ABSTRACT
To better understand the biology of Schizothorax waltoni, the age structure and growth characteristics of S. waltoni were examined. The standard length (SL) range was 41–642 mm and the body weight (BW) range was 1.1–3788.1 g. Otoliths continually grew with increasing SL and increasing age, but asymmetrically over time. One annulus was formed each year, between April and July. Estimated age range was 4–37 yr for males, 4–40 yr for females, and 1–9 yr for those of undetermined sex. The SL–BW relationship was described as BW = 1.365 × 10−5 SL2.984 for females, BW = 1.238 × 10−5 SL2.999 for males, and BW = 3.664 × 10−5 SL2.800 for undetermined specimens. The von Bertalanffy function was used to model the observed length-at-age data as Lt = 644.3 {1 − exp [−0.084(t − 0.247)]} for females, and Lt = 586.2 {1 − exp [−0.084(t + 2.250)]} for males. Females grew faster than males. Knowledge of this species’ characteristics of slow growth and a long life will be useful for establishing reasonable management practices for its conservation.
1. Introduction
The subfamily Schizothoracinae is the predominant group of endemic fishes living in high-elevation rivers and lakes on the Qinghai-Tibetan Plateau (Cao et al. Citation1981). They are characterized by restricted distributions, slow growth rates, low fecundity, and late maturity, which may result from their surrounding rigorous environment (Chen & Cao Citation2000). These life-history characteristics make them more vulnerable to intense exploitation (Buxton Citation1993). These species have been subjected to intensive anthropogenic pressures such as indiscriminate fishing, habitat modification resulting from dam construction, and biological invasions (Huo et al. Citation2012).
Schizothorax waltoni (Cyprinidae, Schizothoracinae), generally known as marinka baileyova, is an endemic fish to Tibet. It is distributed only in the middle reaches of the Yarlung Tsangpo River and is of economic importance in this region (Chen & Cao Citation2000). Despite the S. waltoni population supporting a valuable fishery, little is known about the biology and ecology of this species. To date, there has been only one study of S. waltoni. Qiu and Chen (Citation2009) estimated the age of S. waltoni using otoliths, but the standard length (SL) range was only 202–580 mm and the body weight (BW) range was 96–2256 g. The estimated age range was 4–28 yr for all specimens and the maximum observed age was only 18 yr for males and only 28 yr for females in their study, owing to difficulties in collecting sufficient samples of smaller and larger specimens and the need to develop appropriate methods for ageing older fish. This ageing problem often led to age underestimation, resulting in overly optimistic estimates of growth and mortality rates, which can contribute to overexploitation of a population or species (Campana Citation2001). The growth model estimates are greatly affected by the lack of very young or old individual, extra space in juveniles (age classes 1–4) (Cailliet & Goldman Citation2004).
Accurate estimates of age and growth are prerequisites for understanding population dynamics and maintaining sustainable yields in fisheries (Campana & Thorrold Citation2001). Anal scales (Tsao & Wu Citation1962; Zhao et al. Citation1975; Ren & Sun Citation1982), vertebrae (Lal & Mishra Citation1980), and opercles (Sunder Citation1985) have all been used to determine age in previous studies of schizothoracine fishes. Recent studies have demonstrated that schizothoracine age can be estimated reliably from the sections of lapillus otoliths (Huo et al. Citation2012; Ma et al. Citation2010; Chen et al. Citation2006). For S. waltoni, sectioned lapillus showed a clear pattern of alternating opaque and hyaline zones (Qiu & Chen Citation2009).
Some researchers focused on relationships of otolith size with fish size and age, and suggested that age can be estimated from the otolith weight and fish length (Boehlert Citation1985). Experimental studies using fish of known ages showed that for juveniles, the otolith growth is in fact isometric with somatic growth (Secor & Dean Citation1989), but for slowly growing individuals, otoliths grow asymmetrically through time, having relatively heavy otoliths for their body size, which might be attributed to an increase in otolith thickness (Reznick et al. Citation1989).
The overall goal of our study was to characterize the age and growth of S. waltoni in the Yarlung Tsangpo River of Tibet, China, by a large number of specimens with a wide SL and BW. The specific research content included: (1) to verify the annual periodicity of the growth increments in otoliths, (2) to determine age structure of the population through otolith analysis, and (3) to model the growth for both sexes of the population.
