculated EBI was greater () for the birds that received 800 or 900 mg kg−1 of BG in their diets compared to the other treatments.
Figure 1. Effect of BG on mortality rate of broiler chickens during 1–42 days of age. Means without a common superscript (a-b) differ significantly (P < .05).
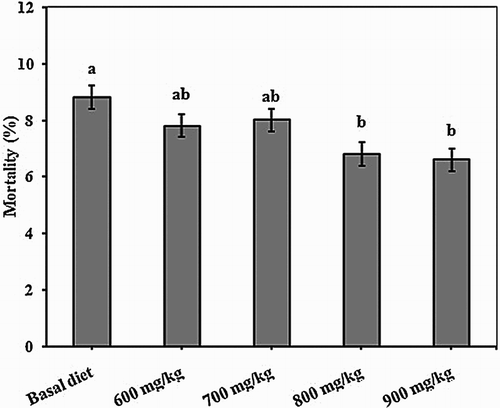
Figure 2. Effect of BG on average European broiler index in broiler chicks at 42 days of age. Means without a common superscript (a-b) differ significantly (P < .05).
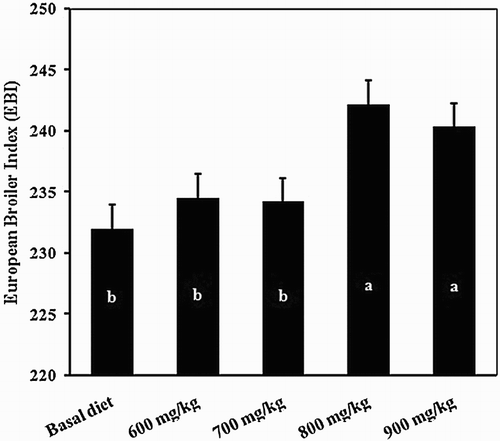
Table 2. Effect of BG on body weight, ADG, ADFI, and feed to gain ratio (FCR) of broiler chicks.
Addition of 800 mg kg−1 BG significantly increased CW and CY compared with those in control birds (P < .05; ). There was no significant effect on AF percentage in broilers at day 42 of age.
Table 3. Effect of BG on CW, CY, AF-to-CW ratio (AF:CW) in broiler chicks at 42 days of age.
No change in relative weight of proventriculus, gizzard, and gall bladder was observed with dietary BG, while inclusion of 800 and 900 mg kg−1 of BG in the diet significantly increased the relative weight of crop (P < .05) compared with birds fed the BD (). Inclusion of BG supplementation did not have a significant effect on the relative length of different segments of small intestine (duodenum, jejunum, and ileum).
Table 4. Effect of BG on weight and length percentage in certain gastrointestinal-tract segments of broiler chicks at 42 days of age.
The effects of BG supplementation on relative weight of some visceral organs are summarized in . The results indicated that inclusion of 900 mg kg−1 BG significantly increased relative weight of spleen and bursa (P < .05). However, the BG did not affect relative weights of liver and pancreas compared with those of the control group.
Table 5. Effect of BG on relative weight of liver, pancreas, spleen, and bursa (g/100 g BW) in broiler chicks at 42 days of age.
As shown in , no change in serum HDL concentration was observed with dietary BG, whereas inclusion of 900 mg kg−1 of BG in the diet significantly decreased the levels of triacylglycerides, cholesterol, LDL, and LDL:HDL ratio compared with birds fed the control diet. The effect of BG on immune status of broiler chickens was also evaluated (). Inclusion of BG in the diet significantly increased (P < .05) antibody response against SRBC at 35 days of age. Serum IgG and IgM levels were significantly higher in the groups receiving 700 to 900 mg kg−1 BG when compared with the control (P < .01).
Table 6. Effect of BG on some blood parameters (at 42 days) and immune response (at 35 days) in broiler chicks.
4. Discussion
The present study indicated that dietary inclusion of BG improved the performance of broilers compared to the unsupplemented. These findings confirm those reported by Attia et al. (Citation2014) who found that BG can be used in broiler feeds as natural growth promoters. The mode of action of BG not only may be due to a strong effect of antibacterial action, but also may be related to the presence of micronutrients with positive effects on bird’s health and metabolism, and consequently improvement in broiler performance (Canogullari et al. Citation2009). The European broiler index (EBI) created by Euribrid (Citation1994) is calculated based on final BW, FCR, and mortality. The calculated index for the treated birds with 800 and 900 mg kg−1 of BG was greater compared to other groups. The advantages of diet supplemented with 800 mg kg−1 BG in ADG, improved FCR in 1–42 days of age and lowered mortality rate was reflected as the improved EBI index. These results suggest that 800 mg kg−1 can be recommended as the optimum dosage for addition of BG to broiler diets. In relation to the results obtained in this experiment, the increasing dressing percentage in supplemented treatments can be attributed to the greater BW at slaughter.
