ABSTRACT
The aim of this study was to evaluate the physiological responses of adult male Red Sokoto goats (n = 10; indigenous to the Guinea Savannah climate) and Sahel goats (n = 10; indigenous to the Sahel climate) at the peak of the cold-dry (CDS), hot-dry (HDS) and rainy seasons prevailing in the Guinea Savannah zone of Nigeria. Results revealed that Red Sokoto goats had significantly higher rectal temperature (RT), PCV, red blood cell (RBC), Hb, thyroxin (T4), but lower respiratory rate (RR) and total leucocyte count (TLC) than Sahel goats during the CDS. Comparison within breeds for the three seasons showed that both breeds exhibited the highest (P < .05) PR, RT, neutrophil-lymphocyte ratio (N:L), but lower mean corpuscular volume and triiodothyronine (T3) during the HDS. In addition, Red Sokoto goats showed higher (P < .05) RR and T4, but lower RBC, Hb, lymphocyte counts and T3:T4. However, Sahel goats exhibited higher (P < .05) PCV, RBC, erythrocyte osmotic fragility, but lower circulating T4. It was concluded that the cold and hot seasons exerted different physiological effects on the two breeds of goats with marked variation in RT, composition of blood cellular components, thyroid physiology and erythrocyte membrane integrity.
1. Introduction
Globally, about 80% of goat production is confined to low-income countries, particularly in tropical Africa and Asia (Morand-Fehr et al. Citation2004). In developing countries of the world, small ruminants are described as ‘village bank’, while goats are known as the poor man’s cow (Ikwuebu et al. Citation1994; Haldar & Ghosh Citation2014). Promoting small-scale livestock ownership has been reported to be a potential way of reducing poverty and improving human nutrition in rural Africa (Azzarri et al. Citation2014). The Red Sokoto and Sahel goats are important breeds of goat that are widely distributed in the West Africa subcontinent. Red Sokoto goats are indigenous to Niger Republic and the savannah region of Nigeria, while the Sahel goats are found in West Africa around the Sahel belt, south of the Sahara (Gall Citation1996). However, adaptation of Sahel goats to the Northern Guinea Savannah zone of Nigeria, mainly by institutional and commercial farms, and unregulated livestock markets has been observed in recent years (Makun et al. Citation2013).
The three distinct seasons of the Northern Guinea Savannah zone of Nigeria are: the cold-dry (CDS; harmattan), hot-dry (HDS) and rainy (RAS) seasons (Igono & Aliu Citation1982; Dzenda et al. Citation2011). These seasons have different combination of meteorological parameters, and domestic animals must adjust to the periodic changes in these parameters in order to adapt and remain productive. Similarly, these seasonal differences also affect the availability of feed since the growth of plants occur, mainly during the RAS, and the animals must be supplemented with hay and/or concentrate during the dry seasons (Scogings et al. Citation2015). High (Gaughan et al. Citation2013) or low (Zhao et al. Citation2013) ambient temperatures (ATs) as an aftermath of seasonal changes are well known to induce stress, with negative consequences on the physiology and productivity of livestock. Over the years, scientists have put intense efforts into understanding how domestic animals respond to climate stressors. However, most of these studies were conducted in developed countries with a significant amount of data generated on adaptation and the impact of environmental stress on the production, reproduction and health of animals (Scholtz et al. Citation2013). On the other hand, little is known about the adaptation of animals to the rapidly changing environmental conditions in developing countries of the arid and semiarid regions, where the stressors are different and the intensity of expected changes from one season to the other is greater (Thornton et al. Citation2007). Physiological changes in blood cellular components, free radical biology, as well as endocrine, respiratory and cardiovascular systems have been used as important parameters to evaluate the adaptation of animals to a given geographical location (Sejian et al. Citation2014; Ribeiro et al. Citation2015). This may help in the selection of animals that are capable of producing satisfactorily in harsh environments and outside the zone of thermal comfort (Ribeiro et al. Citation2015). Therefore, this study was carried out to evaluate the adaptation of Red Sokoto and Sahel bucks to the seasons of the Northern Guinea Savannah zone of Nigeria by evaluating the changes in physiological variables, haematology, erythrocyte osmotic fragility (EOF) as a biomarker of oxidative stress and serum thyroid hormones concentration.
2. Materials and methods
2.1. Experimental design and animal management
The animals were sourced from the Small Ruminant Research Programme, National Animal Production Research Institute (NAPRI), Ahmadu Bello University, Shika, Zaria, Nigeria located on latitude 110 12’ N, longitude 70 33’ E, and altitude 610 m.
Twenty apparently healthy Red Sokoto (n = 10) and Sahel (n = 10) male goats aged between 1.5 and 3.5 years, body weight ranging between 20 and 25 kg and body condition score of 2.90 ± 0.10 were used in all the seasons during the study period. Body condition was scored by the same person adopting a 6-point scale (0–5) method with 0.5 increment (Santucci et al. Citation1991). An animal of body condition score of 1.0 is extremely thin with no fat reserves and body condition score of 5.0 represents an obese animal. In each season, the animals were balanced for age, body condition score and body weight. The animals were managed intensively in a well-ventilated pen in east–west orientation with dimension of 20, 20 and 7.3 ft for length, width and height, respectively. The roof of the pen was made of galvanized metal sheets, while the floor was made of concrete. The pens had a stocking density of 20 goats. Animals in the farm were routinely screened for haemoparasites and helminths (using floatation and sedimentation tests). Only clinically healthy animals were used for the study. The animals were fed on Digitaria smutsi hay as basal diet and supplemented with concentrate ration of ground maize (20%), cotton seed cake (30%), wheat offal (40%), bone meal (5%) and salt (5%) at 300 g/head/day. The animals were provided with water ad libitum.