2. Material and methods
2.1. Collection of samples
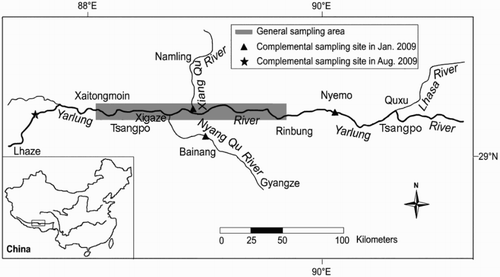
The S. waltoni individuals were obtained from the Yarlung Tsangpo River and its tributaries (Xiang Qu and Nyang Qu) monthly from August 2008 to August 2009 by means of floating gillnets, bottom gillnets, and trap nets (). The standard length (SL) of each fresh specimen was measured to the nearest 1 mm using a tapeline, and body weight (BW) was measured to the nearest 0.1 g with an electronic balance. Specimens were classified as male, female, or undetermined sex by macroscopic examination of gonads. Lapillus otoliths were extracted from each fish, washed with 95% ethanol, air-dried, and then stored in labelled tubes.
2.2. Otolith preparation
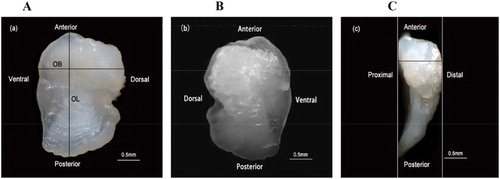
The terms anterior, posterior, dorsal, ventral, proximal, and distal faces refer to the position of the otolith corresponding to its original orientation in the fish (Reñones et al. Citation2007, ). To study otolith size and growth, the following measurements were made in a sample of 1061 whole otoliths (): length (OL: maximum anterior-posterior distance), breadth (OB: dorsal-ventral distance at the widest point), thickness (OT: distal-proximal maximum distance), and weight (OW: measured after being dried at 60°C for 24 h) (Reñones et al. Citation2007). OL and OB were measured to the nearest 0.01 mm using Image-Pro Plus software (version 6.0; Media Cybernetics, USA) after taking photos with Leica Application Suite (Leica EZ4D dissecting microscope). The OT was measured to the nearest 0.01 mm with digital calipers and OW to the nearest 0.1 mg with an electronic balance.
Figure 2. Proximal (b), distal (a), and dorsal (c) faces of a whole lapillus under a dissecting microscope with reflected light, from an 8-yr old S. waltoni (338 mm standard length (SL)). The axes along which the otolith length (OL) and breadth (OB) were measured are indicated. The otolith (c) was placed inside digital calipers to measure the thickness (OT). Scale bars = 0.5 mm.
A lapillus was mounted with the proximal face on a glass slide using nail polish, ground from the distal face using wet sandpaper (600–2000 grit) and polished with alumina paste (3 μm) until the core was visible under a compound microscope. Then the otolith was removed from the glass slide by dissolving the polish with acetone, and the section was re-affixed with the polished surface down, ground, and polished until the core was exposed again (He et al. Citation2008).
2.3. Marginal increment ratio (MIR)
A marginal increment ratio (MIR) analysis was used to verify the period of opaque zone formation in the otoliths. Monthly variations of the MIR (1–8 annuli) were established using the following equation:where R is the otolith radius, Rn is the distance from the focus to the outer edge of the last annulus formed, and Rn−1 is the distance from the focus to the outer edge of the penultimate complete annulus (Haas & Recksiek Citation1995). Measurements were conducted along the anterior growth axis using an image analysis system (Leica Application Suite EZ, Heerbrugg, Switzerland) with a direct data feed between the dissecting microscope (Leica EZ 4D) and a computer.
2.4. Age estimation
Each fish was assigned to an age class assuming 1 March as the birth date, which approximately corresponds to the peak spawning season (which occurs in March, unpublished data). When a fish sampled after the assumed birth date had no new ring mark, the annulus should plus 1 in the age estimation (Granada et al. Citation2004).
Otolith readings were made along the anterior growth axis. The reader had no prior knowledge of the length and sex before the age estimation. All ages were determined twice by the same interpreter after a considerable time (3 wk). Readings were only accepted if both counts by the same examiner were in agreement. If the two readings differed, then the otolith was recounted, and the final count was then accepted as the agreed age. If the 3rd reading had no consensus with either of the previous two readings, the sample was discarded.
The index of the average percentage error (IAPE) and coefficient of variation (CV) were calculated to measure the ageing precision between the two readings. The equations (Campana Citation2001) are expressed as follows:
where N is the number of fish aged, R is the number of times each fish was aged, Xij is the ith age determination of the jth fish, and Xj is the mean age calculated for the jth fish.
