ABSTRACT
The present study was designed to find the effect of an antibiotic, an organic acid and a probiotic on performance traits, blood biochemical parameters and antioxidant status during the starter phase exposed to Salmonella typhimurium challenge. A total of 300 day-old broiler chicks were randomly allocated to control (basal diet), T1: infected with Salmonella enteric subsp. Typhimurium; T2: infected + avilamycin; T3: infected + organic acid; T4: infected + Bacillus subtilis; T5: infected + organic acid + probiotic. The results showed that body weight, feed conversion ratio and production efficiency factor did not differ (P > .05) between the control and treated groups. Blood albumin and aspartate aminotransferase increased significantly (P < .05) in birds in T5 during the first week. Similarly, total protein and triglyceride concentration increased significantly (P < .05) in T4 and T5. The total antioxidant capacity in the second week decreased significantly in T4 compared to the control. Thiobarbituric acid reactive substances during the first and second weeks did not differ significantly (P > .05) between the control and treated groups. We concluded that the effect of organic acid blend and B. subtilis was similar to that of the antibiotic in broilers during the starter phase exposed to S. typhimurium challenge.
1. Introduction
Salmonellosis is one of the most frequent zoonotic disease caused by the bacterium Salmonella having more than 2500 serotypes (Calenge et al. Citation2010). Some of the Salmonella stereotypes can attack domestic animals, including poultry, and can cause severe clinical signs ranging from gastroenteritis to death. This bacterium can also be easily transferred to humans through the consumption of contaminated eggs and meat and causes food poisoning (Calenge et al. Citation2010; Bajpai et al. Citation2012). The Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) stressed on the need of effective interventions to reduce the incidence of Salmonellsis in broilers (FAO & WHO Citation2009). In poultry, a number of efforts have been undertaken to control the disease such as prophylactic measures, vaccination, the use of antibiotics and others (Calenge et al. Citation2010; Bajpai et al. Citation2012; Abudabos et al. Citation2015); however, fruitful results have not been achieved.
Antimicrobial growth promoters (AGPs) have been used in animal production for the last few decades to improve the growth and reduce mortality. The use of AGPs in controlling Salmonella infection is successful to a large extent; however, acquired resistance of these antimicrobial agents is a major concern (Bajpai et al. Citation2012; Dhama et al., Citation2015) and has been phased out by the European Union (Kabploy et al. Citation2016). Despite the ban, many countries in the world, including the USA, recommends the use of avilamycin in poultry at sub-therapeutic doses (Kabploy et al. Citation2016). Some studies have shown that the use of AGPs failed to reverse the number of pathogenic bacteria and failed to improve the performance of animals (La-ongkhum et al. Citation2011; Kabploy et al. Citation2016).
As a result of these circumstances, the poultry researchers are focusing on the alternative to antibiotics to optimize gut health and improve animal performance (Wu et al. Citation2011; Chand et al., Citation2016; Khan et al., Citation2016; Raza et al., Citation2016). One such alternative possessing growth-promoting characteristics is organic acid, which can maintain gut health and improve animal performance by balancing normal gut flora (Sultan et al. Citation2015). Organic acids and their derivatives are considered safe and increase the profitability by providing healthy meat to the consumers (Hayat et al. Citation2014). In addition, many researchers have documented that the use of Bacillus subtilis as a probiotic plays an essential role in animal performance and health by adjusting the intestinal ecological imbalance (Abudabos et al. Citation2013; Khan & Naz Citation2013). Bacillus species have been considered safe in animal production and can be easily administered to animals as an oral dose for weight gain and feed efficiency (Wu et al. Citation2011; Nguyen et al. Citation2015).
The objective of the present study was to assess the effectiveness of the Bacillus bacteria and an organic acid blend in comparison with the standard antimicrobial drug, avilamycin, in broilers exposed to experimentally induced Salmonella typhimurium challenge during the starter phase.
