ABSTRACT
A potential relationship between an increased risk of cancer and increased levels of serum insulin-like growth factor-1 (IGF-1), and presence of cancer and high levels of C-reactive protein (CRP) has been previously reported. This study evaluated the influence of a single intramuscular injection of plasma rich in growth factors (PRGF) on serum concentrations of IGF-1 and CRP in dogs. Two groups of eight healthy beagles were injected with two different doses of PRGF in lumbar muscles. For each treatment, IGF-1 and CRP were analysed from blood samples obtained at baseline and the following three days post injection. No differences were found when IGF-1 and CRP were compared among times in the two protocols. Local application of PRGF at clinical doses did not cause significant changes in systemic concentrations of IGF-1 or detectable inflammation.
1. Introduction
Plasma rich in growth factors (PRGF) is an endogenous therapeutic technology that is gaining interest in regenerative medicine due to its potential to stimulate and accelerate tissue healing and bone regeneration (Anitua et al. Citation2012). PRGF is being widely used for the treatment of different injuries of the locomotor system in both human and veterinary medicine (Grageda et al. Citation2005; Fahie et al. Citation2013). Some of its beneficial effects have been attributed to insulin-like growth factor-1 (IGF-1), since it is involved in the regulation of growth and metabolism, mediating many of the anabolic effects of growth hormone in different tissues (Philippou et al. Citation2007).
Regarding systemic effects of growth factors, some authors (Grimberg and Cohen Citation2000; Grimberg Citation2003; Renehan et al. Citation2004; Lim et al. Citation2015; Maniscalco et al. Citation2015) reported in human and veterinary patients positive associations between high circulating levels of IGF-1 and risk for different types of cancer, as well as an ergogenic effect, taking part of the prohibited list of substances of the World Anti-Doping Agency (WADA).
C-reactive protein (CRP) rises up to 100 times in inflammatory responses, although levels could show high variability in dogs (Christensen et al. Citation2015); CRP could also play a significant role in the detection of the secondary inflammation due to cancer development (Allin et al. Citation2009; Crossley et al. Citation2010).
Our hypothesis was that intramuscular PRGF injection into healthy muscle in dogs would not have systemic effects, as determined by measuring serum levels of IGF-1 and CRP post injection in order to exclude potential cancer risk.
2. Materials and methods
2.1. Animals
The study was conducted with two groups of eight healthy adult Beagle dogs – five males and three females in each group, with ages ranging from 3 to 4 years and weights from 10 to 18 kg. Animals were subjected to a thorough clinical examination, including a complete blood count, basic chemistry panel, and blood serology to ensure that animals were healthy.
The animals were housed individually in a controlled room during the full duration of the study; exercise was avoided. The dogs had ad libitum access to water and were fed twice a day with the same feed. The study protocol was approved by the Ethics Committee for Animal Welfare at the University CEU-Cardenal Herrera of Valencia (CEBA/2013; date of approval 7 May 2010).
2.2. Preparation of PRGF and inoculation
To prepare the PRGF, blood samples were processed under previously published protocols (Anitua Citation1999).
All dogs were injected in healthy left lumbar muscles (lumbar multifidus, dorsi lumbar, and iliocostal lumbar) at the level of the 5th lumbar vertebrae on the left side with two different doses: Treatment 1: Single dose of 1 mL PRGF with 0.05 mL 10% calcium chloride, and Treatment 2: Single dose of 3 mL PRGF (HPRGF) with 0.15 mL 10% calcium chloride.
2.3. Determination of IGF-1 and CRP concentrations
Blood samples were obtained directly from the jugular vein in aseptic conditions with a closed vacuum system (Vacutainer, BD, Spain). In order to measure IGF-1 and CRP, blood was sampled at baseline (before PRGF injection) and 1 min, 15 min, 30 min, 1 h, 6 h, 12 h, 1 day, and 3 days post injection.
Total IGF-1 was analysed by an automated immunoassay system (Immulite, Siemens, Spain). CRP was determined by human immunoturbidimetric assay (CRP OSR 6147 Olympus, Ireland), validated for use in dogs (Martinez-Subiela and Cerón Citation2005).
2.4. Statistical analysis
For the analysis of data, a linear mixed effects model was considered. Parameters in this model were estimated by using the package nlme in the R software (https://www.r-project.org/). Significance in IGF-1 and CRP levels between periods of observation was tested by analysis of variance. Post hoc comparisons between fixed effects were performed using the Tukey procedure. For assessing the validity of the model, a Shapiro–Wilk test was applied for testing normality of the residuals, and a Levene’s test was used to test homoscedasticity. The significance level was set at p < .05 for all tests.