2.5. Environmental factors
To discuss the relationship between the water temperature and the deposition of the opaque zone, water temperature was measured and recorded twice a day (8:00 and 20:00) at Xigaze, the middle reaches of Yarlung Tsangpo River. The mean values each month were used in our analysis.
2.6. Data analysis
Relationships of otolith dimensions with fish length and age were studied by linear regression analysis. Relationships between BW and SL were estimated independently for females and males using a power regression analysis, described by BW = a × SLb, where a and b are parameters. The traditional von Bertalanffy growth function (von Bertalanffy Citation1938) was used to fit the observed length at age of S. waltoni: Lt = L∞ {1 − exp [−k (t − t0)]} where Lt is the length at age t, L∞ is the asymptotic length, k is the growth coefficient, t is the age (year from birth), and t0 is the age at length 0. The growth performance index was calculated using the equation of Munro and Pauly (Citation1983): Ø = log10 k + 2 log10 L∞. Ø was used to compare growth parameters obtained in this study with those reported by other authors for schizothoracine fishes.
The BW–SL relationship and von Bertalanffy function were calculated by a non-linear regression analysis (the Levenberg–Marquardt method; Levenberg Citation1944). The difference in the BW–SL relationship between the sexes was compared by an analysis of covariance (ANCOVA). Deviation of the allometric coefficient, b, from the theoretical value of isometric growth (b = 3) was tested by a t-test (Pauly Citation1984). A residual sum of squares analysis (ARSS) was used to determine whether any significant difference existed in the von Bertalanffy equations for males and females (Chen et al. Citation1992).
The data were presented as the mean ± standard deviation (SD). Differences were regarded as significant when p < .05. The analysis was carried out using SPSS 18.0 (Chicago, IL, USA), Origin 8.0 (Originlab, Northampton, USA), Microsoft Excel 2003 (Redmon, WA, USA), and Photoshop CS2 Extended (Adobe, San Jose, USA).
3. Results
3.1. Length–frequency distributions and SL–BW relationship
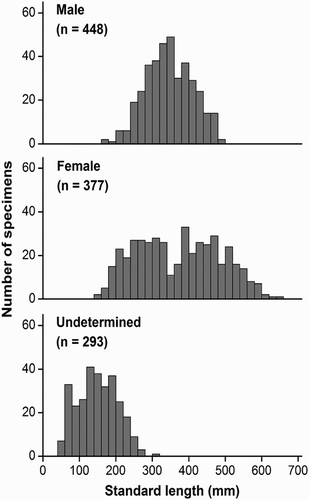
The SL of specimens’ range was 41–642 mm, and the BW range was 1.1–3788.1 g. Of the 1118 S. waltoni sampled, 448 were females with SL of 151–642 mm, 377 were males with SL of 176–499 mm, and 293 were the undetermined sex with SL of 41–303 mm. Length–frequency distributions significantly differed between males and females (Kolmogorov–Smirnov test, D = 3.556, p < .05) (). SL of captured fish was mainly 100–500 mm (88%), and females were significantly larger than males.
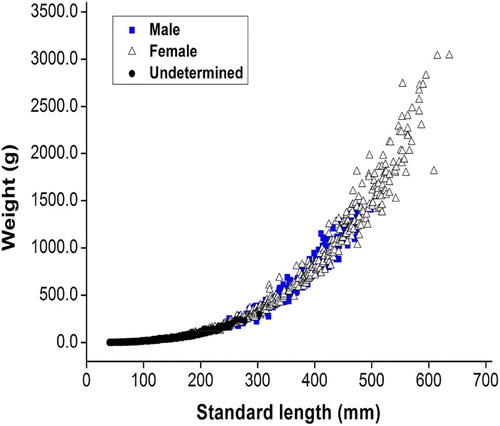
The SL–BW relationships are plotted separately for males, females, and undetermined sex in . No statistically significant difference was detected for SL–BW relationships between males and females by both the slopes and the intercepts (ANCOVA, F = 0.226, p > .05). So, all the individuals were pooled together. The regression equations were shown as follows:
Female BW = 1.365 × 10−5 SL2.984 (R2 = .990, n = 448),
Male BW = 1.238 × 10−5 SL2.999 (R2 = .974, n = 377),
Undetermined sex BW = 3.664 × 10−5 SL2.800 (R2 = .993, n = 293); and
Total BW = 2.274 × 10−5 SL2.896 (R2 = .995, n = 1118).
