ABSTRACT
The primordial anlage of mandibular salivary gland was seen at 2 cm CVRL (37th day) whereas primary ducts were first observed at 11.5 cm CVRL (80th day), in the form of cords. However, the capsule formation around the gland was initiated at 21.1 cm CVRL (121st day). The lobulation was appeared at 15.3 cm CVRL (97th day). The duct system was completed at 21.1 cm CVRL (121st day). At 18.6 cm CVRL (112nd day), the terminal tubules (TT) attained the structure of the acini. The myoepithelial cells were first appeared as flattened basal cells initially around the developing acinar cells at 28.5 cm CVRL (138th day). The typical compound tubulo-acinar nature of the gland was first observed at 21.2 cm CVRL (122nd day). The parenchyma showed predominantly mucous type of cells from 28.3 cm CVRL (137th day) onwards, while initial appearance of serous demilunes was also observed at this stage. In neonatal age groups, the mandibular gland was of compound tubulo-acinar nature with a well-defined capsule. Localization of acidic as well as neutral mucopolysaccharides was observed in mucous cells and goblet cells in prenatal and neonatal age groups. Fine lipid droplets were observed in intralobular as well as interlobular connective tissue, however, phospholipids were observed in the cell membrane of secretory cells and ducts.
1. Introduction
The salivary glands play an important role in the ruminants and are classified as major and minor. The mandibular gland, one of the major salivary glands, contributes to substantial amount of saliva secreted into the mouth. Ruminant saliva is an isotonic, bicarbonate phosphate buffer with high pH. This well-buffered solution is necessary for neutralizing acids formed by fermentation in the rumen and to maintain the acid–base equilibrium of the ruminal contents. During mastication, the saliva is mixed with food, aiding in the formation of the bolus and acting as lubricant during swallowing.
The mandibular salivary gland has multifaceted dimension in digestion, as it provide lubrication for eating and supply saliva for pH buffering (Moghaddam et al. Citation2009). The salivary secretion contains water, various enzymes, mucopolysaccharides and lubricating glycoproteins. In general, the major salivary glands of the herbivores are better developed than those of the carnivores. Saliva is secreted into the oral cavity via a series of ducts in the ductal system. Dysfunction of salivary secretion (hyposalivation) causes xerostomia (dry mouth) and sequentially leads to severe dental caries as well as oral mucosal disorders (Featherstone Citation2000). This paucity and the precious role of mandibular salivary gland in digestion prompted to study the histogenesis which may be serving as a tool in future research on stem cell analysis of primordia of salivary gland. The study of prenatal development is prerequisite to understand the normal developmental biology of an organ. The documentation of normal foetal growth can serve as a guide for understanding the consequence of harmful influences at various stages of gestation.
Various prenatal as well as postnatal studies have been done on this gland in cat (Knospe & Bohme Citation1995), human (Chi Citation1996) and pig (Pospieszny et al. Citation2010) however, there is no detailed information about the histogenesis of mandibular salivary gland of buffalo during prenatal as well as neonatal life, therefore, the present work was undertaken with an aim to highlight the ontogenetic events in histogenesis of mandibular salivary gland of foetal and neonatal buffalo.
2. Materials and methods
The present study was conducted on mandibular salivary gland of 36 buffalo foetuses, during different stages of prenatal development, as well as six neonates. Immediately after collection, the foetuses were measured for their curved crown rump length (CVRL) in centimetres with a calibrated inelastic thread. The approximate age of foetuses was calculated by using the following formula given by Soliman (Citation1975) in buffalo:
where Y is age in day(s) and X is curved CVRL in centimetres. Depending upon CVRL, foetuses were divided into three groups with a minimum of 12 samples in each group:
Immediately after measuring CVRL, the foetuses as well as head of neonates were fixed in 10% neutral buffered formalin (NBF). Group IV comprised of buffalo neonates and their age was estimated by dentition. After gross observations, tissue samples were collected from the mandibular salivary gland of buffalo foetuses and neonates, and fixed in 10% NBF for 48 h. After fixation, the tissue samples were processed for paraffin sectioning (5–6 μm thickness) and frozen sectioning (10–12 µm thickness) techniques. These sections were stained with Haematoxylin and Eosin for general histomorphology (Luna Citation1968), Masson’s trichrome stain for collagen fibres (Luna Citation1968), Gridley’s method for reticular fibres (RF) (Sheehan & Hrapchak Citation1973), Verhoeff’s method for elastic fibres (Sheehan & Hrapchak Citation1973), Holme’s method for nervous tissue (Luna Citation1968), Alcian blue stain (at pH 1.0 and 2.5) for acidic mucopolysaccharides (Luna Citation1968), Periodic Acid Schiff’s (PAS) method for neutral mucopolysaccharides (Luna Citation1968), Combined PAS-Alcian Blue method for mucosubstances (Luna Citation1968), Colloidal iron method for acidic mucopolysaccharides (Luna Citation1968), Mayer’s mucicarmine method for mucin (Luna Citation1968), Best’s carmine stain for glycogen (Luna Citation1968), Bromphenol blue method for basic proteins (Chayen et al. Citation1969), Sudan black ‘B’ method for lipids (Luna Citation1968), Oil-red-o method for lipids (Luna Citation1968) and Acid haematin method for phospholipids (Chayen et al. Citation1969). Lipids and phospholipids were demonstrated on fresh cryostat sections. The diameter of mucous acini as well as serous acini, diameter of intercalated ducts, striated ducts and large excretory ducts of mandibular salivary gland of buffalo foetuses and neonates, was measured and the data were tabulated and statistically analysed.
3. Results and discussion
The primordial anlage of mandibular salivary gland was first noticed as a solid epithelial bud from the oral epithelium, at the base of the tongue in linguo-gingival space at 2 cm CVRL (37th day). The glandular mass was composed of undifferentiating basophilic epithelial cells that sprouted from the linguo-gingival lamina. The epithelial bud was attached to oral epithelium by a single epithelial stalk. These findings were in agreement with the description of Arey (Citation1965) in humans and Young and Van Lennep (Citation1978), Latshaw (Citation1987) and McGeady et al. (Citation2006) in domestic animals.
At 2.5 cm CVRL (40th day), the tip of the epithelial bud had migrated into the surrounding mesenchyme as a solid club shaped structure with groups of primordial cells. The surrounding mesenchyme was dense and eosinophilic and the formation and deepening of epithelial cleft was observed at this stage. At 3 cm CVRL (42nd day), the glandular mass began to branch into several terminal bulb like structures that is, terminal epithelial buds and were surrounded by large amount of mesenchymal tissue (MT).