2.2. Meteorological parameters
The values of AT and relative humidity (RH) were collected from the Data Processing Unit, Institute of Agricultural Research, Ahmadu Bello University; Zaria is located about 6 km away from the experimental site. The AT and RH were recorded daily during the morning (08:00–10:00 h) and afternoon (13:00–16:00 h) hours in each season of study. The temperature-humidity index (THI) was used to evaluate the level of heat stress induced by the environment and was calculated using the equation reported by Ravagnolo et al. (Citation2000):where T is the ambient temperature (°C) and RH the relative humidity (%).
2.3. Determination of physiological variables
The physiological variables recorded were: rectal temperature (RT), respiratory rate (RR) and pulse rate (PR). They were measured between 9:00 and 11:00 h two times at four days interval at the peak of each season. RR was obtained by counting the movement of the right flank at the paralumbar fossa per unit time and presented as number of breaths per minute. PR was obtained by counting the pulsations felt in the femoral artery per unit of time and presented as number of beats per minute. The body temperature of the goats was determined by measuring the RT using a digital thermometer (Tro-Digitatherm, Hamburg, Germany) and presented in °C.
2.4. Blood sample collection and determination of haematological parameters
The bucks were sampled once in each season at the peak of the CDS, HDS and RAS seasons. This period coincided with the months of December, April and August, respectively. Blood sampling was done between the hours of 8 and 9 am and immediately transported (within an hour) in ice pack to the laboratory. In each season, 3 mL of blood was collected via jugular venipuncture into vacutainer tubes containing ethylene diamine tetraacetic acid (K3EDTA) for haematological analysis and determination of EOF. Another 3 mL of blood was collected in anticoagulant-free tubes and allowed to clot, followed by centrifugation at 2000 × g for 15 min. The serum was then harvested for thyroid hormones analysis.
Packed cell volume (PCV), red blood cell (RBC) count, total leucocyte count (TLC) and differential TLCs were determined as described by Dacie and Lewis (Citation1991). Haemoglobin concentration (Hb) was determined using a haemoglobin meter (XF-1C Haemoglobin meter, China). Erythrocytic indices were calculated from PCV and RBC count as described by Schalm et al. (Citation1975).
2.5. Determination of EOF
EOF was determined according to the method described by Oyewale (Citation1992) using a 1% NaCl stock solution prepared with phosphate buffer (3.22 g/L) at a pH of 7.4. The absorbance was measured at 540 nm using a spectrophotometer (Spectronic-20, Philip Harris Limited, Shenstone, England). Distilled water was used as blank to read the absorbance of supernatants in hypotonic and isotonic saline. Taking the haemoglobin concentration in the 0.0% NaCl solution as 100% haemolysis, haemolysis percentage values were calculated and their curves were drawn.
2.6. Determination of serum thyroid hormones
Thyroid hormones (total T4 and T3) were measured from serum using a commercial enzyme-linked immunoassay kit (AccuBind ELISA Microwells; Monobind Inc® USA). Assay sensitivities for total T4 and T3 were 3.2 ng/well and 0.04 ng/well, respectively. The intra- and inter-assay coefficients of variation for both T4 and T3 were less than 10%. All assays were performed in accordance with the manufacturer’s instructions.
2.7. Data analysis
The values obtained were expressed as mean (±SEM). The data were subjected to student’s t-test for comparison between breeds and repeated measure analysis of variance followed by Tukey’s post-hoc test to compare values between the three seasons. Pearson’s correlation test was used to determine the relationship between variables. The statistical package used was GraphPad Prism version 5.01 for windows (2007) from GraphPad Software, San Diego California, USA (www. graphpad.com). Values of P < .05 were considered significant.
3. Results
3.1. Meteorological parameters
Mean values of meteorological parameters in the study area are presented in . The highest mean AT (39.21°C) and temperature-humidity index (THI; 84.62) were obtained in the afternoon hours of the HDS. The THI in the morning hours was lower than the THI during the afternoon hours in all the seasons. The highest RH (77.36%) was recorded during the RAS in the morning hours. The CDS in the morning hours had the lowest THI (56.76).
Table 1. Mean values of meteorological parameters during the cold-dry (CDS), hot-dry (HDS) and rainy (RAS) seasons.
3.2. Effects of breed and season on physiological parameters
Mean (±SEM) values of RR, PR and RT in Red Sokoto and Sahel goats during the CDS, HDS and RAS are presented in –, respectively. The RR was significantly lower, while the RT was higher in Red Sokoto compared to Sahel goats during the CDS. The Sahel goats had higher (P < .05) PR compared to Red Sokoto goats during the HDS.
Figure 1. Mean (±SEM) values of respiratory rate of T4 in adult bucks of RSG and SHG during the cold-dry, hot-dry and rainy seasons (n = 10). RSG – Red Sokoto goats; SHG – Sahel goats. Bars with different alphabets are statistically significant (P < .05). x,y: between seasons, a,b between breeds.
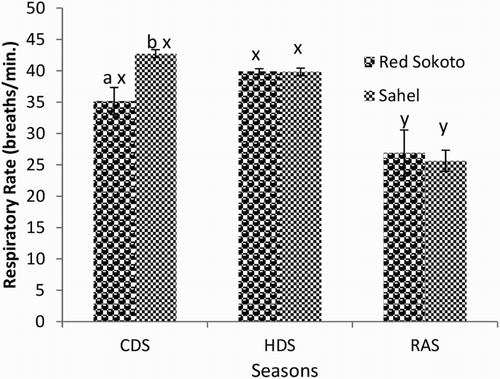
Figure 2. Mean (±SEM) values of pulse rate in bucks of RSG and SHG during the cold-dry, hot-dry and rainy seasons (n = 10). RSG – Red Sokoto goats; SHG – Sahel goats. Bars with different alphabets are statistically significant (P < .05). x,y: between seasons, a,b: between breeds.