3.2. Age validation and annual periodicity
Under polarized transmitted light, alternate, concentric opaque and hyaline bands were observed in the anterior area of the otolith, which facilitated the reading of growth markers.
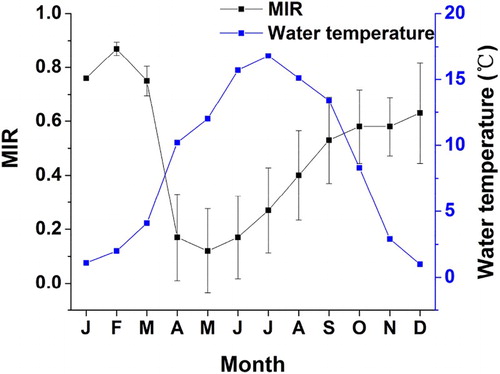
For otolith sections with 1–8 annuli, monthly changes of the MIR gradually increased from July to February, and appeared to peak at 0.871 in February followed by a gradual decrease to 0.124 in May (). Significant differences were found in MIR values among months (one-way ANOVA, F = 11, p < .001). Tukey’s post-hoc pair-wise comparisons revealed that the MIR between April and July significantly differed from that from September to March (p < .05). These results indicated that the opaque band of the otoliths was laid down once a year between March and July
3.3. Relationships between otolith dimensions and fish length/age
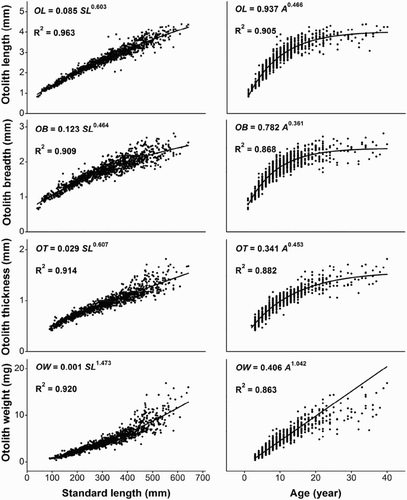
The relationships between otolith dimensions (OL, OB, OT, and OW) and fish length/age are shown in . The lines of best fit between OL/OB/OT/OW and SL/age were power functions. The growth rate of OL/OB/OT declined as SL/age increased, that is, OL/OB/OT increased with increasing SL at a fixed rate up to a size of 400 mm or 15 yr, and at a slightly slower rate thereafter. OL was the best predictor of fish length (R2 = .963) and age (R2 = .905). Otoliths dimensions were more highly correlated with fish length than age. Variability in the data substantially increased for all variables beyond 450 mm SL or 20 yr.
3.4. Age structure
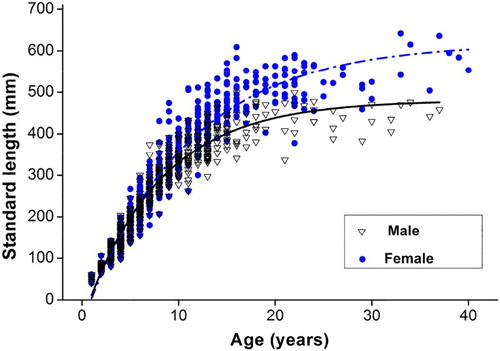
Sectioned otoliths of S. waltoni showed the typical pattern observed in teleosts under transition light, with an alternating sequence of broad opaque and narrow hyaline bands that became progressively narrower and of similar widths as the number of bands increased (). Of the 1118 simples of otoliths examined, there were successfully read for a total of 1061 specimens, only 57 (approximately 5.1%) were discarded due to fragmentation and unidentifiable annulus deposition. Age estimates had low IAPE (0.49%) and CV (0.65%), reflecting concordance in the readings and thus intrinsic reliability. The estimated age range was 1–40 yr. Assigned ages of fish for the undetermined sex were in the range 1–9 yr, for males the range was 4–37 yr, and for females the range was 4–40 yr. Standard lengths-at-age of the 1061 specimens are presented in .
Figure 7. Sectioned lapillus of S. waltoni with 467 mm SL, estimated to be 22 yr old under transmitted light using the compound microscope. Dots represent annuli. Scale bar = 0.1 mm.

Table 1. Number of specimens and Mean ± SD and range of standard length at age of S. waltoni.