3. Results
Weight of the animals was (mean ± SD) 14.8 kg ± 3.1 in PRGF-injected dogs and 14.9 kg ± 2.5 in HPRGF-injected dogs (p = .330 between groups). Mean, standard deviation, minimum, maximum, and confidence intervals for IGF-1 and CRP values in the PRGF and HPRGF groups are showed in and , respectively.
Table 1. Mean, standard deviation, minimum and maximum values, and inferior (Inf) and superior (Sup) 95% confidence intervals for IGF-1 serum levels (ng/mL) in PRGF- and HPRGF-injected dogs at each study period.
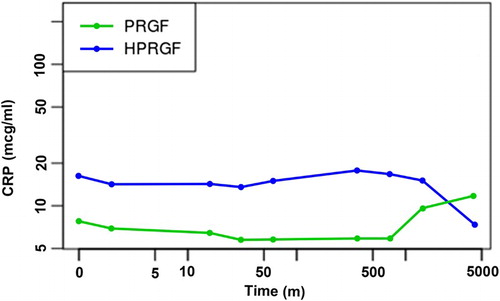
Table 2. Mean, standard deviation, minimum and maximum values, and inferior (Inf) and superior (Sup) 95% confidence intervals for CRP serum levels (µg/mL) in PRGF- and HPRGF-injected dogs at each study period.
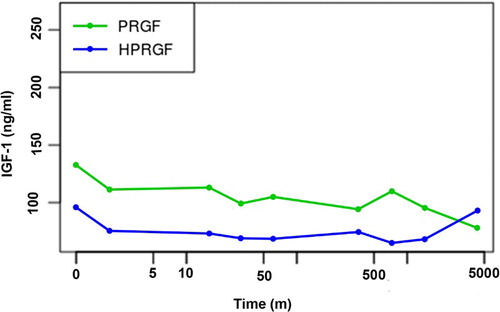
For IGF-1 (), no statistically significant differences existed between basal status (0 min) and any of the periods after inoculation of PRGF or HPRGF (p = .5282), or when PRGF and HPRGF groups were compared (p = .248). On the other hand, mean levels remained between published interquartile normal ranges (Dabrowski et al. Citation2015). The validity of the model fit was assessed by testing normality and homoscedasticity of the residuals. Both of the following assumptions could be accepted: the Shapiro–Wilk test for normality, p = .1876, and the Levene’s test for homoscedasticity, p = .2836.
Figure 1. Evolution of IGF-1 levels in PRGF- and HPRGF-injected dogs at various time periods (logarithmic scale) up to three days post injection.
As occurred with IGF-1, CRP () was not statistically different between baseline and any of the study periods (p = .05). Between PRGF and HPRGF, differences were also not significant (p = .093). In addition, levels remained between published normal ranges for this breed Otabe et al. Citation1998). Normality and homoscedasticity were also confirmed (Shapiro–Wilk, p = .095 and Levene’s test, p = .2131).
4. Discussion
Some authors have indicated a potential carcinogenic effect of high levels of IGF-1 in humans (Grimberg Citation2003). In veterinary medicine, two recent studies demonstrated IGF-1 receptor expression and its role in canine osteosarcoma (Maniscalco et al. Citation2015) and mammary gland carcinoma (Lim et al. Citation2015). However, in our study, a single intramuscular PRGF or HPRGF injection at clinical doses did not result in an increase in circulating IGF-1 levels in any of the test periods. This could be because most of the PRP systems do not concentrate IGF-1 (Schippinger et al. Citation2011); in addition, IGF-1 in plasma has a low half-life (20 h) in humans (Grahnen et al. Citation1993) and, although we could not find published data, in dogs should be similar. Our results differ from those in a previous study, in which serum levels of IGF-1 increased for 48 h after a PRP-derivate injection, although their product contained a high number of leukocytes (Wasterlain et al. Citation2013).
There were no significant differences in CRP levels between groups and no variations in levels over time, indicating that the administration of PRGF did not cause any inflammation. Some authors have reported an inflammatory response, but such a response could be due to the presence of white blood cells in the preparation (Grahnen et al. Citation1993). For our PRGF protocol, we completely discarded the plasma fraction containing white blood cells.
PRGF was injected only once, which could be enough to help repair small muscular tears. In contrast, larger tears or osteoarthritis could require two or three applications of PRGF (Anitua et al. Citation2014). Theoretically, the outcome might change when PRGF is used repeatedly. However, taking into account that the HPRGF used in this study contained three times the dose of PRGF and that no significant changes in serum IGF-1 were found, two or three single applications separated in time likely would not alter IGF-1 serum levels.
5. Conclusion
No significant changes in systemic concentrations of IGF-1 and CRP after intramuscular application of PRGF were found, and then cannot produce cancer or inflammation in dogs.