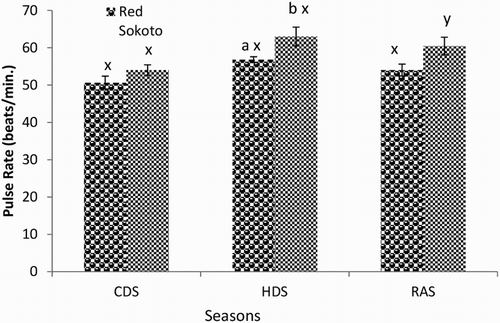
Figure 3. Mean (±SEM) values of rectal temperature in bucks of RSG and SHG during the cold-dry, hot-dry and rainy seasons (n = 10). RSG – Red Sokoto goats; SHG – Sahel goats. Bars with different alphabets are statistically significant (P < .05). x,y: between seasons, a,b between breeds.
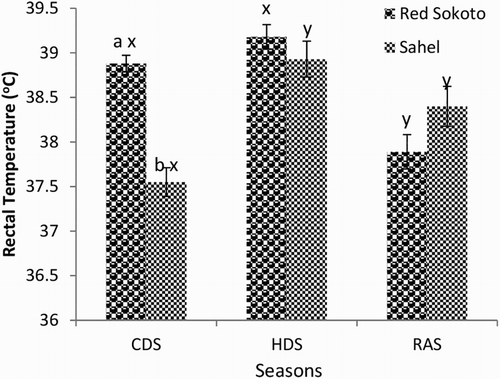
In the Red Sokoto goats, RR and RT were significantly lower in the RAS compared to the CDS and HDS, whereas the pulse was significantly higher during the HDS compared to the CDS. In Sahel goats, significantly (P < .05) higher RR, but lower RT was observed during the CDS compared to the RAS. The PR was significantly lower in the CDS compared to the HDS in Sahel goats.
3.3. Effects of breed and season on haematological parameters
Mean (±SEM) values of haematological parameters of Red Sokoto and Sahel goats during the CDS, HDS and RAS are presented in . Red Sokoto goats had significantly higher PCV, RBC and Hb than Sahel goats during the CDS. However, the MCH was significantly higher in Sahel than Red Sokoto goats during the CDS. Values of WBC in Red Sokoto goats were significantly higher during the HDS, but lower during the RAS compared to Sahel goats.
Table 2. Seasonal changes in haematological parameters of male Red Sokoto and Sahel goats.
In Red Sokoto goats, the RBC was significantly (P < .05) higher in the CDS compared to the HDS and RAS. The Hb in Red Sokoto goats was significantly lower during the HDS compared to the CDS and RAS. In HDS, however, Sahel goats had significantly higher (P < .05) PCV and RBC than CDS. The mean corpuscular volume (MCV) in Red Sokoto goats was higher (P < .05) during the RAS compared to CDS and HDS. Similarly, the MCV in Sahel goats was significantly (P < .05) higher during the RAS compared with the CDS.
Results of the leucocyte parameters in Red Sokoto goats indicated significantly higher WBC and neutrophil-lymphocytes ratio (N:L) during the HDS compared to the CDS and RAS. The Red Sokoto goats had significantly (P < .05) higher lymphocyte counts (LYMPH), but lower neutrophil (NEUT) counts in the RAS compared to the CDS and HDS.
Although, the TLC and lymphocyte counts were higher and neutrophil counts lower in Sahel goats during the HDS as compared to the CDS and RAS, the difference was not statistically significant (P > .05). However, the values of N:L of Sahel goats was significantly higher in the HDS compared to the CDS.
3.4. Effects of breed and season on EOF
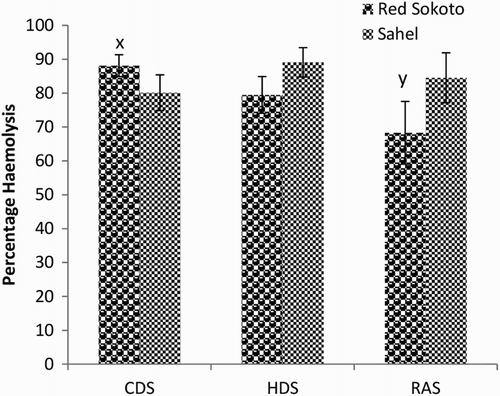
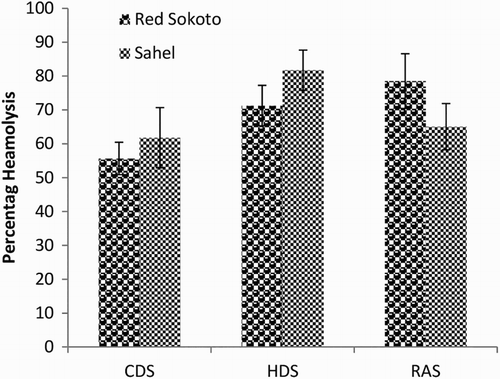
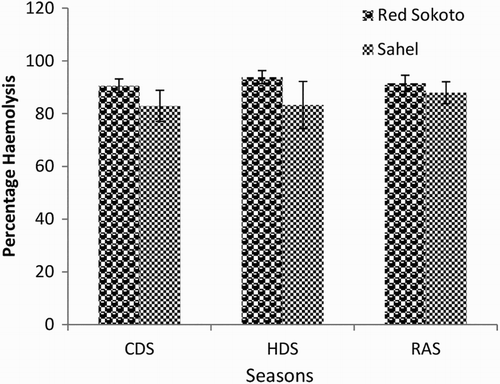
Mean (±SEM) values of EOF in male Red Sokoto and Sahel goats at 0.1, 0.3, 0.5 and 0.7% NaCl are presented in –, respectively during the CDS, HDS and RAS. Erythrocytes of Red Sokoto goats were significantly (P < .05) more fragile during the CDS compared to the RAS at 0.7% NaCL (). In Sahel goats, the percentage haemolysis was significantly higher (P < .05) in the HDS compared with the CDS at 0.3% NaCl (). There was no significant (P > .05) effect of breed on EOF.
