ABSTRACT
Pathogens of different kinds may infect amphibians at various stages of their life and can be the immediate causes of their mortality. Salamanders are hosts to a variety of parasites, including intracellular bacteria in the order Rickettsiales. All bacteria in this order are obligate, intracellular (usually within erythrocytes) organisms that are transmitted by arthropod vectors. By examining stained blood smears of Neurergus kaiseri under a light microscope, we encountered single or multiple round, clear to faint purple with dark purple ring inclusions within the cytoplasm which were similar to intraerythrocytic rickettsial inclusion bodies. Reported here is a case of the rickettsial-like inclusions in N. kaiseri in a captive breeding facility for the first time in Iran.
Three species of the genus Neurergus have been reported from Iran: N. crocatus, N. microspilotus and N. kaiseri (Restegar-Pouyani et al. Citation2008). The endangered Kaiser’s mountain newt, N. kaiseri, is a species endemic to the southern Zagros Range in Iran. Until now, N. kaiseri had been reported from 13 localities (Sharifi et al. Citation2013). N. kaiseri has been designated a critically endangered species by the International Union for Conservation of Nature (IUCN) because of its highly fragmented breeding habitat and also because it occupies a small range during its reproductive period (Sharifi et al. Citation2009). According to the IUCN evaluation, the most important threats to this species are likely to be illegal trade and the presence of non-native fish as a result of the damming of Dez River, which extends the reservoir close to the known localities of N. kaiseri. This species has also been included in Appendix I of the Convention of International Trade on Endangered Species (CITES, https://cites.org/eng/app/appendices.php; Sharifi et al. Citation2009). Although N. kaiseri amendment to Appendix I of CITES has effectively stopped international trade of this species, illegal collection of this species for national trade is continuing (Sharifi et al. Citation2013).
Disease risks are an inherent part of captive breeding and reintroduction programmes and of research involving contact between researchers and animals. Thus, quantitative assessment of the risks of disease should be an important part of developing conservation strategies (Ballou Citation1993; Spitzen van der Sluijs et al. Citation2001). The development of amphibian medicine and welfare comprise a continuing science that reflects the life history of these animals. The growing knowledge of amphibian diseases is due in part to the successful maintenance of captive amphibians for culture, public display, the pet industry, and breeding programmes for endangered species (Densmore & Green Citation2007). Amphibians are notoriously difficult in terms of captive care requirements, and the majority of diseases of amphibians maintained in captivity will relate directly or indirectly to husbandry and management. Investigators have described many infectious and noninfectious diseases that occur among various species of captive and wild amphibians, and there is considerable overlap in the diseases of captive versus free-ranging populations (Densmore & Green Citation2007).
Very little is known regarding diseases occurring in newts of the genus Neurergus. As in most urodelans, inadequate nutrition is probably the most important predisposing factor for disease (Bogaerts et al. Citation2012). Ranavirosis has been described in N. crocatus imported from Iraq (Stöhr et al. Citation2013). Chytridiomycosis (a fungal disease) is caused by Batrachochytrium dendrobatidis and is considered the most important infectious driver of worldwide amphibian declines. It is of utmost importance that (as for ranavirosis) captive populations of Neurergus are negative for the fungus. It is at present not clear whether this fungus causes clinical problems in newts of the genus Neurergus. In other amphibians, the course of a B. dendrobatidis infection may vary from asymptomatic to apathy, skin disorders, and death. Recently, B. dendrobatidis infection was demonstrated in N. kaiseri and N. crocatus (Spitzen van der Sluijs et al. Citation2001; Bogaerts et al. Citation2012). In addition to other factors which are known to have deleterious effects on amphibians, there have been reports of disease in the species of the genus Neurergus in Iran, including high prevalence of Chytridiomycosis (Parto et al. Citation2013; Sharifi et al. Citation2014) in N. microspilotus and also red leg disease in N. kaiseri (Parto et al. Citation2014).
Pathogens of different kinds may infect amphibians at various life stages and can be the immediate causes of mortality or can lead to sublethal damage such as severe developmental and physiological abnormalities (Voyles et al. Citation2012). Salamanders are hosts to a variety of parasites, including helminths (McAllister et al. Citation2008), arthropods (Westfall et al. Citation2008), and in particular, intracellular bacteria in the order Rickettsiales (Davis et al. Citation2009). The reported incidence of rickettsial disease is increasing worldwide because of increased public health concern and improvement in diagnostic technology (Davis & Cecala Citation2010; Parola et al. Citation2013). Presence of intraerythrocytic rickettsial inclusions has not been reported in any species of amphibian in Iran. In this study, we report cases of the rickettsial inclusions in N. kaiseri in a captive breeding facility for the first time.