The improved performance cannot be explained by effects of the BG supplements on the relative size of the gastrointestinal organs because there were no differences related to the digestive or absorptive capacities of these organs. Only the relative weight of the crop was influenced by the BG supplementation, and this influence may have been related to the increased feed intake that was observed simultaneously.
The results of the present study suggest that lymphoid compartments differ in their responses to BG probably due to the different roles in immune system. Lymphoid tissues play an important role in the body defence against microorganisms. The bursa and spleen are sites of B cells and T cells differentiation, respectively (Fasina et al. Citation2006). The relative lymphoid organs weight is used to investigate the immune status of birds and changes in weight may be associated to alterations in the function of lymphoid organs (Cooper et al. Citation1966). Because BG is rich in nutrients (amino acids, flavonoids, carotenoids, and phytosterols), it promotes faster cell proliferation and differentiation in the immune system of broilers (Hegazi et al. Citation2012). It seems that dietary BG stimulates the T lymphocytes formation, as well as the division, proliferation and activity of thymus cells. Hegazi et al. (Citation2012) found that BG is able to increase proliferation of lymphocyte, and impacting on immune function and resistance ability to disease. In this study, high level BG supplementation had positive effects on immune function of broilers. Nevertheless, the lower weight of lymphoid organs may not necessarily be related to a lower proliferation of these organs; therefore, it is necessary to correlate this measurement with other immunological parameters.
Reduction in cholesterol and triacylglycerides concentrations is the most beneficial effect on the lipid profile. The decrease in plasma lipids and cholesterol can be due to phospholipids and PUFA, particularly linolenic acid (Xu et al. Citation2009). The results of this study are in agreement with those reported by Kolankaya et al. (Citation2002) and Fuliang et al. (Citation2005). Our study revealed that the BG positively affected the LDL level and LDL:HDL ratio of the broilers. But, there is a clear need to clarify the constituents of BG in order to evaluate its biological activities.
The antibody level in serum is an important indicator for humoral immunity in poultry. Our findings showed that serum IgG and IgM concentrations in the birds receiving 700, 800, and 900 mg kg−1 of BG were significantly higher than those in control birds, suggesting that high levels of BG could modulate humoral immunity in broilers. Park et al. (Citation2004) found that BG supplementation activates the immune system in broilers, raising macrophage and natural killer cells activities, and increasing levels of cytokines (interleukin-1, interleukin-2, and interleukin-4). These cytokines enhance B-lymphocytes activities, which would be able to produce immunoglobulins (Banskota et al., Citation2000). Therefore, in the current study, the increased levels of IgG and IgM in birds of groups given dietary BG at higher levels may be related to the stimulation of B-lymphocytes by these cytokines. In contrast with the present results, Ziaran et al. (Citation2005) found that low levels of dietary BG increased antibody titre, whereas high levels of BG decreased antibody titre, thereby exhibiting a bell-shaped dose–response relation. In that study, they observed a relatively negative effect of a higher level of BG on humoral immunity of broilers and concluded that immune system of broilers may respond to BG on a critical dosage. Furthermore, Scheller et al. (Citation1988) reported that high dose of BG or in the frequency of its intake has an inhibitory effect on the immunity status of mouse. Regarding the humoral immune response, studies have shown that BG is able to increase antibody production in SRBC-immunized broilers (Scheller et al. Citation1988). In birds, few studies have investigated the immunostimulatory effect of BG (Kong et al. Citation2004; Wang et al. Citation2005). Regarding these present results, a possible mechanism involved in the increased levels of antibodies produced in BG-treated broilers is probably related to increased expression of IL-2 and γ-interferon (Wang et al. Citation2005). Although the mechanism of action of BG on the immune system of mammals has been investigated in recent years (Sforcin Citation2007), further studies are necessary to investigate the effects of high levels of BG on natural antibody titres in chickens.
To our knowledge, there are no published data on effect of the BG on antibody response to SRBC. The SRBC, a non-pathogenic antigen, is classified as a thymus-dependent antigen that obviously needs the help of T lymphocytes to produce antibodies. BG that is rich in flavonoids extends the activity of vitamin C, acts as antioxidant and may therefore enhance the immune function.