3.5. Growth
The mean length-at-age did not significantly differ between sexes for age classes 5–8 (two-sample t-test, all p > .05). Therefore, the length-at-age data of undetermined specimens (except for two 9-yr-old individuals) were included in the von Bertalanffy models for both sexes. The von Bertalanffy functions fitted to the observed length-at-age are given as follows:
Lt = 586.2 {1 − exp [−0.084(t + 2.250)]} (R2 = .970) for males and,
Lt = 644.3 {1 − exp [−0.084(t − 0.247)]} (R2 = .966) for females.
4. Discussion
It has been argued that otolith size is highly correlated with fish size, because they are both controlled by the same metabolic processes (Gauldie Citation1988). Otoliths, as measured along various axes, show asymmetrical growth through time, but nevertheless grow throughout the life of the fish (Fowler Citation1990). Asymmetries occur in otolith growth because the new material is mainly deposited on the distal face of the otolith which thickens the structure, particularly in slow-growing, long-lived species (Boehlert Citation1985; Reñones et al. Citation2007). Such a growth pattern might affect the application of back-calculation which assumes that otolith growth is isometric with somatic growth through time (Campana Citation1990). In the present study, otoliths grew symmetrically along the three axes, but the growth rate slightly declined after the SL longer than 400 mm.
Pino et al. (Citation2004) reported that otolith weight could be correlated with the age of some fish, and it may be a useful, simple tool for rapidly estimating the age of individuals (Araya et al. Citation2001). However, in some studies, the increased variability of OW in older fish indicated that this variable may produce less-reliable age estimates in older fish (Tuset et al. Citation2004). Gunn et al. (Citation2008) and Ma et al. (Citation2010) pointed out that OW was an imprecise predictor of age beyond 10 or 15 yr. In the present study, a similar trend was observed, with variability of OW in S. waltoni significantly increasing beyond 450 mm SL or 20 yr.
Application of the precision measures (IAPE and CV) presented satisfactory consistency in age determination between the two readings, because the estimated value was below the precision point of 5.5% established by Campana (Citation2001) for ageing studies.
Although the exact mechanisms of growth increment formation are poorly understood, they are thought to involve some exogenous and endogenous factors, which may include temperature, season, feeding, and reproductive cycles (Beckman & Wilson Citation1995; Morales-Nin Citation2000; Tserpes & Tsimenides Citation2001; Grandcourt et al. Citation2006; Liu et al. Citation2009). In this study, deposition of the opaque zone in otoliths of S. waltoni occurred in the spring and early summer, whereas the hyaline zone was formed in the winter months. A similar phenomenon in Scizothoracinae fishes has been demonstrated for O. stewartii (Jia & Chen Citation2009; Huo et al. Citation2012), P. dipogon (Li et al. Citation2009), S. waltoni (Qiu & Chen Citation2009), and S. o’connori (Ma et al. Citation2010). Deposition of the opaque zone occurs between March and July, corresponding well to the reproductive activity and water temperature changes, and deposition of the hyaline zone occurs during winter months, when there is reduced metabolic activity. Formation of the opaque zone in otoliths of S. waltoni appeared to be closely associated with the abrupt increase in seasonal water temperature in April–July and August. Moreover, formation of the opaque zone in otoliths of S. waltoni may be partially related to reproductive cycle. Peak reproductive activity may promote a redirection of energy to reproduction, with a consequent reduction in somatic growth, which could possibly affect the physiology of otolith growth. Similar influences by water temperature and reproduction on other fishes were reported in past studies (Morales-Nin & Ralston Citation1990; Mann & Buxton Citation1997; Pajuelo et al. Citation2003; Bustos et al. Citation2009; Beckman & Wilson Citation1995). Thus, formation of the annulus in otoliths of S. waltoni could be due to an interaction between water temperature and reproduction.
The maximum ages estimated for S. waltoni in this study were 40 yr for females and 37 yr for males. Li and Chen (Citation2009) recorded 45 yr for females and 24 yr for males of P. dipogon based on an interpretation of sectioned otoliths. Chen et al. (Citation2009) found 18 yr for females and 16 yr for males of S. younghusbandi younghusbandi by means of otoliths. Yao et al. (Citation2009) obtained 24 yr for females and 18 yr for males of S. o’connori using otoliths. Ma et al. (Citation2010) reported 50 yr for females and 40 yr for males of S. o’connori based on otolith observations. Huo et al. (Citation2012) reported 25 yr for females and 17 yr for males of O. stewartii based on otolith observations. Those studies including the present paper confirm that relatively high longevity is a common trait in schizothoracine fishes and the females live longer than males.
