ABSTRACT
Echocardiography is a well-established method for evaluating the heart and its function. The knowledge of normal echocardiographic anatomy and dimensions represents the basis for the diagnosis of abnormal cardiac affections. The present study was performed on 11 adult buffaloes that were divided into 2 groups according to their body weights. The technique used in the study was an adaptation from the technique used in cattle. The standardized technique used here allowed the collection of reliable and repeated echocardiographic images. The echocardiographic dimensions and functional indices for the buffalo’s heart were recorded. No significant difference was noticed between 2-D and M-mode measurements of cardiac dimensions. A significant correlation was noticed between the body weight and echocardiographic measurements. The echocardiographic dimensions in buffaloes were smaller than that previously recorded in cattle of similar body weights. The study could be used as a reference for further echocardiographic studies in buffaloes and represents a step in the identification and interpretation of cardiac affection in buffaloes.
Introduction
The water buffalo (Babalus bubalis) is a species of great potential for meat and milk production. There are about 185 million buffaloes in the world, of which 5.3 million are present in Egypt according to FAO statistics 2011 (Fooda et al. Citation2011).
Echocardiography is a simple, straight-forward method for evaluating the heart size and function (Braun et al. Citation2001; Braun Citation2009; Buczinski Citation2009). The knowledge of normal echocardiographic anatomy represents the basis for the identification and interpretation of abnormal findings (Schiller et al. Citation1989; Weyman Citation1994; Kriz & Rose Citation1996; Young Citation1999; Braun et al. Citation2001; Braun Citation2009). The two-dimensional (2-D) display mode allows the assessment of the spatial relationships of cardiac structures, whereas M-mode is suitable for investigating cardiac dimensions and function as well as valve, chamber and septum movement (Carlsten Citation1987). However, in any species, for echocardiography to distinguish between normal subjects and subjects suffering from cardiac disease, it is essential to establish reliable reference values for the species (Boon Citation2011). Ultrasonography of the normal bovine heart has been described and used mainly as the method of choice for diagnosing traumatic pericarditis (Braun et al. Citation2001; Braun Citation2009). The methods and normal values for echocardiography in normal dairy cattle (Hallowell et al. Citation2007), dromedary camel (Tharwat et al. Citation2012) and donkeys (Hassan & Torad Citation2015) were reported. Up till now, normal echocardiographic measurements in buffalo are not established yet. So, the purpose of this paper was to report the normal echocardiographic dimensions and functional indices of adult buffalo based on the techniques adapted from echocardiographic studies in cattle (Boon Citation2011).
Materials and methods
The study was carried out at the Surgery Department, Faculty of Veterinary Medicine, Cairo University, after approval of the study protocol by the care and use ethical committee of the Faculty of Veterinary Medicine, Cairo University.
Animals
Eleven adult buffaloes (8 non-pregnant females and 3 males) aged 2–7 years and weighing 230–570 kg were used in this study. According to the weight, the animals were divided into two groups: small buffaloes (including buffaloes with body weight ≤400 kg) (n: 5) and large buffaloes (including buffaloes with body weight > 400 kg) (n: 6). These buffaloes were the property of the Faculty of Veterinary Medicine, Cairo University. The animals’ ages were obtained from the animal’s record and they were weighed before echocardiographic procedures. All buffaloes were apparently healthy on physical examination.
Study protocol
Non-sedated buffaloes were restrained in a stanchion with adequate securing by ropes. The animals were allowed 15 minutes for acclimatization to this environment. A small area just caudal to the elbow on both the right and left hemithorax was clipped and surgical spirit was used to clean the skin. Ultrasound coupling gel was applied and allowed to soak into the skin for 5 minutes before echocardiographic examination. Application of gel was repeated as the buffalo skin absorbed the gel.
Echocardiographic examination
All echocardiographic measurements were performed in standing position. A phased-array probe was used at frequency 2–4 MHz attached to Samsung Madison (SONO ACE R3®-Korea) ultrasound machine with a maximum depth of 30 cm. The focus of the transducer was fixed at 5–10 cm depth (5 cm for small buffaloes and 10 cm for large buffaloes), while the maximum transducer angle was 110°. The transducer was applied approximately 5–10 cm dorsal to the olecranon process in the 3rd and 4th right intercostal spaces (group 1) and the 4th and 5th left intercostal spaces (group 2).