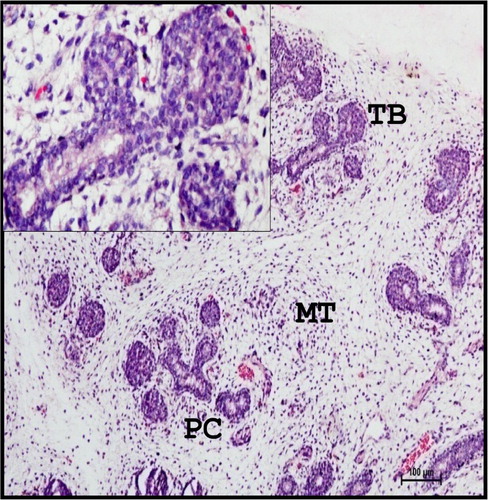
The gland was formed by groups of luminized terminal buds and primary cords with loose mesenchyme at 11.5 cm CVRL (80th day) (). The cell clusters of terminal buds were formed by multilayered polyhedral cells with deep basophilic cytoplasm and darkly stained spherical nuclei. Differentiation of intercalated and striated ducts was observed at 11.5 cm CVRL (80th day). These observations were in accordance with the findings of Latshaw (Citation1987) in domestic animals and Mohandes et al. (Citation1987) in human beings.
Figure 1. Photomicrograph of 11.5 cm CVRL (80th day) buffalo foetus showing groups of luminized, multilayered TB and primary cords (PC) with loose mesenchymal tissue (MT) in mandibular gland. Haematoxylin and Eosin method ×100.
The terminal buds (TB) and primary ducts were weak positive for neutral mucopolysaccharides at same stage of gestation.
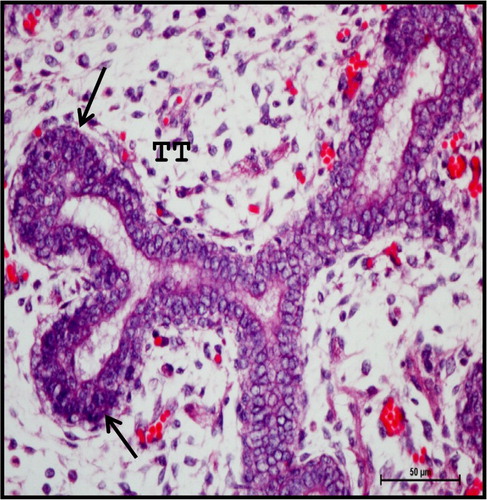
Extensive branching of TT with rich vascular network was seen at 13.2 cm CVRL (88th day) (). Most of the TT were lined with two to three layered epithelium with central lumen. The branching pattern of the gland was dichotomotous (). The differentiation of embryonic MT into stroma was observed first in foetal mandibular gland at this stage. The ducts and acini differentiated themselves into primitive lobules, separated by stroma with collagen fibres. The lobules of the gland were reported to be formed at 60th day in human beings (Velasco et al. Citation1993) and 20th day of prenatal development in cat (Knospe & Bohme Citation1995).
Figure 2. Photomicrograph of 13.2 cm CVRL (88th day) buffalo foetus showing extensive branching of TT with rich vascular network (arrows) in mandibular gland. (PD-primary duct). Haematoxylin and Eosin method ×100.
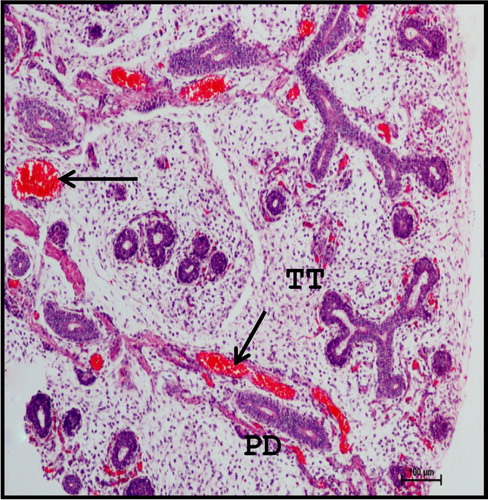
Figure 3. Photomicrograph of mandibular salivary gland of 13.2 cm CVRL (88th day) buffalo foetus showing two to three layered epithelium lining (arrows) of the TT with central lumen. Haematoxylin and Eosin method ×400.
At 15.3 cm CVRL (97th day), the lobulation of the gland was distinct. The intercalated ducts and striated ducts were lined by a double layered epithelium with inner low columnar or cuboidal cells and outer flattened cells.
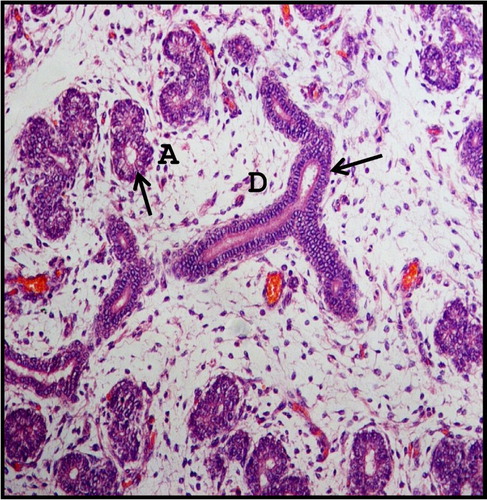
The TT attained the structure of acini at 18.6 cm CVRL (112nd day), in which the lining epithelium was changed to single layer. The cytoplasm of the acinar cells was lightly basophilic and the nucleus was spherical and situated at the basal end of the cells ().
Figure 4. Photomicrograph of 18.6 cm CVRL (112th day) buffalo foetus showing single layer epithelial lining (arrows) of acinar cells (A) and ducts (D) of mandibular gland. Haematoxylin and Eosin method ×200.