Cailliet and Goldman (Citation2004) reported that growth model estimates are greatly affected by the lack of very young or old individuals, extra space in juveniles (age classes 1–4) collected from traps provided a meaningful estimate of the VBGF parameter, t0. More data points from juvenile fish would tend to shift t0 values higher and closer to 0 (Paul & Horn Citation2009). Since the mean length-at-age exhibited no significant difference between sexes up to an age of 8 yr, the length-at-age data of undetermined specimens were used to fit models for both sexes (Peres & Haimovici Citation2004). Moreover, the presence of several specimens of >600 mm SL also provided a reliable estimate of the other VBGF parameter, L∞ (644.2 mm for females, which was larger than the maximum observed size of 642 mm).
Regarding the estimation of VBGF parameters, a low estimate of k and a high L∞ indicated that S. waltoni is a slow-growing, long-lived fish. Females attained a bigger size (L∞) than males. Comparing our results of the growth of S. waltoni with those from the previous study (), the L∞ obtained in our study was lower the study by Qiu and Chen (Citation2009) in the Yarlung Tsangpo River (), the L∞ obtained in this study was lower, but the k value was higher. Differences among all of the estimated parameters could be attributed to several factors: (1) different environmental conditions (such as altitude and water temperature) (2) different size distributions (probably caused by different types of sampling gear), and (3) different age groups employed to fit the VBGF function (groups whereas here in individuals were age of 4 to 28).
Table 2. Comparison of growth characters of Schizothoracinae fish in different studies.
The von Bertalanffy growth coefficient (k) is a useful index to address the potential vulnerability of stocks to excessive mortality (Musick Citation1999). Comparing the parameters of several Schizothoracinae fishes, Li and Chen (Citation2009) suggested that they are slow-growing species with k values of around 0.1. Slow-growing, long-lived fishes tend to be particularly vulnerable to excessive exploitation and exhibit rapid stock collapse, after which population turnover may be lower than expected, and their responses to rehabilitation measures slower than predicted (Musick Citation1999). In this study, the estimated maximum age was 40 yr, and the k value was well below .1, indicating that S. waltoni is a very slow-growing, long-living species. The growth traits of S. waltoni may be adaptations to cold water temperatures and food deficiencies on the Qinghai-Tibetan Plateau (Chen et al. Citation2009). Therefore, it is necessary to establish science-based management of this resource to guarantee its sustainable use. Fishery regulations for S. waltoni should aim to prevent growth overfishing through minimum landing sizes, and also prevent recruitment overfishing by protecting the oldest population components especially in the spawning season.
Acknowledgements
The authors wish to express their gratitude to Charles P. Madenjian for suggesting revisions to the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Araya M, Cubillos LA, Guzmán M, Peñailillo J, Sepúlveda A. 2001. Evidence of a relationship between age and otolith weight in the Chilean jack mackerel, Trachurus symmetricus murphyi (Nichols). Fish Res. 51:17–26.
- Beckman DW, Wilson CA. 1995. Seasonal timing of opaque zone formation in fish otoliths. In: Secor DH, Dean JM, Campana SE, editors. Recent development in fish otolith research. Columbia: University of South Carolina Press; p. 27–43.
- von Bertalanffy L. 1938. A quantitative theory of organic growth (inquiries on growth laws II). Hum Biol. 10:181–213.
- Boehlert GW. 1985. Using objective criteria and multiple regression models for age determination in fishes. Fish Bull. 83:103–117.
- Bustos R, Luque Á, Pajuelo JG. 2009. Age estimation and growth pattern of the island grouper, Mycteroperca fusca (Serranidae) in an island population on the northwest coast of Africa. Sci Mar. 73:319–328.
- Buxton CD. 1993. Life-history changes in exploited reef fishes on the east coast of South Africa. Environ Biol Fish. 36:47–63.
- Cailliet GM, Goldman KJ. 2004. Age determination and validation in Chondrichthyan fishes. In: Carrier J, Musick JA, Heithaus M, editors. The biology of sharks and their relatives. New York, NY: CRC Press; p. 399–447.
- Campana S. 1990. How reliable are growth back-calculations based on otoliths? Can J Fish Aquat Sci. 47:2219–2227.
- Campana SE. 2001. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol. 59:197–242.
- Campana SE, Thorrold SR. 2001. Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can J Fish Aquat Sci. 58:30–38.