The echocardiographic techniques used in this study are an adaptation from the technique used in cattle (Long et al. Citation1992; Weyman Citation1994; Boon Citation2011; Hallowell et al. Citation2007; Buczinski Citation2009). Four two-dimensional (2-D) right and three 2-D left parasternal images were recorded. The 2-D images were used to guide the placement of the curser to obtain accurate M-mode images. Additionally, M-mode images were obtained from the right and left sides. On the right side, the echocardiographic images were obtained in the following order: a caudal long-axis view of both the right and left ventricles (four-chamber view), a caudal long-axis view of the left ventricular outflow tract (LVOT), a caudal short-axis view of the ventricles and a cranial long-axis view of the right ventricular outflow tract (RVOT). The three 2-D images obtained on the left side include the caudal long-axis view (four-chamber view), the caudal long-axis view of the LVOT and the caudal short-axis view of the ventricles.
In small buffaloes, the following measurements were recorded from the right parasternal caudal long-axis (four-chamber view) with the probe placed at the 4th intercostal space with slight clockwise rotation or perpendicular on the 3th intercostal space: left ventricular diameter in systole (LVDs) and diastole (LVDd), interventricular septal thickness in systole (IVSs) and diastole (IVSd), left ventricular wall thickness in systole (LVWs) and diastole (LVWd), right ventricular diameter in diastole (RVDd), right atrial diameter in diastole (RADd) and left atrial diameter in diastole (LADd). In large buffaloes, the same measurements were obtained from the left parasternal long-axis view with the transducer positioned in the 5th or 4th intercostal space and positioned caudodorsally. The aortic root diameter (AOD) was measured in diastole using an M-mode parasternal short-axis view at the aortic valve (AV) level. End-diastolic aortic diameters were also recorded by 2-D echocardiography from the parasternal long-axis view of the ventricular outflow tract at the base of the valve leaflets (ABS), at the level of the sinus of Valsalva (ASV) and at the sino-tubular junction (AJT). An M-mode echocardiogram of the mitral valve (MV) was obtained from the parasternal short-axis view for the measurement of the distance between the maximal opening of the septal leaflet of the MV in early diastole (the E point) and the maximal excursion of the septum (EPSS).
A simultaneous electrocardiogram (ECG) was recorded for the correct timing of measurements within the cardiac cycle. End-diastolic measurements were taken at the onset of the QRS complex and end-systolic measurements were taken during the maximum excursion of the interventricular septum (IVS). All echocardiographic examinations were performed by the same operator. At least three cardiac cycles were measured and the mean value for each parameter was obtained.
Estimation of functional indices
where LVEDV is the left ventricular end-diastolic volume and LVESV is the left ventricular end-systolic volume calculated using the Teichholz formulae:
Fractional wall thickening (FWT%):
Data collection and statistical analysis
All data were stored digitally and analysed offline by two evaluators. Data presented as mean ± standard deviation were calculated from the measurements of both observers, and both measurements were compared for inter-observer variability. The statistical analysis included comparison of 2-D and M-mode measurements within the group of small or large buffaloes and between both groups using non-parametric Mann–Whitney U test. Differences between compared measurements were considered significant when P < 0.05.
Results
The echocardiographic examination was carried out from the right side in small buffaloes where all cardiac structures were clearly visualized. In large buffaloes, the examination was performed from the left side as the entire heart could not be imaged from the right side. All standard images required pulling of the front leg forward. Some buffaloes were not co-operative with the pulling of the forelimb and required meticulous attempts until standing comfortable.
In the right parasternal caudal long-axis view (four-chamber view), the right ventricle (RV) and right atrium (RA) were seen at the near field of the image separated by the tricuspid valve (TCV). The left ventricle (LV) and left atrium (LA) were seen at the far field separated by the MV. Both ventricles are separated by clearly visualized IVS, while both atria are separated by interatrial septum (). A cross-sectional image of the right coronary artery in the centre of the screen was also noticed. In the right parasternal caudal long-axis view of the LVOT, the RV, LV, AV and LVOT were seen in all buffaloes. This image was obtained by placing the probe in the 4th intercostal space with rotation of the probe between 0° and 30°. The right parasternal caudal short-axis view was obtained by placing the probe at the 4th intercostal space approximately 6–8 cm above the olecranon process and angled dorsally with the transducer rotated 90°. The right parasternal short-axis view revealed the RV and LV in all animals. The papillary muscles were visible and symmetrically positioned in all animals. Symmetrical position of the papillary muscles was obtained when both muscles appeared as hyperechoic structures on both sides of the image with equal thickness. The MV, TCV and the AV with its characteristic cusps (right coronary, left coronary and non-coronary cusps) were seen.