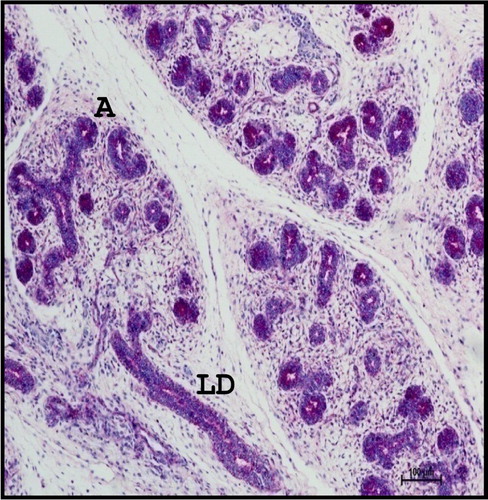
Dense lobulation of the gland with rapid increase in the number of lobules was observed from 21.2 cm CVRL (122nd day) onwards. Rich vascularity of the lobes and lobules was also noticed. The typical compound tubulo-acinar nature of the gland was attained first at 21.2 cm CVRL (122nd day) of foetal life. The capsule formation was evident first at this stage. The parenchyma of the gland was predominant of acinar cells. All types of ducts, that is, intercalated, striated and interlobular ducts were observed in the developing mandibular gland from 21.2 cm CVRL (122nd day) onwards. The compound tubulo-acinar nature of the gland was also reported by Trautmann and Fiebiger (Citation1957), Frandson and Spurgeon (Citation1992), Banks (Citation1993) and Dellmann and Eurell (Citation1998) in adult domestic animals. However, a fully developed capsule around the mandibular gland was reported to be observed at 85th day of the gestation in human beings by Holsinger and Bui (Citation2007). Cutler and Chaudhry (Citation1973) stated that the differentiation of striated and intercalated ducts and TB could occur at the time of the birth in rat. Contrary to this, Mohandes et al. (Citation1987) reported that the intercalated and striated ducts were distinguished at 16th week in human beings. The septa formation was evident first at this stage of gestation. From the capsule, septa of connective tissue traversed by vessels, nerves and ducts penetrated and divided and subdivided the parenchyma into lobes, lobules and acini, which was in conformity with findings of Narasimhan et al. (Citation1999) in goat.
The acinar cells and large excretory ducts with goblet cells were moderate to strong positive for acidic as well as neutral mucopolysaccharides at this stage of prenatal life ( and ). Similar findings were reported in human beings (Mohandes et al. Citation1987) and rat (Cutler & Chaudhry Citation1973; Jayasinghe et al. Citation1990). Combined PAS-AB method showed the presence of both acidic and neutral mucopolysaccharides in acinar cells and goblet cells, whereas moderate to strong Alcianophilic reaction was observed in connective tissue. Mucinous substances were found in acinar cells; however, these were not observed in ducts.
Figure 5. Photomicrograph of mandibular salivary gland of 21.2 cm CVRL (122nd day) buffalo foetus showing acinar cells (A) and large excretory ducts (LD) with goblet cells (arrows) were moderate to strong positive for acidic mucopolysaccharides. Alcian Blue 2.5 method ×100.
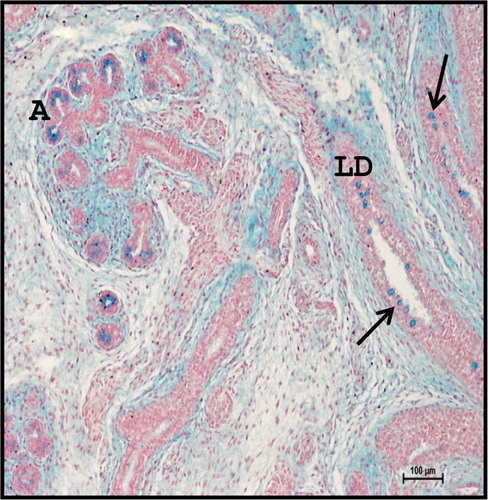
Figure 6. Photomicrograph of 21.2 cm CVRL (122nd day) buffalo foetus showing localization of neutral mucopolysaccharides in acinar cells (A) and large ducts (LD) of mandibular gland. Periodic Acid Schiff’s method ×100.
From 28.3 cm CVRL (137th day) onwards, the gland was predominantly of mucous type (). The cells of the mucous acini were pyramidal in shape with distinct cell borders and basement membrane. The cytoplasm was lightly basophilic and showed darkly stained flattened nucleus situated against the basal margin of the cell. Initial appearance of serous demilunes was also observed at this stage. The cells of serous acini were pyramidal in shape with spherical and darkly stained nuclei. The cytoplasm of the serous acinar cells was eosinophilic and their nuclei were situated in the basal half of the cells. Fine RF were seen around the lobules of the gland (). The parenchyma of the gland contained non-glandular constituents such as lymphocytes (), whereas ganglion cells were found in the capsule of the gland. Striated ducts were lined with double layered cuboidal epithelium and were closer nearest to the secretory end pieces (). Double layered striated ducts composed of inner and outer cuboidal cells were also evident at this stage of gestation. The mature striated ducts were lined by a layer of tall inner columnar cells. The inner cells lining the striated ducts showed intense eosinophilic cytoplasm and darkly stained nuclei. These ducts showed a well-developed adventitia with abundant vasculature.
Figure 7. Photomicrograph of mandibular salivary gland of 28.3 cm CVRL (137th day) buffalo foetus showing predominantly mucous acinar cells (M) and less serous cells (S) in parenchyma of the gland. (D-duct). [Inset: Mucous acinar cells]. Haematoxylin and Eosin method ×100.
![Figure 7. Photomicrograph of mandibular salivary gland of 28.3 cm CVRL (137th day) buffalo foetus showing predominantly mucous acinar cells (M) and less serous cells (S) in parenchyma of the gland. (D-duct). [Inset: Mucous acinar cells]. Haematoxylin and Eosin method ×100.](/cms/asset/4e1e7457-7276-4ac7-a4f6-934c9868286f/taar_a_1195392_f0007_c.jpg)
Figure 8. Photomicrograph of mandibular salivary gland of 28.3 cm CVRL (137th day) buffalo foetus showing fine reticular fibres (RF) around the lobules. (M-mucous acini; D-duct). Gridley’s method ×100.
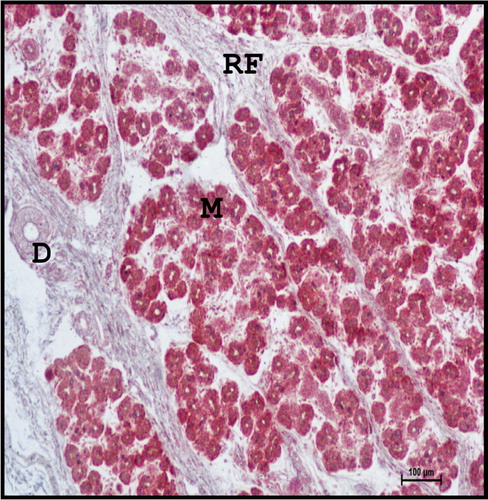
Figure 9. Photomicrograph of 28.3 cm CVRL (137th day) buffalo foetus showing the infiltration of lymphocytes (arrows) in the parenchyma of mandibular gland. (M-mucous acini; D-duct). Haematoxylin and Eosin method ×100.