- Cao WX, Chen YY, Wu YF, Zhu SQ. 1981. Origin and evolution of schizothoracine fishes in relation to the upheaval of the Xizang Plateau. In: CAS, editor. The comprehensive scientific expedition to the Qinghai-Xizang Plateau, studies on the period, amplitude and type of the uplift of the Qinghai-Xizang Plateau. Beijing: Science Press; p. 118–130 (in Chinese).
- Chen YF, Cao WX. 2000. Schizothoracinae. In: Yue PQ, editor. Fauna sinica osteicthtyes cypriniformes III. Beijing: Scince Press; p. 273–388 (in Chinese).
- Chen F, Chen YF, He DK. 2009. Age and growth of Schizopygopsis younghusbandi younghusbandi in the Yarlung Zangbo River in Tibet, China. Environ Biol Fish. 86:155–162.
- Chen Y, Jackson DA, Harvey HH. 1992. A comparison of von Bertalanffy and polynomial functions in modelling fish growth data. Can J Fish Aquat Sci. 49:1228–1235.
- Chen DQ, Zhang X, Xiong F, Liu SP, Tang HY. 2006. Studies on growth characteristics of Gymnocypris przewalskii przewalskii. Acta Hydrobiol. 30:173–179 (in Chinese).
- Fowler A. 1990. Validation of annual growth increments in the otoliths of a small, tropical coral reef fish. Mar Ecol Prog Ser. 64:25–38.
- Gauldie R. 1988. Function, form and time-keeping properties of fish otoliths. Comp Biochem Phys. 91:395–402.
- Granada VP, Masuda Y, Matsuoka T. 2004. Age and growth of the yellowbelly threadfin bream Nemipterus bathybius in Kagoshima Bay, southern Japan. Fish Sci. 70:497–506.
- Grandcourt EM, Abdessalaam TZA, Francis F. 2006. Age, growth, mortality and reproduction of the blackspot snapper, Lutjanus fulviflamma (Forsskål, 1775), in the southern Arabian Gulf. Fish Res. 78:203–210.
- Gunn JS, Clear NP, Carter TI, Rees AJ, Stanley CA, Farley JH, Kalish JM. 2008. Age and growth in southern bluefin tuna, Thunnus maccoyii (Castelnau): direct estimation from otoliths, scales and vertebrae. Fish Res. 92:207–220.
- Haas RE, Recksiek CW. 1995. Age verification of winter flounder in Narragansett Bay. Trans Am Fish Soc. 124:103–111.
- He WP, Li ZJ, Liu JS, Li YX, Murphy BR, Xie SG. 2008. Validation of a method of estimating age, modeling growth, and describing the age composition of Coilia mystus from the Yangtze Estuary, China. ICES J Mar Sci. 65:1655–1661.
- Huo B, Xie CX, Ma BS, Yang XF, Huang HP. 2012. Age and growth of Oxygymnocypris stewartii (Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River, Tibet, China. Zool Stud. 51(2):185–194.
- Jia YT, Chen YF. 2009. Otolith microstructure of Oxygymnocypris stewartii (Cypriniformes, Cyprinidae, Schizothoracinae) in the Lhasa River in Tibet, China. Environ Biol Fish. 86:45–52.
- Jia YT, Chen YF. 2011. Age structure and growth characteristics of the endemic fish Oxygymnocypris stewartii (Cypriniformes: Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River, Tibet. Zool Stud. 50:69–75.
- Lal MM, Mishra M. 1980. Ecobiological studies of some hill-stream fishes of Grahwal Himalayas 4. Length-weight relationship of Schizothorax richardsonii (Heckel). Indian J Zoot. 21:83–85.
- Levenberg K. 1944. A method for the solution of certain nonlinear problems in least squares. Q Appl Math. 2:164–168.
- Li XQ, Chen YF. 2009. Age structure, growth and mortality estimates of an endemic Ptychobarbus dipogon (Regan, 1905) (Cyprinidae: Schizothoracinae) in the Lhasa River, Tibet. Environ Biol Fish. 86:97–105.
- Li XQ, Chen YF, He DK, Chen F. 2009. Otolith characteristics and age determination of an endemic Ptychobarbus dipogon (Regan, 1905) (Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River, Tibet. Environ Biol Fish. 86:53–61.
- Liu KM, Lee ML, Joung SJ, Chang YC. 2009. Age and growth estimates of the sharptail mola, Masturus lanceolatus, in waters of eastern Taiwan. Fish Res. 95:154–160.