Figure 1. Right parasternal 2-D four-chamber long-axis view of small buffalo heart at the cordal (cordae tendinae) level in end diastole (a) and systole (b) showing IVSs, interventricular septal thickening in systole; LA, left atrium, LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; LVWd, left ventricular wall thickness in diastole; LVWs, left ventricular wall thickness in systole; RA, right atrium; RV, right ventricle; MV, mitral valve; TCV, tricuspid valve.
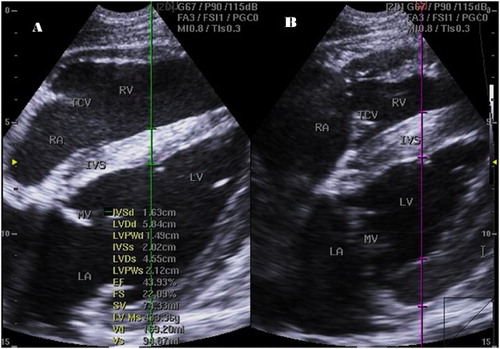
The M-mode image of the LV was obtained by placing the cursor perpendicular to the IVS and left ventricular free wall at the level of chordae tendinae between the tips of MV leaflets and the papillary muscles of the LV. The M-mode image has the RV at the top of the image followed by the IVS, the LV and the wall of the LV at the bottom. The maximum opening of the MV (E-point) was measured by placing the cursor over the tip of the MV leaflets, and the E-point to septal separation was then estimated (EPSS) ( and ).
Figure 2. Right parasternal M-mode short-axis echocardiogram of small buffalo demonstrating IVS, interventricular septum; LV, left ventricle; LVW, left ventricular wall; MV, mitral valve; RV, right ventricle; EPSS, distance between septal leaflet and IVS at the maximal excursion of the valve (red line).
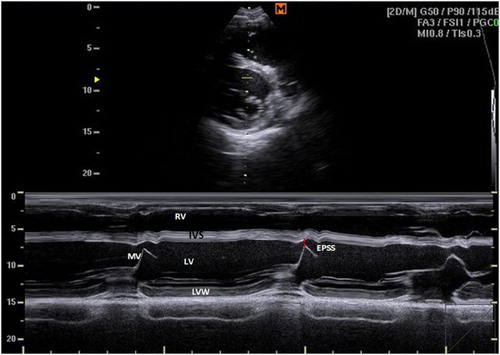
Figure 3. Right parasternal M-mode short-axis echocardiogram of small buffalo at the cordal (cordae tendinae) level showing IVSd, interventricular septal thickening in diastole; IVSs, interventricular septal thickening in systole; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; LVWd, left ventricular wall thickness in diastole; LVWs, left ventricular wall thickness in systole.
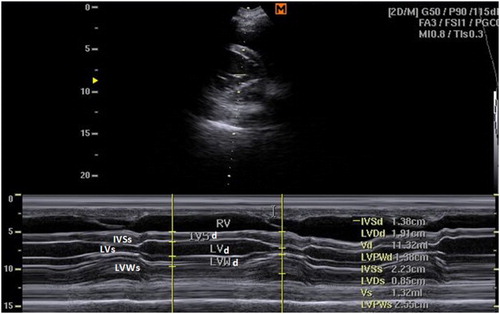
In large buffaloes, the left parasternal caudal long-axes view allowed the visualization of the RV, LV as well as RA and LA. This view was obtained by placing the probe perpendicularly at the 5th intercostal space or the 4th intercostal space with caudal direction of the probe in all animals. The left parasternal cranial long-axes view was obtained by placing the probe at the 4th intercostal space with cranio-dorsal direction with the transducer rotated 0° to 30°. This view allowed imaging of the LA, LV, RA, RV and LVOT. The right parasternal cranial long-axes view of the RVOT was obtained by placing the probe at the 4th intercostal space with cranio-dorsal angulation of the probe. This view allowed the visualization of the RA, TV, RVOT and aorta in all buffaloes.
The technique of echocardiography in buffalo required physical strength in order to obtain good, standard images as the animals resist the placement of and pressure exerted by the probe. On the other hand, the intercostal spaces were wide enough for the placement of the probe.
The echocardiographic measurements for both 2-D and M-mode in both small and large buffaloes are listed in , where an obvious correlation with the body weights is noticed for all measurements. No significant difference was noticed between 2-D and M-mode measurements for both small and large buffaloes.