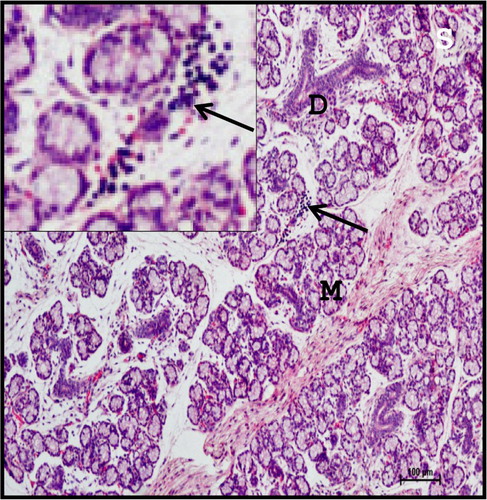
Figure 10. Photomicrograph of mandibular salivary gland of 28.3 cm CVRL (137th day) buffalo foetus showing double layered cuboidal epithelium (arrow) of striated ducts (STD). (M-mucous cell; C-capsule). Haematoxylin and Eosin method ×400.
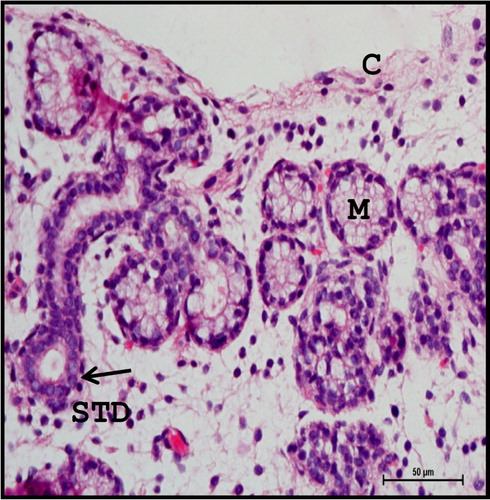
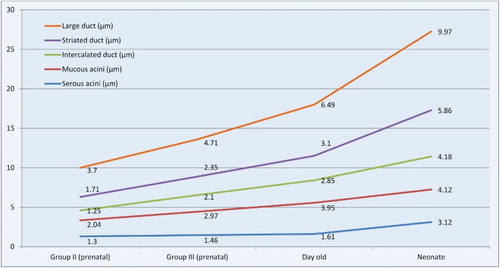
The mean diameter of serous acini, mucous acini, intercalated duct, striated duct and large duct of mandibular gland of Group II foetuses was 1.30 ± 0.1, 2.04 ± 0.1, 1.25 ± 0.1, 1.71 ± 0.1 and 3.70 ± 0.5 µm, respectively ().
Table 1. Mean diameter of acinar cells, intercalated ducts, striated ducts and large ducts of mandibular salivary gland of buffalo foetuses and neonates.
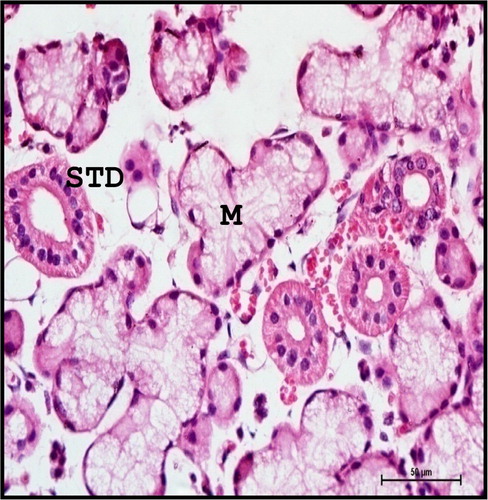
At 40.5 cm CVRL (165th day), the groups of mature intralobular ducts were lined with stratified cuboidal or columnar epithelium with abundant goblet cells (). The typical tree-like branching pattern of gland was evident at this stage (). The connective tissue was well developed around the groups of double layered intralobular ducts.
Figure 11. Photomicrograph of 40.5 cm CVRL (165th day) buffalo foetus showing stratified cuboidal epithelium (arrow) of intralobular ducts (ILD) of mandibular gland. (M-mucous cell). Haematoxylin and Eosin method ×400.
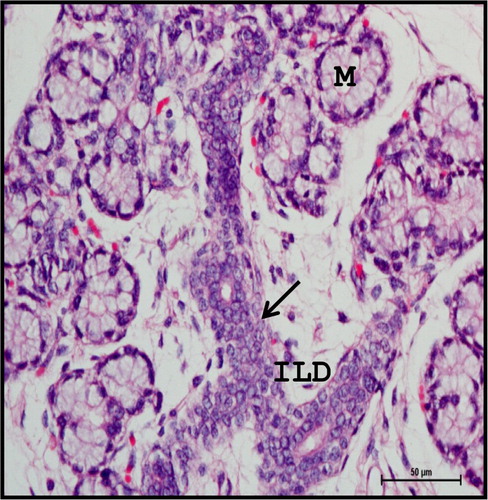
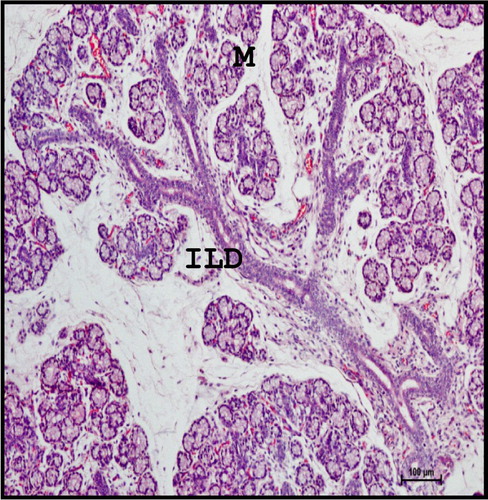
Figure 12. Photomicrograph of mandibular salivary gland of 40.5 cm CVRL (165th day) buffalo foetus showing typical tree-like branching pattern with well-developed intralobular ducts (ILD) (M-mucous cell). Haematoxylin and Eosin method×100.