- Ma BS, Xie CX, Huo B, Yang XF, Huang HP. 2010. Age and Growth of a Long-Lived Fish Schizothorax o’connori in the Yarlung Tsangpo River, Tibet. Zool Stud. 49:749–759.
- Mann B, Buxton C. 1997. Age and growth of Diplodus sargus capensis and D. cervinus hottentotus (Sparidae) on the Tsitsikamma coast, South Africa. Cybium. 21:135–147.
- Morales-Nin B. 2000. Review of the growth regulation processes of otolith daily increment formation. Fish Res. 46:53–67.
- Morales-Nin B, Ralston S. 1990. Age and growth of Lutjanus kasmira (Forskål) in Hawaiian waters. J Fish Biol. 36:191–203.
- Munro JL, Pauly D. 1983. A simple method for comparing the growth of fishes and invertebrates. ICLARM Fish byte. 1:5–6.
- Musick JA. 1999. Ecology and conservation of long-lived marine animals. Am Fish Soc Symp. 23:1–10.
- Pajuelo JG, Lorenzo JM, Domínguez-Seoane R. 2003. Age estimation and growth of the zebra seabream Diplodus cervinus cervinus (Lowe, 1838) on the Canary Islands shelf (central-East Atlantic). Fish Res. 62:97–103.
- Paul LJ, Horn PL. 2009. Age and growth of sea perch (Helicolenus percoides) from two adjacent areas off the east coast of South Island, New Zealand. Fish Res. 95:169–180.
- Pauly D. 1984. Fish population dynamics in tropical waters: a manual for use with programmable calculators. ICLARM Stud Rev. 8:325. https://www.researchgate.net/publication/37909647_Fish_Population_Dynamics_in_Tropical_Waters_A_Manual_for_Use_with_Programmable_Calculators
- Peres MB, Haimovici M. 2004. Age and growth of southwestern Atlantic wreckfish Polyprion americanus. Fish Res. 66:157–169.
- Pino CA, Cubillos LA, Araya M, Sepúlveda A. 2004. Otolith weight as an estimator of age in the Patagonian grenadier, Macruronus magellanicus, in central-south Chile. Fish Res. 66:145–156.
- Qiu H, Chen YF. 2009. Age and growth of Schizothorax waltoni in the Yarlung Tsangpo River in Tibet, China. Ichthyol Res. 56:260–265.
- Ren ML, Sun L. 1982. An investigation of the fish resource and exploitation problems in Namucuo Lake, in Tibet. Freshwater Fishery. 2:1–10 (in Chinese).
- Reñones O, Piñeiro C, Mas X, Goñi R. 2007. Age and growth of the dusky grouper Epinephelus marginatus (Lowe 1834) in an exploited population of the western Mediterranean Sea. J Fish Biol. 71:346–362.
- Reznick D, Lindbeck E, Bryga H. 1989. Slower growth results in larger otoliths: an experimental test with guppies (Poecilia reticulata). Can J Fish Aquat Sci. 46:108–112.
- Secor D, Dean J. 1989. Somatic growth effects on the otolithfish size relationship in young pond-reared striped bass, Morone saxatilis. Can J Fish Aquat Sci. 46:113–121.
- Sunder S. 1985. Length–weight relationship of Schizothorax curvifrons Heckel from Jhelum Srinagar. Geobios New Rep. 4:131–136.
- Tsao WX, Wu XW. 1962. An investigation of the fish biology and fishery problems in Ganze–Apa region of western Szechwan Province. Acta Hydrobiol. 2:79–111.
- Tserpes G, Tsimenides N. 2001. Age, growth and mortality of Serranus cabrilla (Linnaeus, 1758) on the Cretan shelf. Fish Res. 51:27–34.
- Tuset VM, González JA, Lozano IJ, García-Díaz MM. 2004. Age and growth of the blacktail comber, Serranus atricauda (Serranidae), off the Canary Islands (centraleastern Atlantic). Bull Mar Sci. 74:53–68.
- Yao JL, Chen YF, Chen F, He DK. 2009. Age and growth of an endemic Tibetan fish, Schizothorax o’connori, in the Yarlung Tsangpo River. J Freshwater Ecol. 24:343–345.
- Zhao LH, Wang SH, Zhao TQ. 1975. The age and growth of Gymnocypris przewalskii przewalskii (Kessler). In: Institute of Biology of Qinghai Province, editor. The fish fauna of Qinghai Lake region and biology of Gymnocypris przewalskii przewalskii (Kessler). Beijing: Science Press; p. 37–45 (in Chinese).