2. Materials and methods
This experiment was approved by the Departmental Board of Studies on Ethics, Methodology and Welfare, King Saud University, Kingdom of Saudi Arabia.
2.1. Experimental design and management of birds
A total of 300 day-old broiler chicks (Ross 308) were weighed and randomly allocated to six treatments. Each group was further divided into five replicates having eight birds per replicate. On arrival, all chicks were confirmed for the absence of Salmonella spp. The experiment was conducted in an environmentally controlled experimental station where the temperature was maintained at 35 ± 0.5°C during the first week and 25 ± 0.3°C in the following week. A typical starter diet (1–14 days) consisting of isocaloric and isonitrogenous contents was offered in mash form as recommended by the National Research Council (NRC Citation1994). The ingredients and composition of feed are presented in . Upon arrival, the chicks were randomly distributed to one of the six treatments as follows: Control (basal diet); T1: infected with Salmonella enteric subsp. Typhimurium; T2: infected + avilamycin at the rate of 0.2 g/kg (Maxus, Vienna, Austria); T3: infected + organic acid blend containing phenolic compounds stimulating the release of butyrate, medium chain fatty acids and organic acids (Presan, Trouw Nutrition, Ireland); T4: infected + consisting of a probiotic having viable spores (2 × 107 CFU/g) of B. subtilis (ATCC PTA-6737) (Clostat, Kemin Industries, Inc., Des Moines, IA, USA); T5: infected + Maxus + Presan.
Table 1. Dietary composition of broiler chicks during starter.
2.2. Challenge inoculum
In this experiment, the chicks were challenged with S. enteric subsp. typhimurium (MicroBiologics, St. Cloud, MN, USA) as described by Abudabos and Al-Mufarrej (Citation2014). The strain was known for efficient colonization of the gut of broilers. The viability of the bacteria was confirmed before and after the inoculation. Briefly, stored at −80°C, the bacteria were retrieved and plated twice on tryptone soy agar for 24 h at 37°C. A single colony of the bacteria was transferred into sterile prewarmed tryptone soy broth and incubated at 37°C for 18 h. The challenge inoculum was diluted and adjusted to 3 × 109 CFU/ml. Count of viable bacteria was confirmed before and after inoculation.
2.3. Performance measurements
Feed intake (FI) on a daily basis was calculated in the post-infection period by subtracting the amount of feed rejected from the feed offered. Total FI was computed for each group at the end of the first and second weeks. FI and weight gain for each group were subjected to adjustment in case of mortality of birds. Feed conversion ratio (FCR) was computed for each group by using the following formula: FCR = FI/weight gain.
Production efficiency factor (PEF) as suggested by Griffin (Citation1979) was determined as follows:
PEF = (liveability × live weight (kg)/(age in days × FCR) × 100.
2.4. Biochemical measurements of blood
At the end of the first and second weeks, two blood samples (3 ml) were obtained from the wing vein of the bird per replicate and centrifuged at 3000 rpm for 10 min. Serum was harvested and then transferred into clean plastic tubes and stored at −20°C until analysis. Serum total antioxidant capacity (TAC) and thiobarbituric acid reactive substances (TBARS) were measured by using ELISA kits (Cayman Chemical Company, MI, USA). Total protein, albumin, glucose, alanine transaminase (ALT) and aspartate aminotransferase (AST) were measured by using enzymatic calorimetric kits (M di Europa GmbH Wittekamp 30, Hannover, Germany). All the analyses were carried out in duplicate.
All statistical analyses was performed using the Statistical Analysis System (SAS Citation2003). The overall level of statistical significance was set at P < .05. All values were expressed as statistical means ± standard error of the mean (SEM).
3. Results
3.1. Performance traits
The findings of FI, body weight (BW), FCR and PEF during the first and second weeks are presented in and . The results showed that the performance traits did not change significantly (P > .05) during the experiment. In infected birds (T1), the FI and corresponding weight gain, FCR and PEF decreased numerically. It is further important to note that the performance traits were not significantly different in antibiotic-treated (T2) and other AGPs.