At this stage, sulphomucins were not localized in mucous cells while goblet cells were moderate to strong positive for sulphomucins (), which was in agreement with the reports of Mohandes et al. (Citation1987) in human beings. Mucous acinar cells as well as large ducts with goblet cells showed strong positive reaction to acidic mucopolysaccharides () as well as neutral mucopolysaccharides (); however, neutral mucopolysaccharide content was not observed in intralobular ducts. Similar observations were recorded by Suzuki et al. (Citation1983) in bovines. Combined PAS-AB method revealed the presence of acidic and neutral mucopolysaccharides in mucous acinar cells, while strong Alcianophilic reaction was observed in connective tissue (). Mucous acinar cells were strong positive for mucinous substances.
Figure 13. Photomicrograph of 40.5 cm CVRL (165th day) buffalo foetus showing moderate to strong reaction for sulphomucins in goblet cells (arrows), whereas mucous cells (M) of mandibular gland were weak positive. Alcian Blue 1 method ×100.
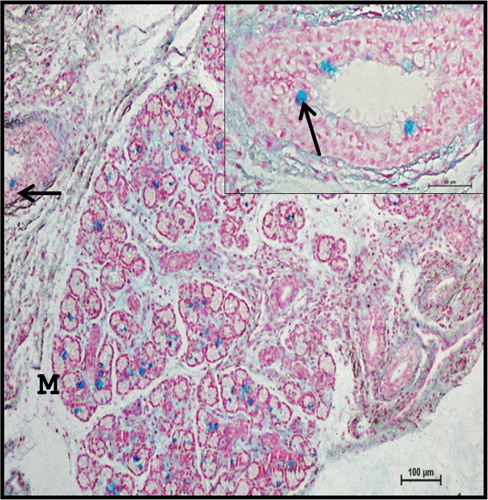
Figure 14. Photomicrograph of 40.5 cm CVRL (165th day) buffalo foetus showing localization of acidic mucopolysaccharides in goblet cells (arrows) of mandibular gland. (M-mucous cell). Alcian Blue 2.5 method ×40.
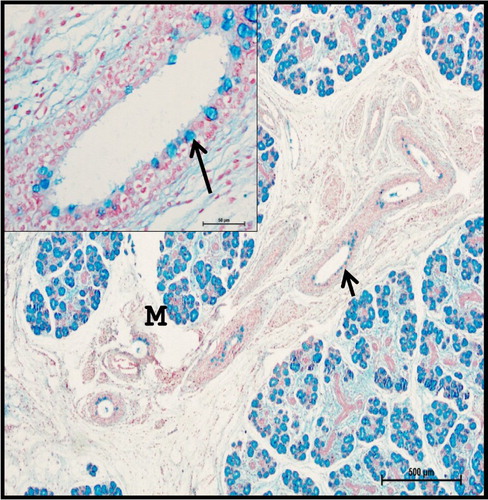
Figure 15. Photomicrograph of 40.5 cm CVRL (165th day) buffalo foetus showing goblet cells (arrows) of mandibular gland were strong positive to neutral mucopolysaccharides. (M-mucous cell). Periodic Acid Schiff’s method ×100.
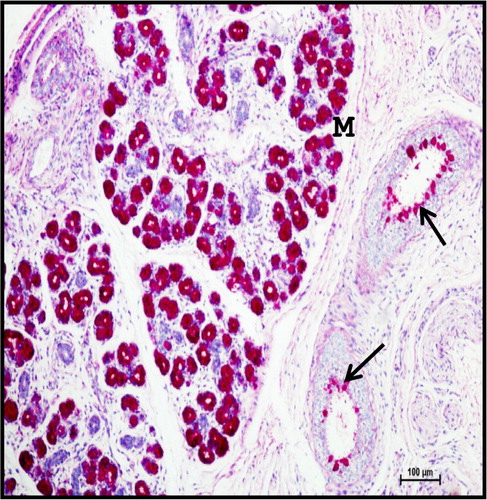
Figure 16. Photomicrograph of mandibular salivary gland of 40.5 cm CVRL (165th day) buffalo foetus showing strong mixed reaction in mucous acinar cells (M). (D-duct). Periodic Acid Schiff’s -Alcian Blue method ×100.
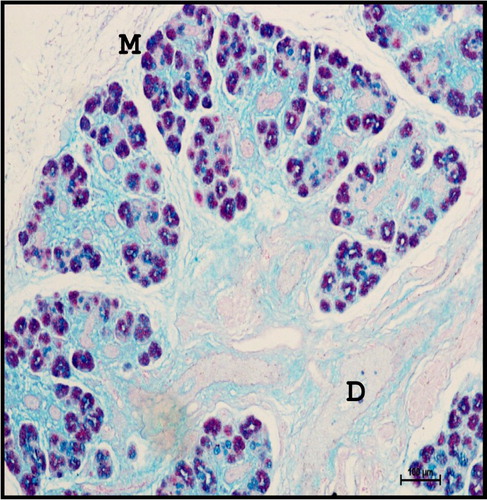
Fine Sudanophilic and oil-red-o positive lipid droplets were observed in the interlobular spaces ( and ). The presence of phospholipids was observed in the cell membrane of secretory cells as well as ducts of the gland ().
Figure 17. Photomicrograph of 40.5 cm CVRL (165th day) buffalo foetus showing presence of fine lipid droplets (arrows) in the interlobular spaces of mandibular gland. (M-mucous cell; D-duct). Sudan Black B method ×100.
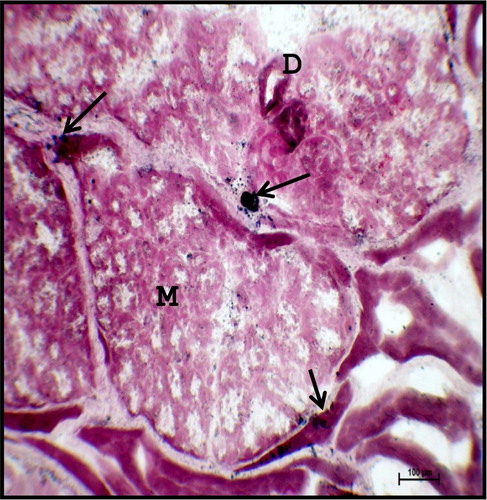
Figure 18. Photomicrograph of mandibular salivary gland of 40.5 cm CVRL (165th day) buffalo foetus showing lipid droplets (arrows) in the interlobular septa. (M-mucous cell; D-duct). Oil-red-o method ×100.