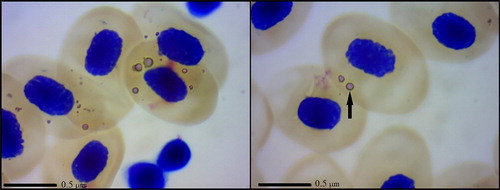
N. kaiseri used in this study were obtained from a pet shop in Tehran. No information was available about the locality where these newts were collected. Since there was not a real choice for us to release these newts into their habitats, we used them to gain experience for the newt husbandry and health (Parto et al. Citation2013, Citation2014; Sharifi et al. Citation2014; Sharifi & Vaissi Citation2014). These include seven adult N. kaiseri (three male and four female) that are kept in one aquarium (75 × 45 × 35 cm). Four days after transfer to the captive breeding, salamanders showed signs of inflammatory skin lesions in their legs and fingers. The individuals who are severely infected show lethargy and loss of appetite. Based on these symptoms, samples were used for microbiological, hematological and pathological diagnosis. The individuals who are worst infected were killed with an overdose of chloroform. Immediately after death, each salamander was decapitated, the blood obtained directly from the heart was dripped onto a clean microscopic slide, and the smears were prepared and samples from skin and internal organ were prepared for pathological examinations. Air-dried blood smears were stained with Giemsa and in our survey, we encountered intraerythrocytic inclusion body. To determine the prevalence of rickettsial infections in the collected salamanders, all blood smears were examined under ×6000 (oil immersion) magnification in a standard light microscope. Twenty fields of view per slide were examined and the numbers of RBC with inclusions for each salamander were recorded. Photomicrographs were taken using a digital camera and the length and width of each RBC and the inclusions within were measured using Dino-capture software (http://www.dinolite.us/downloads).
In N. kaiseri, the affected erythrocytes stained with Giemsa method showed ***single or multiple round, clear to faint purple with a dark purple ring inclusions within the cytoplasm, which were randomly distributed within the cytoplasm. The size of the inclusions was approximately about 2.00 ± 0.32 μm. Intraerythrocytic inclusion bodies were found in 5 of 7 (71.43% of 100%) salamanders we examined. The average number of erythrocytes with inclusions was 15 per 20 fields of view. The staining characteristics, the size and the nuclear position were similar in both affected and unaffected erythrocytes ().
Amphibians in their natural habitats are exposed to a vast variety of parasitic infestations, including haematogenous pathogens. Bacteria in the order Rickettsiales are obligate, intracellular organisms which are transmitted by arthropod vectors (Rikihisa Citation2006). Most infections can be seen by examining stained blood smears under a light microscope and noting the presence of the characteristic inclusions that are formed within blood cells (Davis & Cecala Citation2010). In the present study, examination of stained blood smears with a light microscope and observation of the characteristic inclusions within the red blood cells is the key feature of rickettsial identification. The characteristics of inclusions we described here were consistent with previous investigations in other salamanders and amphibians (Rankin Citation1937; Davis et al. Citation2009; Davis & Cecala Citation2010). The mode of transmission of Rickettsia among amphibians is still unclear (Davis & Cecala Citation2010). The life cycle of adult N. kaiseri is characterized by periodic migrations between two critical habitats (aquatic for breeding and terrestrial for foraging) (Sharifi et al. Citation2013). As stated by Davis et al. (Citation2009), the rickettsial bacteria in salamander were likely transmitted by a terrestrial arthropod vector. Although some Rickettsia species faculatively infect humans and other vertebrates, their primary hosts are bloodsucking arthropods that can act as disease vectors (Davis & Cecala Citation2010).
Determination of the prevalence of rickettsial infection in wild population of salamanders is the first step in assessment of the impact of the organism on these populations. Presently little is known about the prevalence of the rickettsia in aquatic salamanders. Although it is globally known that pet shops are an important source of various diseases that can even help spread diseases into the natural communities, little attention has been given to salamanders with respect to their parasitic infections in Iran. Because of the risk of the link between such organisms and public health, this warrants additional care and investigations. Finally, we suggest that future investigations into this pathogen of N. kaiseri would be fruitful areas of research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ballou JD. 1993. Assessing the risks of infectious diseases in captive breeding and reintroduction programs. J Zoo Wildl Med. 24(3):327–335.