5. Conclusions
Based on the results of the present work, it was concluded that inclusion of BG at the level 800 mg kg−1 of diet improves productive performance and may stimulates IgG and IgM production in broilers. Therefore, this level may be more effective than low- or high-dose BG, and dosage could be an important factor in using BG for stimulation of broilers. It could be concluded that dietary use of BG as a feed additive may offer a practical nutritional strategy in broiler production. Further research is needed to understand and clarify the mechanism(s) involved.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Attia YA, Abd Al-Hamid AE, Ibrahim MS, Al-Harthi MA, Bovera F, Elnaggar AS. 2014. Productive performance, biochemical and hematological traits of broiler chickens supplemented with propolis, bee pollen, and mannan oligosaccharides continuously or intermittently. Livestock Sci. 164:87–95. doi: 10.1016/j.livsci.2014.03.005
- Bankova VS, de Castro SL, Marcucci MC. 2000. Propolis: recent advances in chemistry and plant origin. Apidologie. 31:3–15. doi: 10.1051/apido:2000102
- Banskota AH, Tezuka Y, Adnyana IK, Midorikawa K, Matsushige K, Message D, Huertas AA, Kadota S. 2000. Cytotoxic, hepatoprotective and free radical scavenging effects of propolis from Brazil, Peru, the Netherlands and China. J Ethnopharmacol. 72:239–246. doi: 10.1016/S0378-8741(00)00252-X
- Bell RR, Thornber EJ, Seet JL, Groves MT, Ho NP, Bell DT. 1983. Composition and protein quality of honeybee-collected pollen of Eucalyptus marginata and Eucalyptus calophylla. J Nutr. 113:2479–2484.
- Canogullari S, Baylan M, Sahinler N, Sahin A. 2009. Effects of propolis and pollen supplementations on growth performance and body components of Japanese quails (Coturnix coturnix japonica). Archive fur Geflügelkunde. 73:173–178.
- Cooper MD, Raymond DA, Peterson RD, South MA, Good RA. 1966. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 123:75–102. doi: 10.1084/jem.123.1.75
- Euribrid BV. 1994. Technical information for Hybro broilers. Boxmeer, The Netherlands: Euribrid Poultry Breeding Farm, p. 22.
- Fasina YO, Classen HL, Garlich JD, Black BL, Ferket PR, Uni Z, Olkowski AA. 2006. Response of Turkey poults to soybean lectin levels typically encountered in commercial diets. 2. Effect on intestinal development and lymphoid organs. Poult Sci. 85:870–877. doi: 10.1093/ps/85.5.870
- Fuliang HU, Hepburn HR, Xuan H, Chen M, Daya S, Radloff SE. 2005. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res. 51:147–152. doi: 10.1016/j.phrs.2004.06.011
- Hegazi A, Abdou AM, Allah FA. 2012. Egyptian propolis 9- Its effect on chicken productivity and immune response against Newcastle disease vaccine. Brit Poult Sci. 3:25–30.
- Kolankaya D, Selmanogˇlu G, Sorkun K, Salih B. 2002. Protective effects of Turkish propolis on alcohol-induced serum lipid changes and liver injury in male rats. Food Chem. 78:213–217. doi: 10.1016/S0308-8146(01)00400-9
- Kong XF, Hu YL, Rui R, Wang DY, Li XR. 2004. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int Immunopharmacol. 4:975–982. doi: 10.1016/j.intimp.2004.03.008
- NRC. 1994. Nutrient requirements of poultry. Washington (DC): National Academic Press.
- Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, Chung SJ, Paik SU, Oh SU. 2004. Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int Immunopharmacol. 4:429–436. doi: 10.1016/j.intimp.2004.01.013
- SAS. 2006. Statistical Analysis Systems user’s guide. 9,1th ed. Cary (NC): SAS Institute.
- Scheller S, Gazda G, Pietsz G, Gabrys J, Szumlas J, Eckert L, Shani J. 1988. The ability of ethanol extract of propolis to stimulate plaque formation in immunized mouse spleen cells. Pharmacol Res Commun. 20:323–328. doi: 10.1016/S0031-6989(88)80068-7
- Sforcin JM. 2007. Propolis and the immune system: a review. J Ethnopharmacol. 113:1–14. doi: 10.1016/j.jep.2007.05.012
- Sforcin JM, Bankova V. 2011. Propolis: is there a potential for the development of new drugs? (Review). J Ethnopharmacol. 133:253–260. doi: 10.1016/j.jep.2010.10.032
- Van der Zijpp AJ. 1983. The effect of genetic origin, source of antigen, and dose of antigen on the immune response of cockerels. Poult Sci. 62:205–211. doi: 10.3382/ps.0620205
- Vynograd N, Vynograd I, Sosnowski Z. 2000. A comparative multicentre study of the efficacy of propolis, acyclovir and placebo in the treatment of genital herpes (HSV). Phytomedicine. 7:1–6. doi: 10.1016/S0944-7113(00)80014-8
- Wang DY, Hu YL, Sun JL, Kong XF, Zhang BK, Liu JG. 2005. Comparative study on adjuvanticity of compound Chinese herbal medicinal ingredients. Vaccine. 23:3704–3708. doi: 10.1016/j.vaccine.2005.02.011
- Xu X, Sun L, Dong J, Zhang H. 2009. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov Food Sci Emerg. 10:42–46. doi: 10.1016/j.ifset.2008.08.004
- Ziaran HR, Rahmani HR, Pourreza J. 2005. Effect of dietary oil extract of propolis on immune response and broiler performance. Pak J Biol Sci. 8:1485–1490. doi: 10.3923/pjbs.2005.1485.1490