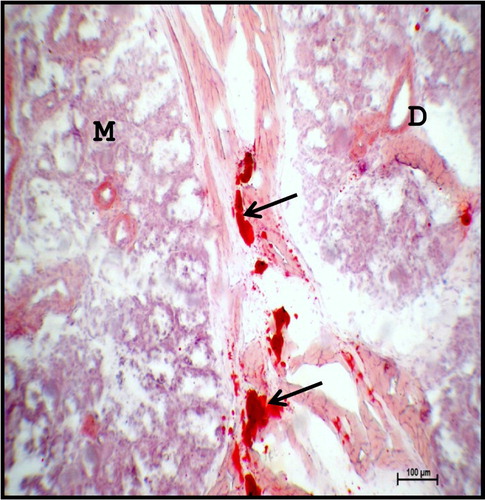
Figure 19. Photomicrograph of 40.5 cm CVRL (165th day) buffalo foetus showing the presence of phospholipids (arrow) in the cell membrane of acinar cells (A) and ducts (D) of the mandibular gland. Acid Haematin method ×40.
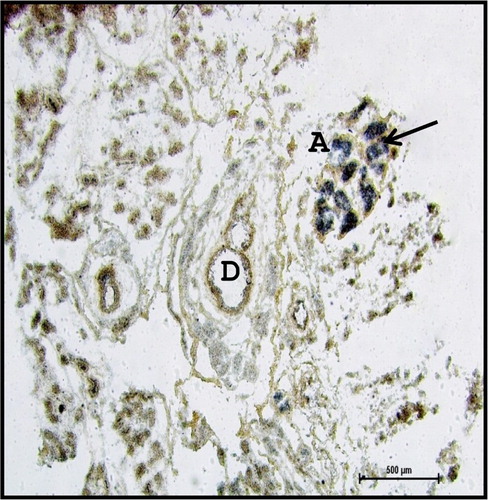
Large amount of collagen fibres and few elastic fibres () were observed at 53.5 cm CVRL (194th day). The connective tissue septae were traversed by several blood vessels (BV), nerves and ducts. These observations were agreed with the findings of the Knospe and Bohme (Citation1995) in cat.
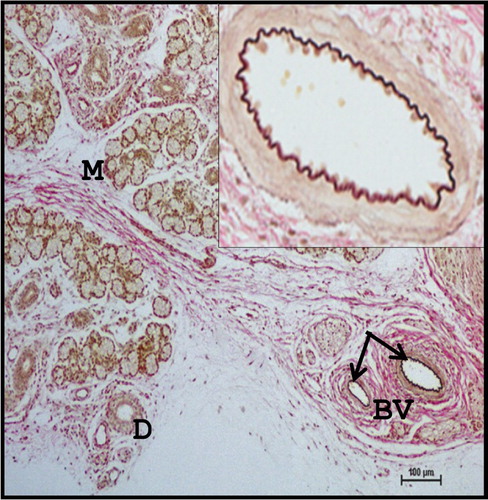
Figure 20. Photomicrograph of 53.5 cm CVRL (194th day) buffalo foetus showing blood vessels having elastic fibres (arrows), around the periphery of the lobes and lobules of mandibular gland. (M-mucous cell; D-duct). Verhoeff’s method ×100.
In Group III foetuses, the mean diameter of serous acini, mucous acini, intercalated duct, striated duct and large duct was 1.46 ± 0.1, 2.97 ± 0.2, 2.10 ± 0.2, 2.35 ± 0.1 and 4.71 ± 0.6 µm, respectively ().
In neonatal buffalo, the mandibular salivary gland was of compound tubulo-acinar type with a well-defined capsule. From the capsule, septal fibres traversed the parenchyma to form lobes and lobules. Nerves, BV and ganglion cells and several order of ducts appeared in the connective tissue stroma. From the ganglion plexus, delicate nerve fibrils penetrated the stroma and innervated the basement membranes of acini and ducts. The nerve endings were found in close relationship with the basement membrane of acinar cells and ducts as quoted by Nagato and Tandler (Citation1986) in avian salivary gland. The stromal content was made of collagen fibres and RF with few elastic fibres. These findings were in total agreement with the observations in farm animals by Banks (Citation1993), Narasimhan et al. (Citation1999) and Dellmann and Brown (Citation1987).
In day-old buffalo, the mean diameter of serous acini, mucous acini, intercalated duct, striated duct and large duct of mandibular gland was 1.61 ± 0.1, 3.95 ± 0.1, 2.85 ± 0.1, 3.10 ± 0.1 and 6.49 ± 0.8 µm, respectively ().
The parenchyma of the mandibular gland is composed of mixed acini with more number of mucous acini and few serous acini and serous demilunes along with several orders of ducts distributed in the stroma. These findings were in total agreement as reported in buffalo calves (Venkatakrishnan & Mariappa Citation1969) and mammals (Young & Van Lennep Citation1978). The mucous acini were surrounded by crescent-shaped serous cells forming serous demilunes. The mucous acini were composed of five to six pyramidal cells enclosing a narrow indistinct lumen. The nuclei were flattened and located in the basal part of the cytoplasm. The mucous acini appeared vacoulated due to mucin dissolved during tissue processing. Purely serous acini were few and smaller in size and were composed of two to three pyramidal cells with spherical nuclei and acidophilic granulated cytoplasm ().
Figure 21. Photomicrograph of mandibular salivary gland of neonatal buffalo showing mucous acini (M) having narrow indistinct lumen. The nucleus is flattened and located in the basal part of the cytoplasm. Haematoxylin and Eosin method ×1000.
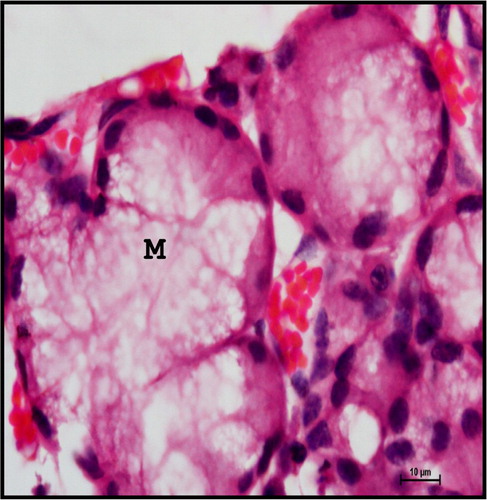
Stellate-shaped myoepithelial cells with cytoplasmic process were observed around the base of mucous and serous acinar cells. The myoepithelial cells were scattered around the acini as well as the intercalated and striated ducts (). They were dark basophilic in nature. The myoepithelial cells lining the acini were stellate shape with long cytoplasmic processes found between the basal lamina of the cell and basement membrane of the acini. The myoepithelial cells lining the duct epithelium appeared spindle shaped with few cytoplasmic processes. These findings were in accordance with Banks (Citation1993) in domestic animals, Kishore et al. (Citation1998) in goat, Pedini et al. (Citation2008) in sheep and Elewa et al. (Citation2010) in sheep and goat mandibular salivary gland.