Table 2. The effect of treatments on FI, BW, FCR and PEF of broiler chickens at the end of the first week.
Table 3. The effect of treatments on FI, body weight gain (BWG), FCR and PEF of broiler chickens at the end of the second week.
3.2. Blood biochemical parameters
The effect of treatments on the blood biochemical parameters in broiler during the first week are presented in . The result revealed that blood albumin and AST increased significantly (P < .05) in birds in T5. Blood glucose, protein, globulin, triglyceride and ALT concentration did not differ significantly (P > .05) between the control and treated birds. Similarly, the results of blood biochemical parameters in observed during the second week showed that total protein and triglyceride concentration increased significantly (P < .05) in T4 and T5, respectively. However, no significant changes (P > .05) were observed in glucose, total protein, globulin, AST and ALT concentrations between the control and treated groups.
Table 4. Effect of treatments on blood biochemical parameters and liver enzymes of broiler chickens at the end of the first week given experimental diets.
Table 5. Effect of treatment on blood parameters and liver enzymes of broiler chickens at the end of the second week given experimental diets.
3.3. Serum antioxidant status
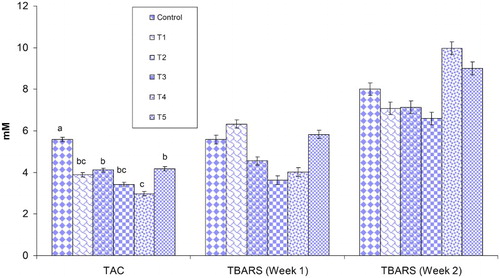
The antioxidant status in the form of TAC (at the end of the second week) and TBAR during the first and second weeks is shown in . The result showed that TAC decreased significantly in T4 compared to the control. However, no significant change (P > .05) was observed in TBAR during the first and second weeks.
Figure 1. Mean TAC at the end of the second week, and TBARS concentration in blood of control and treated groups during the first and second weeks of the experiment. Different superscripts in a column differ significantly (P < .05); T1: Infected; T2: Infected + Maxus; T3: Infected + Presan; T4: Infected + Clostat; T5: Infected + Presan + Clostat.
4. Discussion
The current trend in poultry is to increase the application of non-antibiotic feed additives to improve the growth and feed utilization after banning the use of antibiotics in the feed of animals (Abduabos et al. Citation2015). The use of antibiotics as a feed additive under the sub-therapeutic level has been found to be useful in poultry production; however, a ban has been imposed on their use due to the possible antibiotic resistance and the presence of their residues in meat and milk products. Due to a big push to search for alternatives, several feed additives such as acidifiers, antioxidants, phytogenes, probiotics and prebiotics have been used successfully in poultry production.
In the present study, we found that the addition of an organic acid blend and a probiotic was as effective as the addition of an antibiotic in terms of growth performance and feed utilization, suggesting their possible role as potential alternatives. An improved growth performance and feed utilization fed with probiotics in comparison with antibiotics in broilers challenged with different pathogenic bacteria such as Salmonella, Clostridium perfringens and Escherichia coli have been reported recently (Abudabos et al. Citation2013, Citation2015; Al-Owaimer et al. Citation2014). However, the comparison of an organic acid blend with antibiotic alone or in combination with a probiotic has probably been reported for the first time in this study. The improved performance in birds treated with probiotic bacteria has been attributed to the mechanism of action through which the probiotic bacteria exert their action such as lowering the pH of the gut, suppression of pathogenic bacteria through the production of organic acids, prevention of the colonization of the bacteria through competitive exclusion, production of antibacterial mucin, stimulating the immune system, production of antibacterial enzymes (β-glucosidase and β-glucuronidase), competition for nutrients in the gut and others (Khan & Naz Citation2013). An improved performance in broilers by the addition of organic acid without any bacterial challenge has also been previously reported (Sultan et al. Citation2015). It has been suggested that the addition of organic acid significantly reduced the population of Salmonella by the production of acidic environment in the gut that favours the lowering of pH and arrests the growth and proliferation of harmful bacteria but favours the growth of beneficial bacteria (Samanata et al. Citation2010; Sultan et al. Citation2015). In addition, acidic environment favours the production and secretion of pepsin, gastrin and cholecystokinin, which play a significant role in the nutrient utilization and subsequent growth performance and feed efficiency (Hayat et al. Citation2014).