Figure 4. Mean (±SEM) values of percentage haemolysis at 0.1% NaCl in bucks of RSG and SHG during the cold-dry, hot-dry and rainy seasons (n = 10). RSG – Red Sokoto goats; SHG – Sahel goats. Bars with different alphabets are statistically significant (P < .05). x,y: between seasons.
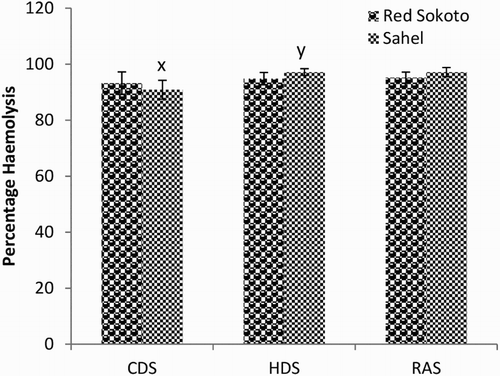
Figure 5. Mean (±SEM) values of percentage haemolysis at 0.3% NaCl in bucks of RSG and SHG during the cold-dry, hot-dry and rainy seasons (n = 10). RSG – Red Sokoto goats; SHG – Sahel goats.
3.5. Effects of breed and season on serum triiodothyronine and thyroxin
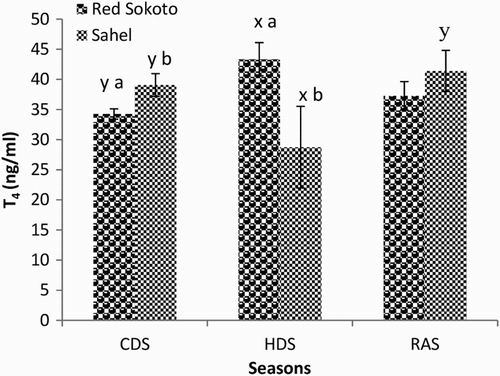
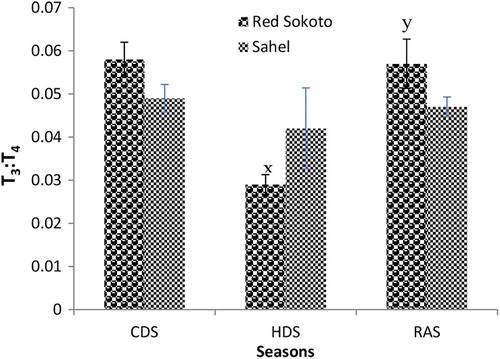
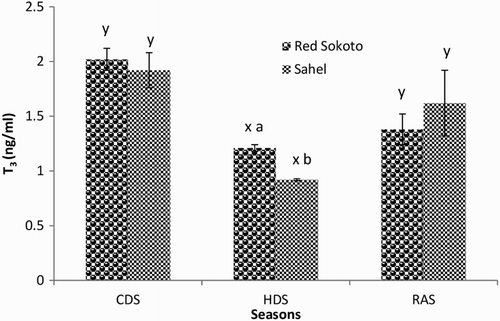
Results on the effect of breed and season on serum T3, T4 and T3:T4 in adult bucks of Red Sokoto and Sahel goats during the CDS, HDS and RAS are shown in –, respectively. Serum concentration of T3 was significantly (P < .05) higher in Red Sokoto than Sahel goats during the HDS. In the HDS, serum T4 was significantly higher (P < .05) in Red Sokoto than Sahel goats, while in the CDS, serum T4 was lower (P < .05) in Red Sokoto than Sahel goats. In both breeds, circulating T3 was significantly lower (P < .05) during the HDS compared with the CDS and RAS. On the other hand, circulating T4 was significantly higher (P < .05) during the HDS compared with the CDS in Red Sokoto goats. However, circulating T4 was significantly lower (P < .05) during the HDS compared with the RAS in Sahel goats. In Red Sokoto goats, T3:T4 was significantly lower (P < .05) in the HDS compared with the RAS. However, there was no significant breed difference (P > .05) in T3:T4 in all the seasons.
Figure 8. Mean (±SEM) serum concentration of T3 in bucks of RSG and SHG during the cold-dry, hot-dry and rainy seasons (n = 10). RSG – Red Sokoto goats; SHG – Sahel goats. Bars with different alphabets are statistically significant (P < .05). x,y: between seasons, a,b: between breeds.
3.6. Relationship between thyroid hormones and physiological parameters
Values of coefficient of correlation (r) between serum thyroid hormones and physiological variables in male Red Sokoto and Sahel goats during the CDS, HDS and RAS are presented in . Significant positive correlation (P < .05) was observed between physiological parameters (RR and RT) and thyroid hormones in Sahel goats during the CDS and RAS. There was a significantly positive correlation (r = 0.942) between T3 and RR during the CDS. Similarly, a positive correlation (P < .05) was observed between T3 and RT (r = 0.736) and T3:T4 and RT (r = 0.942) in the CDS. In the RAS, a significantly (P < .05) positive correlation was observed between T3 and RT (r = 0.736) and between T3:T4 and PR (r = 0.663). There was no significant correlation (P > .05) between thyroid hormones and physiological variables in Red Sokoto goats.