Figure 22. Photomicrograph of neonatal buffalo showing dark basophilic myoepithelial cell (arrow) lining the striated duct (STD) of the mandibular gland. Haematoxylin and Eosin method ×1000.
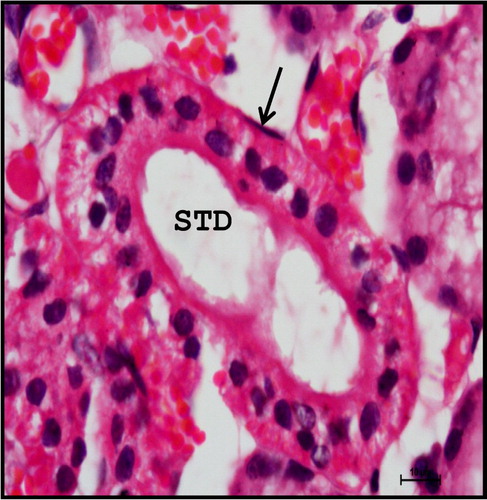
The duct system of mandibular gland is composed of intercalated duct, striated duct, interlobular duct and large excretory duct. The intercalated duct was lined by cuboidal to low columnar epithelium into which the acinar cells drained. The intercalated duct opened into large striated ducts within the lobules. The striated ducts seen within the lobule and periphery of the lobule were lined by simple columnar epithelium. The basal striations which stained basophilic were characteristic of the striated ducts (). The striated duct opened into interlobular ducts and several interlobular ducts opened into large excretory duct. The larger ducts situated in the stromal tissue within the lobule and in between the lobes were lined by pseudostratified columnar epithelium. The goblet cells intermingled between the dark and light cells of the lining epithelium and basal cells were found underlying between them. The main excretory duct was lined by stratified squamous epithelium.
Figure 23. Photomicrograph of neonatal buffalo showing striated ducts (STD) having characteristic basal striations in mandibular gland. (M-mucous cell). Haematoxylin and Eosin method ×400.
With the advancement of age, the acini as well as striated and excretory ducts seemed to have increased in size. Similar observation was reported by Latshaw (Citation1987) in domestic animals who stated that differentiation of the secretory elements occurred in later foetal age and continued postnatally.
In neonatal buffalo, acidic mucopolysaccharides were not localized in the serous cells and stroma of the gland. The mucous as well as goblet cells showed strong to intense positive reaction for acidic mucopolysaccharides and neutral mucopolysaccharides; however, serous cells and demilunes were devoid of these carbohydrates. Combined technique of PAS-AB showed acidic and neutral mucins were localized in acinar cells and large ducts. This was in accordance with the findings of Yamada and Shimizu (Citation1976) in porcine, Garrett and Kidd (Citation1977) in cat, Reid et al. (Citation1978) in bovine and Mohammadpour (Citation2011) in molar salivary glands of cat. In contrary to this finding, Kishore et al. (Citation1998) stated that acidic mucins were not present in the secretory cells and demilunes of goat mandibular gland. Mucous secretory acini showed strong to intense positive reaction for mucinous substances. Mucous acinar cells showed strong positive reaction for acidic mucopolysaccharides by colloidal iron method (). Moderate protein activity was observed in capsule and striated ducts.
Figure 24. Photomicrograph of mandibular salivary gland of neonatal buffalo showing mucous cells (M) were strong positive for acidic mucopolysaccharides. (D-duct). Colloidal Iron method ×100.
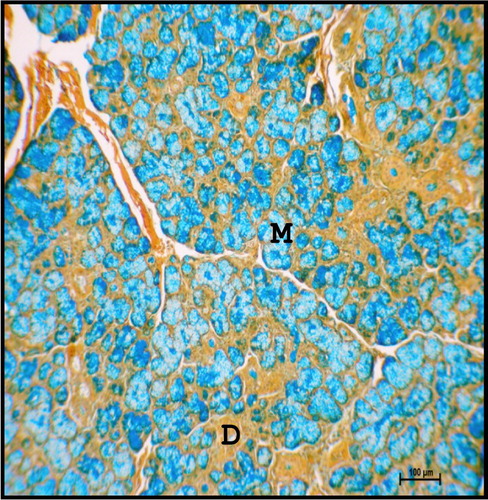
Large fat cells were identified in the septae of neonatal buffalo. The presence of lipid droplets was more in neonates as evidenced by oil-red-o staining. This was supported by Suzuki et al. (Citation1983) in bovines and Narasimhan et al. (Citation1999) in goat. As age increased, more fat droplets were deposited in the interlobular connective tissue septa in buffalo.
The mean diameter of serous acini, mucous acini, intercalated duct, striated duct and large excretory duct of neonatal buffalo was 3.12 ± 0.1, 4.12 ± 0.2, 4.18 ± 0.1, 5.86 ± 0.1 and 9.97 ± 0.9 µm, respectively ().
The mean values of micrometrical parameters of mandibular gland varied significantly between different groups at p ≤ .05 and p ≤ .01 level (). These findings were in total agreement with the reports of Kishore et al. (Citation1998) in goat.
4. Conclusions
It may be concluded that the primordial anlage of mandibular salivary gland was seen at 2 cm CVRL (37th day), whereas primary ducts were first observed at 11.5 cm CVRL (80th day). Lobulation of gland was appeared at 15.3 cm CVRL (97th day); however, the duct system was completed at 21.1 cm CVRL (121st day). At 18.6 cm CVRL (112nd day), the TT attained the structure of the acini. Myoepithelial cells were first appeared as flattened basal cells initially around the developing acinar cells at 28.5 cm CVRL (138th day). The parenchyma of the gland showed predominantly mucous type of cells from 28.3 cm CVRL (137th day) onwards. In neonatal age groups, the mandibular gland was of compound tubulo-acinar nature with a well-defined capsule. The mucous acini were surrounded by crescent-shaped serous cells known as serous demilunes. Localization of acidic as well as neutral mucopolysaccharides was observed in mucous cells and goblet cells of mandibular gland in prenatal and neonatal age groups. Fine lipid droplets were observed in intralobular as well as interlobular connective tissue; however, phospholipids were observed in the cell membrane of secretory cells and ducts of mandibular salivary gland.