In the present study, blood albumin and AST concentration increased significantly in T5 (organic acid + probiotic) during the first week, with no significant difference in the other metabolites. No significant effect on blood metabolites, particularly glucose, in birds fed with organic acid has been previously reported (Mahdavi & Torki Citation2009; Adil et al. Citation2010). In the previous studies, an increase in serum protein and albumin has been reported in probiotic-treated birds (Arslan & Saatci Citation2004; Yesilbag & Colpan Citation2006; Li et al. Citation2011; Rajput et al. Citation2013). We suggest that the increased protein, especially albumin, may be due to the fact that an acidic environment in the gut stimulates peptide-2 (Tappenden & McBurney Citation1998), which may result in better absorption of protein in the gut. A significant increase in AST during the first week and triglyceride in the second week in T5 was observed in the present study. A reduction in the concentration of triglyceride was documented in response to the supplementation of Saccharomyces boulardii and B. subtilis in broilers (Rajput et al., Citation2013). No significant difference in triglyceride was observed in broilers in response to B. subtilis and organic acid in birds in the previously documented reports (Adil et al., Citation2010; Li et al., Citation2011). The discrepancy may be due to the genetic, dose and duration of the agents and experimental conditions.
The TAC reflects accurately the antioxidant status of the organism; therefore, different antioxidant components in serum of the birds were not measured since it is neither possible nor practical. The TAC precisely measures the antioxidant status of any organism (Erel Citation2004). The body usually keeps a balance between the production of free radicals and antioxidants; however, under stress conditions, the balance shifts towards free radicals and oxidative stress is developed, which may harm cellular machinery, enzymes and DNA and protein (Abudabos & Al-Mufarrej Citation2014). In the present study, no significant effect was observed by the treatments on TBARS concentration during the two weeks, however, at the end of the study, TAC decreased significantly in the infected group and infected groups treated with organic acid and probiotic. It is noteworthy that the birds recovered from the infection stress when the organic acid and probiotics were combined (T5), suggesting the synergistic effect of both the entities. Further, an improved TAC concentration was also observed in the birds treated with the antibiotic. Recently the anti-oxidative effect of avilamycin has been linked to its scavenging effect on hydroxyl radicals (Kabploy et al. Citation2016). Improved TAC was also reported in another study in broilers simultaneously challenged with S. enterica subsp. enterica typhimurium and an organic acid (Abudabos & Al-Mufarrej Citation2014) and/or in response to S. boulardii and B. subtilis B10 (Rajput et al. Citation2013). The probiotic bacteria resist oxidation process, scavenge free radical and enhance the antioxidant potential of the organism (Capcarova et al. Citation2010).
5. Conclusion
The authors concluded that probiotic and organic acid could be used successfully in comparison to an antibiotic in maintaining the growth and biochemical profile of broilers challenged with S. typhimurium.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abudabos AM, Al-Batshan HA, Murshed MA. 2015. Effects of prebiotics and probiotics on the performance and bacterial colonization of broiler chickens. South Afr J Anim Sci. 45:419–428. doi: 10.4314/sajas.v45i4.8
- Abudabos AM, Al-Mufarrej SI. 2014. Effects of organic acid supplementation on antioxidant capacity and immune responses of broilers challenged orally with Salmonella enterica subsp. enterica typhimurium. South Afr J Anim Sci. 44:342–349. doi: 10.4314/sajas.v44i4.4
- Abudabos AM, Alyemni AH, Al Marshad MBA. 2013. Bacillus subtilis PB6 based-probiotic (CloSTATTM) improves intestinal morphology and microbiological status of broiler chickens under Clostridium perfringens challenge. Int J Agric Biol. 15:978–982.