Table 1. Echocardiographic measurements for small and large buffaloes.
The echocardiographic functional indices included fractional shortening, left ventricular ejection fraction, FWT% and fractional septum thickening and are tabulated in . No significant differences were recorded between the functional indices of both small and large buffaloes as well as the indices calculated from 2-D or M-mode measurements. No significant difference was found between the measurements performed by the two observers.
Table 2. Functional indices calculated from echocardiographic measurements in small and large buffaloes.
Discussion
Echocardiography is a well-established and rapidly growing method for diagnosing congenital and acquired cardiac diseases in dairy cattle (Zarifi et al. Citation2012; Buczinski et al. Citation2013). The technique used in the present study was an adaptation from the technique developed by Hallowell et al. (Citation2007) for adult dairy cattle. This standardized technique allowed the collection of reliable and repeated echocardiographic images. Little information is known about cardiac disease in buffalo. This may be attributed to the lowest percentage of cardiac disease in buffalo or the absence of reliable means for diagnosis. The knowledge of normal echocardiographic appearance and cardiac dimensions facilitates the identification and diagnosis of cardiac diseases. Early diagnosis represents the first step in the management of these affections. Echocardiography in buffaloes is a practical technique that requires physical strength as buffaloes resist the transducer placement and rotation to obtain the standardized images. Images were obtained from the right side in small buffaloes (less than 400 kg), while in large buffaloes (more than 400 kg) the images were obtained from the left side, where the LV and posterior wall could be imaged properly. The same was mentioned by Slater & Herrtage (Citation1995), Jezei & Flor (Citation2008) and Tharwat & Buczinski (Citation2011). Correct positioning (advancement of the forelimbs) of the buffaloes allowed obtaining good quality images. Holding the legs up was not a good idea where the animals become less comfortable as well as this position led to a more caudal position of the triceps muscle mass, which results in obtaining poor quality images (Slater & Herrtage Citation1995; Tharwat et al. Citation2012; Hussein & Staufenbiel Citation2014). The short-axes view was the most challenging view due to difficulties in obtaining symmetrical images and poor visualization due to hyperechogenicity of the pleural surface, especially in buffaloes with large body weights (Tharwat & Buczinski Citation2011). The relationship between body weight and echocardiographic dimensions was mentioned by Long et al. (Citation1992) in a study performed in a group of national Hunt horses with wide weight range, where no significant difference was found between echocardiographic variables and body weights. The trend for echocardiographic measurements to increase with body weights was mentioned by Slater and Herrtage (Citation1995) who studied this relation in small ponies, large ponies and horses. The authors found little or no relation between echocardiographic dimensions and body weights within the same group when they were studied individually, but when compared together, a strong relationship was reported between body weight and echocardiographic dimensions. In the present study, a statistically significant obvious correlation was noticed between body weight and echocardiographic dimensions. This correlation was found within and between the groups of buffaloes. In the present study, no differences in probe placement were found between buffaloes and that previously mentioned in cattle and other studies (Slater & Herrtage Citation1995; Tharwat & Buczinski Citation2011; Hussein & Staufenbiel Citation2014). The four-chamber view was obtained from the right and left parasternal long-axes view when the probe was placed at the 4th ICS from the right or the 5th ICS from the left. The LVOT and RVOT were obtained by rotation of the probe caudally and cranially, respectively. The echocardiographic dimensions in buffaloes appeared smaller than that previously reported in cattle with similar body weights (Hallowell et al. Citation2007). No significant difference was noticed between the 2-D and M-mode measurements of the same or both observers. As the functional indices were calculated from echocardiographic measurements, no significant differences were noticed between the functional indices calculated from the 2-D and M-mode measurements or that of small and large buffaloes (El-Khodery et al. Citation2010).
The main limitations of the present study were the small number of buffaloes used, and further studies on a large population are recommended. Another limitation of the study was that it was not possible to confirm that these animals have normal cardiac structures at post-mortem examination as all animals were alive and in apparent good health after 6-month follow-up.
Conclusion
The study demonstrated that it is possible to obtain good quality echocardiograms in adult buffaloes depending on the standardized technique used in cattle. The normal cardiac dimensions obtained in this study could be compared with that of diseased animals. The study represents a step that hopefully would allow early diagnosis of cardiac affections in adult buffaloes.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Boon JA. 2011. Veterinary echocardiography. 2nd ed. Ames, Iowa: Wiley-Blackwell, p. 610.