Table 3. Pearson’s correlation coefficient between physiological variables and circulating concentrations of T3, T4 and values of T3:T4 for Red Sokoto and Sahel goats.
4. Discussion
4.1. Meteorological parameters
THI has been used as a measure of the ease with which animals maintain normal body temperature in an environment. In goats, if the THI is below 65 or above 75, the animal may experience thermal stress (Hamzaoui et al. Citation2013). In the present study, the AT and THI were higher than the thermoneutral zone in the afternoon hours of the HDS and RAS, suggesting that the goats experienced heat stress in these seasons. Similarly, in the morning hours during the CDS, the THI was below the thermoneutral zone suggesting that the goats were exposed to cold stress. In the tropics, as seasons change, different breeds of animals undergo some physiological changes necessary for survival (Scholtz et al. Citation2013; Sejian et al. Citation2014). The efficiency of such changes in various physiological systems determines the adaptation of the breeds to the given environment. To the best of our knowledge, this the first study that extensively evaluates the response of Red Sokoto and Sahel breeds of goat to different seasons of the tropical Savannah region of West Africa.
4.2. Physiological variables
Breed difference was observed in RR, PR and RT during CDS and HDS. In agreement with the present study, breed difference has been reported in Indian goats with the RR, PR and RT being lower in breeds adapted to high AT and higher in breeds adapted to low AT (Banerjee et al. Citation2014). High RT in Red Sokoto compared to Sahel goats during the CDS may indicate that the Red Sokoto goats are more adapted to the CDS, which is characterized by low AT, particularly in the morning and evening hours. However, the RR was higher in Sahel compared to Red Sokoto goats during the CDS. This may be a physiological response in Sahel goats to increase oxygen delivery to the lungs, which could improve heat production and subsequently, elevate body temperature. During the cold season, high uptake of oxygen has been observed when compared with the hot season (Nishimura et al. Citation2015). The oxygen, when available, may increase non-shivering thermogenesis, via the actions of thermogenic hormones such as triiodothyronine. The PR was significantly higher in Sahel than Red Sokoto goats during the HDS, while the HDS was characterized by higher PR compared to the CDS and RAS. This may suggest increased rate of blood flow to the periphery and thus improved heat loss via sensible and insensible means (Marai et al. Citation2007; Habibu et al. Citation2014). In the Red Sokoto goats, RT was higher during the CDS and HDS compared to RAS. On the other hand, in Sahel goats, the RT was higher in HDS and RAS compared to the CDS. Since the AT was lowest during the CDS, the lower RT during the CDS in Sahel goats suggests their inability to efficiently maintain homeostasis. This inefficient thermoregulatory response may affect the survival of Sahel goats during the harmattan season in the Northern Guinea Savannah of Nigeria.
4.3. Haematological parameters
Blood cellular parameters recorded in this study were within the normal range reported in earlier studies (Jain Citation1986; Banerjee et al. Citation2014). However, during the CDS, significantly higher PCV, RBC and Hb were observed in Red Sokoto than Sahel goats. Similarly, breed difference in haematological parameters of goats during the winter season has been reported by Ribeiro et al. (Citation2015). The low erythrocyte parameters in Sahel compared to Red Sokoto goats is probably due to the lower body temperature in the Sahel goats. As animal with low body temperature do not sweat and, thus maintain blood volume (Indu et al., Citation2014; Ribeiro et al., Citation2015). In addition, decrease in RBC has been reported in animals exposed to extreme cold and has been associated with reduction in the half-life of erythrocytes (Ruiz et al. Citation2004).
Both increase and decrease in erythrocytic parameters (PCV, RBC and Hb) due to increase in AT has been reported in livestock. The decrease is associated with enhanced water intake due to heat stress, while the increase is related to elevated loss of body fluid through heat stress induced evaporative heat loss (Marai et al. Citation2007; Indu et al. Citation2014). In the present study, both reduction and a rise in values of erythrocytic parameters were observed depending on breed. The decrease in RBC and Hb in Red Sokoto goats during the HDS compared to the CDS and RAS in the present study is in agreement with the findings of Banerjee et al. (Citation2014), where RBC and Hb values in goats were lower in the hot season compared to the cold season. On the other hand, the Sahel goats had high PCV and RBC during the HDS as compared to the CDS. This agrees with the findings of other studies in goats exposed to high AT (Hashem Citation2014; Indu et al. Citation2014). This difference in heamatological response of Red Sokoto and Sahel goats is presumably due to variation in the physiological responses of goat breeds to the seasonal changes in the Guinea Savannah climate.
The MCV as an indirect measure of the average size of individual erythrocytes was lower in both breeds during the HDS in comparison to the CDS and RAS. Similarly, exposure to natural heat stress has been reported to cause an increase in MCV of Brazilian Azul goats (Ribeiro et al. Citation2015) and Piemontese cattle (Mazzullo et al. Citation2014). The decrease in MCV during the heat stress of the HDS may be due to an increase in body temperature, which causes an increase in the number of erythrocyte membrane vesicles formed and shed from the erythrocytes with a resultant decrease in the size of the parent erythrocytes (Foller et al. Citation2010; Moore et al. Citation2013). This suggests that decrease in MCV may have the potential of being used as an animal-based indicator of heat stress in tropical goats naturally exposed to heat stress. On the other hand, the increase in MCV during the RAS agrees with a study in donkeys, conducted in the same region (Zakari et al. Citation2015). This may be due to seasonal changes in the quantity and nutritional quality of availability pasture. During the RAS season, most plant species are richer in nutrients such as N and P and digestibility is high due to low amount of secondary metabolites such as tannins and polyphenols in most grasses found in the savannah regions (Martz et al. Citation2010; Basha et al. Citation2013; Scogings et al. Citation2015).