- Adil S, Banday T, Bhat GA, Mir MS, Rehman M. 2010. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet Med Int. 7:4061–4065.
- Al-owaimer AN, Suliman GM, Alyemni AH, Abudabos AM. 2014. Effect of different probiotics on breast quality characteristics of broilers under Salmonella challenge. Italian J Anim Sci. 13:450–454. doi: 10.4081/ijas.2014.3189
- Arslan C, Saatci M. 2004. Effects of probiotic administration either as feed additive or by drinking water on performance and blood parameters of Japanese quail. Arch Geflügelk. 68:160–163.
- Bajpai VK, Baek K, Kang SC. 2012. Control of Salmonella in foods by using essential oils: a review. Food Res Int. 45:722–734. doi: 10.1016/j.foodres.2011.04.052
- Calenge F, Kaiser P, Vignal A, Beaumont C. 2010. Genetic control of resistance to salmonellosis and to Salmonella carrier-state in fowl: a review. Genet Select Evol. 42:11. Available from http://download.springer.com/static/pdf/304/art%253A10.1186%252F1297-9686-42-11.pdf?originUrl=http%3A%2F%2Fgsejournal.biomedcentral.com%2Farticle%2F10.1186%2F1297-9686-42-11&token2=exp=1470723210∼acl=%2Fstatic%2Fpdf%2F304%2Fart%25253A10.1186%25252F1297-9686-42-11.pdf*∼hmac=bf68ac3c3f8ec66942cc44494de53e661e6af43ae68a1dc847a783ea636ea238 doi: 10.1186/1297-9686-42-11
- Capcarova M, Weis J, Hrncar C, Kolesarova A, Pal G. 2010. Effect of lactobacillus fermentum and Enterococus faecium strains on intestinal milieu, antioxidant status and body weight of broiler chickens. J Anim Physiol Anim Nutr. 94:215–224. doi: 10.1111/j.1439-0396.2010.01010.x
- Chand N, Faheem H, Khan RU, Qureshi MS, Alhidary IA, Abudabos AM. 2016. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ Sci Pollut Res. doi:10.1007/s11356-016-6600-x.
- Dhama K, Latheef SK, Mani S, Abdul Samad H, Karthik K, Tiwari R, Khan RU, Alagawany M, Farag MR, Alam GM, et al. 2015. Multiple beneficial applications and modes of action of herbs in poultry health and production- a review. Int J Pharmacol. 11:152–176. doi: 10.3923/ijp.2015.152.176
- Erel O. 2004. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014
- Food and Agriculture Organization of the United Nations (FAO) & World Health Organization (WHO). 2009. Salmonella and campylobacter in chicken meat: meeting report. Microbiological Risk Assessment Series, 19, 1–42.
- Griffin RM. 1979. The response of cage-reared broiler cockerels to dietary supplements of nitrovin, zinc bacitracin or penicillin used singly or in paired combinations. Br Poultry Sci. 20:281–287. doi: 10.1080/00071667908416580
- Hayat TA, Sultan A, Khan RU, Khan S, Hassan ZU, Ullah R, Aziz T. 2014. Impact of organic acid on some liver and kidney function tests in japanese Quails, Coturnix coturnix japonica. Pak J Zool. 46:1179–1182.
- Kabploy K, Bunyapraphatsara N, Morales NP, Paraksa N. 2016. Effect of antibiotic growth promoters on anti-oxidative and anti-inflammatory activities in broiler chickens. Thai J Vet Med. 46:89–95.