Acknowledgements
The authors thank Thomas Oxlee and Misty Bailey for language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allin KH, Bojesen SE, Nordestgaard BG. 2009. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 27:2217–2224. doi: 10.1200/JCO.2008.19.8440
- Anitua E. 1999. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 14:529–535.
- Anitua E, Alkhraisat MH, Orive G. 2012. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J Control Release. 157:29–38. doi: 10.1016/j.jconrel.2011.07.004
- Anitua E, Sánchez M, Aguirre JJ, Prado R, Padilla S, Orive G. 2014. Efficacy and safety of plasma rich in growth factors intra-articular infiltrations in the treatment of knee osteoarthritis. Arthroscopy. 30:1006–1017. doi: 10.1016/j.arthro.2014.05.021
- Christensen MB, Eriksen T, Kjelgaard M. 2015. C-reactive protein: quantitative marker of surgical trauma and post-surgical complications in dogs: a systematic review. Acta Vet Scand. 57:1–10. doi: 10.1080/03461238.2015.1020856
- Crossley R, Coloma A, Ríos C, Gonzalez C. 2010. Determinación de proteína C-reactiva en hembras caninas con tumores mamarios benignos y malignos. Arch Med Vet. 42:101–105. doi: 10.4067/S0301-732X2010000100014
- Dabrowski R, Szczubial M, Kostro K, Wawron W, Ceron JJ, Tvarijonaviciute A. 2015. Serum insulin-like growth factor-1 and C-reactive protein concentrations before and after ovariohysterectomy in bitches with pyometra. Theriogenology. 83:474–477. doi: 10.1016/j.theriogenology.2014.09.024
- Fahie MA, Ortolano GA, Guercio V, Schaffer JA, Johnston G, Au J, Hettlich BA, Phillips T, Allen MJ, Bertone AL. 2013. A randomized controlled trial of the efficacy of autologous platelet therapy for the treatment of osteoarthritis in dogs. J Am Vet Med A. 243:1291–1297. doi: 10.2460/javma.243.9.1291
- Grageda E, Lozada JL, Boyne PJ, Caplanis N, McMillan PJ. 2005. Bone formation in the maxillary sinus by using platelet-rich plasma: an experimental study in sheep. J Oral Implantol. 31:2–17. doi: 10.1563/0-692.1
- Grahnen A, Kastrup K, Heinrich U, Gourmelen M, Preece MA, Vaccarello MA, Guevara-Aguirre J, Rosenfeld RG, Sietnieks A. 1993. Pharmacokinetics of recombinant human insulin-like growth factor I given subcutaneously to healthy volunteers and to patients with growth hormone receptor deficiency. Acta Paediatr. 82:9–13. doi: 10.1111/j.1651-2227.1993.tb12918.x
- Grimberg A. 2003. Mechanisms by which IGF-I may promote cancer. Cancer Biol Ther. 2:628–633. doi: 10.4161/cbt.2.6.678
- Grimberg A, Cohen P. 2000. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J
- Lim HY, Im KS, Kim NH, Kim HW, Shin JI, Yhee JY, Sur JH. 2015. Effects of obesity and obesity-related molecules on canine mammary gland tumors. Vet Pathol. 52:1045–1051. doi: 10.1177/0300985815579994
- Maniscalco L, Iussich S, Morello E, Martano M, Gattino F, Miretti S, Biolatti B, Accornero P, Martignani E, Sánchez-Céspedes R, et al. 2015. Increased expression of insulin-like growth factor-1 receptor is correlated with worse survival in canine appendicular osteosarcoma. Vet J. 205:272–280. doi: 10.1016/j.tvjl.2014.09.005
- Martinez-Subiela S, Cerón JJ. 2005. Analytical validation of commercial assays for the determination of haptoglobin, C-reactive protein and serum amyloid in dogs. Arch Med Vet. 37:61–66. doi: 10.4067/S0301-732X2005000100009
- Otabe K, Sugimoto T, Jinbo T, Honda M, Kitao S, Hayashi S, Shimizu M, Yamamoto S. 1998. Physiological levels of C-reactive protein in normal canine sera. Vet Res Comm. 22:77–85. doi: 10.1023/A:1006071211779
- Philippou A, Maridaki M, Halapas A and Koutsilieris M. 2007. The role of the insulin-like growth factor 1 (IGF-1) in skeletal muscle physiology. Vivo. 21:45–54.
- Renehan AG, Zwahlen M, Minder C, O’dwye ST, Shalet SM, Egger M. 2004. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3
- Schippinger G, Oettl K, Fankhauser F, Spirk S, Domej W, Hofmann P. 2011. Influence of intramuscular application of autologous conditioned plasma on systemic circulating IGF-1. J Sports Sci Med. 10:439–444.
- Wasterlain AS, Braun HJ, Harris AHS, Kim HJ, Dragoo JL. 2013. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 41:186–193. doi: 10.1177/0363546512466383