A pronounced change in leucocytic parameters was observed during the HDS probably due to heat stress. The values of WBC in Red Sokoto goats during the HDS were higher than values recorded in CDS and RAS; and also higher when compared to values in Sahel goats. The present study agrees with the finding of Omran et al. (Citation2013), who reported an increase in WBC in buffalo calves exposed to high AT. Also, the N:L was higher during the HDS compared to CDS and RAS in both breeds of goats. This is in agreement with the high N:L reported in goats subjected to transport stress (Minka et al. Citation2009), and also suggests that both breeds of goat were exposed to heat stress.
4.4. Erythrocyte osmotic fragility (EOF)
In the present study, the increase in EOF during the HDS compared to the CDS and RAS in Sahel goats may be due to heat stress (Brzezinska-Slebodzinska Citation2001; Habibu et al. Citation2014). Heat stress indicated by increase in body temperature during the HDS may have enhanced the production of free radicals and subsequently, occurrence of lipid peroxidation. The ultimate consequence is an increase in the fragility of the erythrocytes membrane and thus, elevation in EOF (Brzezinska-Slebodzinska Citation2001; Habibu et al. Citation2014; Yaqub et al. Citation2014). Similarly, higher EOF has been reported in animals exposed to transport stress compared to those in which the effect of transport stress was ameliorated using antioxidant (Yaqub et al. Citation2014). On the contrary, higher EOF has been demonstrated during the CDS compared with the HDS in goats in the middle belt of Nigeria (Adenkola et al. Citation2011). This difference is probably due to variation in geographical location and differences in adaptation of the goats. In Red Sokoto goats, however, the erythrocytes were more fragile during the CDS than RAS. This may be attributed to the wide fluctuation in AT between the morning and afternoon hours (16.45 vs. 30.47°C) during the CDS, which may have stimulated the biological stress mechanism of the goats (Adenkola et al. Citation2009).
4.5. Thyroid hormones
As seasons change, animals undergo a highly integrated physiological adjustment aimed at maintaining homeostasis (Sharma et al. Citation2013). In seasons with low AT, the physiological responses are aimed at reducing heat loss and increasing heat production, while in seasons with high AT, the responses aim to decrease heat production and increase heat loss. The primary function of thyroid hormones is to increase metabolic rate in order to maintain a relatively constant body temperature. When the AT increases, serum levels of thyroid hormones decrease and vice versa (Todini Citation2007). Thus, suggesting that as seasons change, breeds that can effectively regulate thyroid hormone secretion may survive better, particularly in the tropical countries where animals are not be provided with special housing according to the prevailing season of the year. In the present study, circulating concentrations of T4 and T3 were lower in the HDS compared to the CDS and RAS in Sahel goats. In Red Sokoto goats, however, serum concentration of T3 and conversion of T4 to T3 (i.e. T3:T4) were lower in the HDS compared to the CDS and RAS. Similarly, the study of Indu et al. (Citation2014) and Ribeiro et al. (Citation2015) in goats demonstrated high T4 and T3 during the cold compared to the hot seasons. The reduction in serum concentration of T4 and T3 during the HDS could reduce metabolism and heat generation so as to prevent a rise in body temperature. Furthermore, the positive correlation between RT and T3 and RT and T3:T4 in Sahel goats during the CDS and RAS (characterized by low ATs, particularly in the morning hours) confirms the role of T3 in thermogenesis. In Red Sokoto goats, serum T4, unlike T3, did not decrease during the HDS compared to CDS and RAS. This is probably due to the reduction in enzymatic conversion of T4 to T3 indicated by decreased in T3:T4. This may suggest a more efficient thyroid hormone metabolism in male Red Sokoto goats aimed at preventing a significant seasonal fluctuation in serum T4.
The result of this study showed that, though Red Sokoto and Sahel goats are assumed to be phylogenetically related and belong to the Savannah or Sahel type of goats, their physiological response differ in different seasons of the year. Missohou et al. (Citation2006) studied the genetic relationship between seven West African goat breeds, including Red Sokoto and Sahel goats. The seven breeds were clearly separated genetically in conformation with their geographical origin rather than their type or morphological grouping into long-legged (Savannah) and dwarf types. The difference in physiological response is presumably due to the fact that the Sahel goats are originally indigenous to the Sahel zone, which is characterized by higher ATs and lower RH (due to the closeness of the Sahel zone to the Sahara desert) when compared to the Guinea Savannah zone of West African.
5. Conclusion
When seasons change, the Red Sokoto and Sahel goats experience an integrated physiological response in thyroid function, blood cellular components, EOF and physiological variables so as to efficiently adapt to the existing meteorological parameters. In both breeds of goats, the CDS and HDS altered the composition of blood cellular components, thyroid physiology and decreased resistance and size of erythrocytes, with the HDS being more stressful. During the CDS, the male Sahel seems to be less adapted to the harsh environmental conditions of Guinea Savannah zone of West African. Thus, it is logical to infer that this may affect the productivity of male Sahel goats in Guinea Savannah climate. The thermoregulatory changes seemed to be strongly associated with changes in serum T3 in Sahel goats.