Acknowledgements
The authors would like to thank Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana for providing all type of necessary facilities to carry out the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arey LB. 1965. Developmental anatomy: a text book and laboratory manual of embryology. Philadelphia (PA): W. B. Saunders Co; p. 221–222.
- Banks WJ. 1993. Applied veterinary histology. 3rd ed. St. Louis, MO: Mosby-Year Book; p. 277–296.
- Chayen J, Bitensky L, Butcher RG, Poulter LW. 1969. A guide to practical histochemistry. Edinburgh: Oliver and Boyol; p. 83–174.
- Chi JG. 1996. Prenatal development of human major salivary glands. Histological and immunohistochemical characteristics with reference to adult and neoplastic salivary glands. J Korean Med Sci. 11:203–216. doi: 10.3346/jkms.1996.11.3.203
- Cutler LS, Chaudhry AP. 1973. Release and restoration of the secretory granules in convoluted granular tubules of the rat submaxillary gland. Anat Rec. 176:405–419. doi: 10.1002/ar.1091760405
- Dellmann HD, Brown BM. 1987. Dellmann’s Textbook of veterinary histology. 3rd ed. Philadelphia (PA): Lee and Febiger; p. 209–253.
- Dellmann HD, Eurell JA. 1998. Digestive system. In: Dellmann’s Text book of veterinary histology. Eurell JA, Dellmann HD, editors. 5th ed. London: William and Wilkins; p. 174–176.
- Elewa YH, Bareedy MH, Abudatta AA, Ichii O, Otsuka S, Kanazawa T, Lee S, Hashimoto Y, Kon Y. 2010. Structural characteristics of goat (Capra hircus) parotid salivary glands. J Vet Res. 58:121–135.
- Featherstone JD. 2000. The science and practice of caries prevention. J Am Dent Assoc. 131:887–899. doi: 10.14219/jada.archive.2000.0307
- Frandson RD, Spurgeon TL. 1992. Anatomy and physiology of farm animals. 5th ed. Philadelphia (PA): Lee and Feibiger; p. 270–280.
- Garrett JR, Kidd A. 1977. Acid phosphatase and peroxidase in resting acinar cells of the major salivary glands of cat and their possible movement into secretory granules. In: Garrett JR, Stowards PJ, editors. Histochemistry of secretory process. London: Chapman and Hall; p. 227–242.
- Holsinger FC, Bui DT. 2007. Salivary glands disorders. Anat Funct Eval Salivary Glands. 1:1–16.
- Jayasinghe NR, Cope GH, Jacob S. 1990. Morphometric studies on the development and sexual dimorphism of the submandibular gland of the mouse. J Anat. 172:115–127.
- Kishore PVS, Sundararao KV, Gopinath S. 1998. Histological and histochemical studies on the parotid salivary gland of goat (Capra hircus). Indian J Vet Anat. 10:88–89.
- Knospe C, Bohme G. 1995. Prenatal development of the mandibular gland and parotid gland in cats. Anat Histol Embryol. 24:1–6. doi: 10.1111/j.1439-0264.1995.tb00001.x
- Latshaw WK. 1987. Salivary gland. In: Vetereinary developmental anatomy. Toronto; p. 90–93.
- Luna LG. 1968. Manual of histologic staining methods of the armed forces institute of pathology. 3rd ed. New York (NY): McGraw Hill Book Co.
- McGeady TA, Quinn PJ, Fitz Patrick ES, Ryan MT. 2006. Text book of veterinary Embryology. Iowa: Blackwell Publishing; p. 278–280.
- Moghaddam YF, Darvish J, Mahdavi SN, Abdulamir AS, Mousavi M, Daud SK. 2009. Comparative histological and histochemical inter-species investigation of mammalian submandibular salivary glands. Res J Appl Sci. 4:50–56.
- Mohammadpour AA. 2011. Histochemical characteristics of cat molar salivary gland. Indian Vet J. 88:89–90.
- Mohandes EA, Botros MQ, Bondok AA. 1987. Prenatal development of the human submandibular gland. Acta Anat. 130:213–218. doi: 10.1159/000146446
- Nagato T, Tandler B. 1986. Ultrastructure of the angularis oris salivary gland in the house sparrow. J Anat. 145:143–154.
- Narasimhan E, Gopinath S, Rao KVS, Chandrasekhararao TS. 1999. Histological studies on the lingual glands of the goat (Capra hircus). Indian J Vet Anat. 11:115–118.
- Pedini V, Dall’Aglio C, Mercati F, Pascucci L, Scocco P. 2008. Glyccocojugates in sheep buccal glands investigated by conventional and lectin histochemistry. J Appl Anim Res. 34:49–54. doi: 10.1080/09712119.2008.9706939
- Pospieszny N, Kuryszko J, Juszczyk M, Adamski M. 2010. Morphological and histological analysis of the mandibular gland and sublingual glands in prenatal period of the pig. B Vet I Pulawy. 54:351–355.
- Reid PE, Culling CFA, Dunn WL. 1978. A histochemical method for the identification of 9-o-acyl sialic acids. An investigation of bovine submaxillary gland and intestinal mucins. J Histochem Cyto. 26:187–192. doi: 10.1177/26.3.632555
- Sheehan DC, Hrapchak BB. 1973. Theory and practice of histotechnology. Saint Louis (MO): The CV Mosby Company; p. 79–115.
- Soliman MK. 1975. Studies on the physiological chemistry of the allantoic and amniotic fluids of buffalo at various periods of pregnancy. Indian Vet J. 52:106–111.
- Suzuki S, Hishinagakawa SH, Otsuka J. 1983. Fine structure of bovine salivary glands. Biological Abstracts. 73:4640.
- Trautmann A, Fiebiger J. 1957. Fundamentals of the histology of domestic animals. Ithaca (NY): Constock Publishing Associates.
- Velasco JA, Montesinos I, Espin J, Garcia JD, Gomez S, Roldan V. 1993. Development of the human submandibular salivary gland. J Dent Res. 72:12–27.
- Venkatakrishnan A, Mariappa D. 1969. Studies on the structure of the salivary glands of the Indian buffalo (Bubalus bubalis). Indian Vet J. 46:768–773.
- Yamada K, Shimizu S. 1976. Concanavalin A-Peroxidase-Diaminobenzidine (ConA-PO-DAB)-Alcian Blue (AB): a reliable method for dual staining of complex carbohydrates. Histochemistry. 47:159–169. doi: 10.1007/BF00492563
- Young JA, Van Lennep EW. 1978. The morphology of salivary glands. London: Academic Press; p. 72–108.