- Khan RU, Naz S. 2013. Application of probiotics in poultry production. World Poult Sci J. 69:621–632. doi: 10.1017/S0043933913000627
- Khan RU, Naz S, Dhama K, Kathrik K, Tiwari R, Abdelrahman MM, Alhidary IA, Zahoor A. 2016. Direct-fed microbial: Beneficial applications, modes of action and prospects as a safe tool for enhancing ruminant production and safeguarding health. Int J Pharmacol. 12(3):220–231. doi: 10.3923/ijp.2016.220.231
- La-ongkhum O, Pingsungvorn N, Amornthewaphat N, Nitisinprasert S. 2011. Effect of the antibiotic avilamycin on the structure of the microbial community in the jejunal intestinal tract of broiler chickens. Poult Sci. 90:1532–1538. doi: 10.3382/ps.2010-01288
- Li WF, Rajput IR, Xu X, Li YL, Lei J, Huang Q, Wang MQ. 2011. Effects of probiotic Bacillus subtilis on laying performance, blood biochemical properties and intestinal Microflora of Shaoxing Duck. Int J Poult Sci. 10:583–589. doi: 10.3923/ijps.2011.583.589
- Mahdavi R, Torki M. 2009. Study on usage period of dietary protected butyric acid on performance, carcass characteristics, serum metabolites levels and humoral immune response of broiler chicken. J Anim Vet Adv. 8:1702–1709.
- National Research Council. 1994. Nutrient requirements of poultry. 9th ed. Washington, DC: National Academy of Sciences.
- Nguyen ATV, Nguyen DV, Tran MT, Nguyen LT, Nguyen AH, Phan TN. 2015. Isolation and characterization of Bacillus subtilis CH16 strain from chicken gastrointestinal tract for use as a feed supplement to promote weight gain in broiler. Lett Appl Microbiol. 60:580–588. doi: 10.1111/lam.12411
- Rajput IR, Li YL, Xu X, Huang Y, Zhi WC, Yu DY, Li W. 2013. Supplementary effects of Saccharomyces boulardii and Bacillus subtilis B10 on digestive enzyme activities, antioxidation capacity and blood homeostasis in broiler. Int J Agric Biol. 15:231–237.
- Raza T, Chand N, Khan RU, Shahid MS, Abudabos AM. 2016. Improving the fatty acid profile in egg yolk through the use of hempseed (Cannabis sativa), ginger (Zingiber officinale), and turmeric (Curcuma longa) in the diet of Hy-Line White Leghorns. Arch Anim Breed. 59:183–190. doi: 10.5194/aab-59-183-2016
- Samanta S, Haldar S, Ghosh TK. 2010. Comparative efficacy of an organic acid blend and bacitracin methylene disalicylate as growth promoters in broiler chickens: effects on performance, gut histology, and small intestinal milieu. Vet Med Int. 645–650. Article ID 645150.
- SAS. 2003. Statistical analysis system user’s guide: statistics version 9.1. Cary, NC: SAS Institute.
- Sultan A, Ullah T, Khan S, Khan RU. 2015. Effect of organic acid supplementation on the performance and ileal microflora of broiler during finishing period. Pak J Zool. 47:635–639.
- Tappenden KA, McBurney MI. 1998. Systemic short-chain fatty acids rapidly alter gastrointestinal structure, function, and expression of early response genes. Digest Dis Sci. 43:1526–1536. doi: 10.1023/A:1018819032620
- Wu BQ, Zhang T, Guo LQ, Lin JF. 2011. Effects of Bacillus subtilis KD1 on broiler intestinal flora. Poult Sci. 90:2493–2499. doi: 10.3382/ps.2011-01529
- Yesilbag D, Colpan I. 2006. Effects of organic acid supplemented diets on growth performance, egg production and quality and on serum parameters in laying hens. Revue Méd Vét. 5:280–284.