The effect of heat stress in Red Sokoto and Sahel goats during the HDS should be mitigated by providing shades, free access to water, and possibly the use of antioxidants. Introduction of Sahel goats to the Northern Guinea Savannah zone of Nigeria should be done strategically to include the possibility of cross-breeding with Red Sokoto goats since they are less efficiently adapted to the cold environmental conditions of the CDS. This will ensure the attainment of optimum production potentials of Sahel goats in the Northern Guinea Savannah climate of West Africa.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adenkola AY, Agbendeh J, Okpe J. 2011. Comparative assessment of erythrocyte osmotic fragility of apparently healthy goat and cattle during the hot-dry and harmattan season in Makurdi Nigeria. J Anim Plant Sci. 11:1474–1480.
- Adenkola AY, Ayo JO, Sackey AKB. 2009. Ascorbic acid-induced modulation of rectal temperature in pigs during the harmattan season. J Therm Biol. 34:152–154. doi: 10.1016/j.jtherbio.2009.01.001
- Azzarri C, Cross E, Haile B, Zezza A. 2014. Does livestock ownership affect animal source foods consumption and child nutritional status? Evidence from Rural Uganda, World Bank Policy Research Working Paper, 7111, 1–30.
- Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh Das TK, De S. 2014. Seasonal variations in physio-biochemical profiles of Indian goats in the paradigm of hot and cold climate. Biol Rhythm Res. doi:10.1080/09291016.2014.984999
- Basha NAD, Scogings PF, Nsahlai IV. 2013. Effects of season, browse species and tannins on gas production kinetics of forages in the sub-humid subtropical savannah, South Africa. J Sci Food Agr. 93:1338e1348 doi: 10.1002/jsfa.5895
- Brzezinska-Slebodzinska E. 2001. Erythrocyte osmotic fragility test as the measure of defense against free radicals in rabbits of different ages. Acta Vet Hung. 49:413–419. doi: 10.1556/004.49.2001.4.5
- Dacie JV, Lewis SM. 1991. Practicalhaematology 7th edition. Edinburgh, Melbourne and New York: Churchill Livingston; p. 24–36.
- Dzenda T, Ayo JO, Lakpini CAM, Adelaiye AB. 2011. Diurnal, seasonal, and sex variations in rectal temperature of African Giant rats (Cricetomys gambianus,Waterhouse). J Therm Biol. 36:255–263. doi: 10.1016/j.jtherbio.2011.03.010
- Foller M, Braun M, Qadri SM, Lang E, Mahmud H, Lang F. 2010. Temperature sensitivity of suicidal erythrocyte death. Eur J Clin Invest. 40:534–540. doi: 10.1111/j.1365-2362.2010.02296.x
- Gall, C. 1996. Goat breeds of the world. Weikersheim: Margraf.
- Gaughan JB, Bonner SL, Loxton I, Mader TL. 2013. Effects of chronic heat stress on plasma concentration of secreted heat shock protein 70 in growing feedlot cattle. J Anim. 91:120–129. doi: 10.2527/jas.2012-5294
- Habibu B, Kawu MU, Makun HJ, Aluwong T, Yaqub LS, Ahmad MS, Tauheed M, Buhari HU. 2014. Influence of sex, reproductive status and foetal number on erythrocyte osmotic fragility, haematological and physiologic parameters in goats during the hot-dry season. Vet Med. 59:479–490.
- Haldar C, Ghosh S. 2014. Immune modulation in goats by melatonin and other hormones: a novel horizon of research. J Immunol Res. 1:1–7.
- Hamzaoui S, Salama AAK, Albanell E, Such X, Caja G. 2013. Physiological responses and lactational performances of late-lactation dairy goats under heat stress conditions. J Dairy Sci. 96:6355–6365. doi: 10.3168/jds.2013-6665
- Hashem ALS. 2014. Effect of summer shearing on thermoregulatory, hematological and cortisol responses in Balady and Damascus goats in desert of Sinai, Egypt. World Appl Sci J. 30:521–533.
- Igono MO, Aliu YO. 1982. Environmental profile and milk production of Friesian-Zubucrosses in Nigerian Guinea Savannah. Int J Biometeorol. 26:115–120. doi: 10.1007/BF02184621
- Ikwuebu OA, Tarawali G, Njwe RM. 1994. The role of the West African Dwarf goats in the economy of the small-holder arable farmer in the sub-humid zone of Nigeria. In: Proceedings of Second Biannual Conference of the African Small Ruminant Research Network AICC, Arusha, Tanzania, 7–11 December, pp. 19–22.
- Indu S, Sejian V, Naqvi SMK. 2014. Impact of simulated heat stress on growth, physiological adaptability, blood metabolites and endocrine responses in Malpura ewes under semiarid tropical environment. Anim Prod Sci. 55:766–776. doi: 10.1071/AN14085
- Jain NC. 1986. Schalm’s veterinary hematology. 4th ed. Philadelphia, PA: Lea and Febiger.
- Makun H, Otaru SM, Dung D. 2013. Effect of management practices on milk yield and live weight changes of indigenous breeds of goats supplemented with groundnut haulms and concentrate in sub humid zone of Nigeria. Sokoto J. Vet Sci. 11:45–50.
- Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM. 2007. Physiological traits as affected by heat stress in sheep: a review. Small Ruminant Res. 71:1–12. doi: 10.1016/j.smallrumres.2006.10.003
- Martz F, Jaakola L, Julkunen-Tiitto R, Stark S. 2010. Phenolic composition and antioxidant capacity of bilberry (Vaccinium myrtillus) leaves in Northern Europe following foliar development and along environmental gradients. J Chem Ecol. 36:1017e1028. doi: 10.1007/s10886-010-9836-9
- Mazzullo G, Rifici C, Cammarata F, Caccamo G, Rizzo M, Piccione G. 2014. Effect of different environmental conditions on some haematological parameters in cows. Ann Anim Sci. 14:947–954. doi: 10.2478/aoas-2014-0049
- Minka NS, Ayo JO, Sackey AKB, Adelaiye AB. 2009. Assessment and scoring of stresses imposed on goats during handling, loading, road transportation and unloading, and the effect of pretreatment with ascorbic acid. Livest Sci. 125:275–282. doi: 10.1016/j.livsci.2009.05.006
- Missohou A, Talaki E, Maman Laminou I. 2006. Diversity and genetic relationships among seven West African goat breeds. Asian Australas J Anim Sci. 19:1245–1251. doi: 10.5713/ajas.2006.1245
- Moore T, Sorokulova I, Pustovyy O, Globa L, Vodyanoy V. 2013. Microscopic evaluation of vesicles shed by rat erythrocytes at elevated temperatures. J Therm Biol. 38:487–492. doi: 10.1016/j.jtherbio.2013.08.001
- Morand-Fehr P, Boutonnet JP, Devendra C, Dubeuf JP, Haenlein GFW, Holst P, Mowlem L, Capote J. 2004. Strategy for goat farming in the 21st century. Small Ruminant Res. 51:175–184. doi: 10.1016/j.smallrumres.2003.08.013
- Nishimura T, Motoi M, Egashira Y, Choi D, Aoyagi K, Watanuki S. 2015. Seasonal variation of non-shivering thermogenesis (NST) during mild cold exposure. J Physiol Anthropol. doi:10.1186/s40101-015-0051-9
- Omran FI, Shafie MM, Ashour GH, Youssef MM, Hassan LR. 2013. Response of buffalo calves exposed to first and second acute thermal shocks. Egyptian J Agric Res. 91:1113–1127.
- Oyewale JO. 1992. Effect of temperature and pH on osmotic fragility of erythrocytes of the domestic fowl (Gallus domesticus) and guinea-fowl (Numida meleagris). Res Vet Sci. 52:1–4. doi: 10.1016/0034-5288(92)90049-8
- Ravagnolo O, Misztal I, Hoogenboom G. 2000. Genetic component of heat stress in dairy cattle, development of heat index function. J Dairy Sci. 83:2120–2125. doi: 10.3168/jds.S0022-0302(00)75094-6
- Ribeiro NL, Pimenta Filho EC, Arandas JKG, Ribeirob MN, Saraiva EP, Bozzi R, Costa RG. 2015. Multivariate characterization of the adaptive profile in Brazilian and Italian goat population. Small Ruminant Res. 123:232–237. doi: 10.1016/j.smallrumres.2014.12.010
- Ruiz G, Rosenmann M, Cortes A. 2004. Thermal acclimation and seasonal variations of erythrocyte size in the Andean mouse Phyllotis xanthopygus rupestres. Comp Biochem Phys. 139:405–409. doi: 10.1016/j.cbpb.2004.03.003
- Santucci PM, Branca A, Napoleone M, Bouche R, Aumont G, Poisot F, Alexandre G. 1991. In: body condition scoring of goats in extensive conditions. Goat nutrition. Wageningen: EAAP Publication, Pudoc III. p. 240–256.
- Schalm OW, Jain NC, Caroll EJ. 1975. Veterinary haematology. 3rd ed. Philadelphia, PA: Lea and Febiger.
- Scholtz MM, McManus C, Leeuw KJ, Louvandini H, Seixas L, de Melo CB, Theunissen A, Neser FWC. 2013. The effect of global warming on beef production in developing countries of the southern hemisphere. Nat Sci. 5:106–119.
- Scogings PF, Hattas D, Skarpe C, Hjalten J, Dziba L, Zobolo A, Rooke T. 2015. Seasonal variations in nutrients and secondary metabolites in semi-arid savannas depend on year and species. J Arid Environ. 114:54–61. doi: 10.1016/j.jaridenv.2014.11.003
- Sejian V, Singh AK, Sahoo A, Naqvi SMK. 2014. Effect of mineral mixture and antioxidant supplementation on growth, reproductive performance and adaptive capability of Malpura ewes subjected to heat stress. J Anim Physiol Anim Nutr. 98:72–83. doi: 10.1111/jpn.12037
- Sharma S, Ramesh K, Hyder I, Uniyal S, Yadav VP, Panda RP, Maurya VP, Singh G, Kumar P, Mitra A, Sarkar M. 2013. Effect of melatonin administration on thyroid hormones, cortisol and expression profile of heat shock proteins in goats (Capra hircus) exposed to heat stress. Small Ruminant Res. 112:216–223. doi: 10.1016/j.smallrumres.2012.12.008
- Thornton P, Herrero M, Freeman A, Mwai O, Rege E, Jones P, McDermott J. 2007. Vulnerability, climate change and livestock—research opportunities and challenges for poverty alleviation. SAT J. 4:1–23.
- Todini L. 2007. Thyroid hormones in small ruminants: effects of endogenous, environmental and nutritional factors. Animal. 1:997–1008. doi: 10.1017/S1751731107000262
- Yaqub LS, Mishelia P, Ayo JO. 2014. Erythrocyte osmotic fragility and hematological responses of horses administered ascorbic acid and exposed to road transportation. J Equine Sci. 34:1324–1328. doi: 10.1016/j.jevs.2014.09.015
- Zakari FO, Ayo JO, Rekwot PI, Kawu MU. 2015. Effects of age and season on haematological parameters of donkeys during the rainy and cold–dry seasons. Int J Biometeorol. 59:1813–1824. doi: 10.1007/s00484-015-0989-7
- Zhao F, Zhang Z, Wang C, Zhang B, Yao H, Li S, Xu S. 2013. The role of heat shock proteins in inflammatory injury induced by cold stress in chicken hearts. Cell Stress Chaperones. 18:773–783. doi: 10.1007/s12192-013-0429-8