- Braun U. 2009. Traumatic pericarditis in cattle: clinical, radiographic and ultrasonographic findings. Vet J. 182:176–186. doi: 10.1016/j.tvjl.2008.06.021
- Braun U, Schweizer T, Pusterla N. 2001. Echocardiography of the normal bovine heart: technique and ultrasonographic appearance. Vet Rec. 148:47–51. doi: 10.1136/vr.148.2.47
- Buczinski S. 2009. Cardiovascular ultrasonography in cattle. Vet Clin North Am Food Anim Pract. 25:611–632. doi: 10.1016/j.cvfa.2009.07.010
- Buczinski S, Tolouei M, Rezakhani A, Tharwat M. 2013. Echocardiographic measurements of cardiac valvular thickness in healthy cows, cows with bacterial endocarditis and cows with cardiorespiratory diseases. J Vet Cardiol. 15(4):253–261. doi: 10.1016/j.jvc.2013.08.001
- Carlsten JC. 1987. Two-dimensional real-time echocardiography in the horse. Vet Radiol. 28:76–87. doi: 10.1111/j.1740-8261.1987.tb01730.x
- El-Khodery SA, Nassif MN, Hassan HY. 2010. Two dimension echocardiography in normal buffaloes (Bubalus bubalis). J Appl Anim Res. 37(1):57–61. doi: 10.1080/09712119.2010.9707094
- Fooda TA, Elbeltagy AR, Laila RH. 2011. Assessment of Egyptian buffalo crossing with Pakistani and Italian buffaloes for some production traits. J Am Sci. 7(1):183–192.
- Hallowell G, Potter TJ, Bowen IM. 2007. Methods and normal values for echocardiography in adult dairy cattle. J Vet Cardiol. 9:91–98. doi: 10.1016/j.jvc.2007.10.001
- Hassan EA, Torad FA. 2015. Two-dimensional and M-mode echocardiographic measurements in the healthy donkeys (Equus asinus). J Equine Vet Sci. 35(4):283–289. doi: 10.1016/j.jevs.2015.01.016
- Hussein AH, Staufenbiel R. 2014. Clinical presentation and ultrasonographic findings in buffaloes with congestive heart failure. Turk J Vet Anim Sci. 38:534–545. doi: 10.3906/vet-1404-111
- Jezei AA, Flor MI. 2008. B-mode and M-mode ultrasonography of the heart in female buffaloes (Bubalus bubalis). Philip J Vet Med. 45:7–13.
- Kriz NG, Rose RJ. 1996. Effect of detraining on cardiac dimensions and indices of cardiac function in horses. Proceeding of 42nd AAEP Annual Conference. 42:96–97.
- Long KJ, Bonagura JD, Darke PG. 1992. Standardised imaging technique for guided M-mode and Doppler echocardiography in the horse. Equine Vet J. 24:226–235. doi: 10.1111/j.2042-3306.1992.tb02820.x
- Schiller NB, Shah PM, Crawford M., Schiller NB, Maurer G, Ritter SB, Armstrong WF, Spotnitz H, Cahalan M, Quinones M, Meltzer R, Feinstein S, Konstadt S, Seward J 1989. Recommendations for quantitation of the echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocard. 2:358–367. doi: 10.1016/S0894-7317(89)80013-6
- Slater JD, Herrtage ME. 1995. Echocardiographic measurements of cardiac dimensions in normal ponies and horses. Equine Vet J. 19:28–32.
- Tharwat M, Al-Sobayil F, Ali A, Buczinski S. 2012. Echocardiography of the normal camel (Camelus dromedarius) heart: technique and cardiac dimensions. BMC Vet Res. 8:130–137. doi: 10.1186/1746-6148-8-130
- Tharwat M, Buczinski S. 2011. Clinicopathological findings and echocardiographic prediction of the localisation of bovine endocarditis: 36 cases. Vet Rec. 169:180–186. doi: 10.1136/vr.d4346
- Weyman AE. 1994. Principles and practice of echocardiography, 2nd ed. Philadelphia. Cross-sectional scanning: technical principles and instrumentation, pp. 29–56.
- Young LE. 1999. Cardiac responses to training in 2 year old thoroughbreds: an echocardiographic study. Equine Vet J. 30:195–198.
- Zarifi M, Buczinski S, Rezakhani A, Dezfouli M, Khonsha A. 2012. Effect of location on function and morphological echocardiographic variables in adult dairy cows. J Vet Cardiol. 14(3):415–421. doi: 10.1016/j.jvc.2011.11.009