- Bogaerts S, Janssen H, Macke J, Schultschik G, Ernst K, Maillet F, Bork C, Pasmans F, Wisniewski P. 2012. Conservation biology, husbandry, and captive breeding of the endemic Anatolia newt, Neurergus strauchii Steindachner (1887) (Amphibia: Caudata: Salamandridae). Amphibian Reptile Conserv. 6(4):9–29.
- Davis AK, Cecala K. 2010. Intraerythrocytic rickettsial inclusions in Ocoee salamanders (Desmognathus ocoee): prevalence, morphology and comparisons with inclusion of Plethodon cinereus. Parasitolo Res. 107:363–367. doi: 10.1007/s00436-010-1869-z
- Davis AK, DeVore JL, Milanovich JR, Cecala K, Maerz JC, Yabsley MJ. 2009. New findings from an old pathogen: intraerythrocytic bacteria (Family: Anaplasmatacea) in Red-Backed Salamanders Plethodon cinereus. Ecohealth. 6(2):219–228. doi: 10.1007/s10393-009-0250-0
- Densmore CL, Green DE. 2007. Diseases of amphibians. ILAR J. 48:235–254. doi: 10.1093/ilar.48.3.235
- McAllister M, Payne K, MacLeod R, Nicholls S, Donnai D, Davies L. 2008. What process attributes of clinical genetics services could maximize patient benefits? Eur J Hum Genet. 16:1467–1476. doi: 10.1038/ejhg.2008.121
- Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier PE, Raoult D. 2013. Update on tickborne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 26:657–702. doi: 10.1128/CMR.00032-13
- Parto P, Haghighi ZMS, Vaissi S, Sharifi M. 2014. Microbiological and histological examinations in endangered Neurergus kaiseri tissues displaying red-leg syndrome. Asian Herpetol Res. 5(3):204–208. doi: 10.3724/SP.J.1245.2014.00204
- Parto P, Vaissi S, Farasat H, Sharifi M. 2013. First report of Chytridiomycosis (Batrachochytrium dendrobatidis) in endangered Neurergus microspilotus in western Iran. Global Vet. 11(5):547–551.
- Rankin JS. 1937. An ecological study of parasites of some North Carolina salamanders. Ecol Monogr. 7:169–269. doi: 10.2307/1943289
- Rastegar-Pouyani N, Kami HG, Rajabzadeh M, Shafiei S, Anderson SC. 2008. Annotated checklist of amphibians and reptiles of Iran. Iranian Journal of Animal Biosystematics. 4(1):7–30.
- Rikihisa Y. 2006. New findings on members of the family Anaplasmataceae of veterinary importance. Ann NY Acad Sci. 1078:438–445. doi: 10.1196/annals.1374.083
- Sharifi M, Farasat H, Barani-Beiranvand H, Vaissi S, Foroozanfar E. 2013. Notes on the distribution and abundance of the endangered Kaiser’s mountain newt Neurergus kaiseri (Caudata: Salamandridae) in Southwestern Iran. Herpetol Conserv Biol. 8:724–731.
- Sharifi M, Farasat H, Vaissi S, Parto P, Siavosh Haghighi ZM. 2014. Prevalence of the amphibian pathogen Batrachochytrium dendrobatidis in endangered Neurergus microspilotus (Caudata: Salamandridae) in Kavat Stream, western Iran. Global Vet. 12(1):45–52.
- Sharifi M, Papenfuss T, Rastegar-Pouyani N, Anderson S, Kuzmin S. 2009. Neurergus kaiseri. The IUCN red list of threatened species 2009: e.T59450A11943697 [downloaded 2016 Jul 10]. Available from: http://dx.doi.org/10.2305/IUCN.UK.2009.RLTS.T59450A1194367.en
- Sharifi M, Vaissi S. 2014. Captive breeding and trial re-introduction of the endangered yellow spotted mountain newt Neurergus microspilotus (Caudata: Salamandridae) in western Iran. Endangered Species Res. 23:159–166. doi: 10.3354/esr00552
- Spitzen-van der Sluijs A, Martel A, Wombwell E, Van Rooij P, Zollinger R, Woeltjes T, Rendle M, Haesebrouck F, Pasmans F. 2001. Clinically healthy amphibians in captive collections and at pet fairs: a reservoir of Batrachochytrium dendrobatidis. Amphibia-Reptilia. 32(2011):419–423.
- Stöhr AC, Fleck J, Mutschmann F, Marschang RE. 2013. Ranavirus infection in a group of wild-caught Lake Urmia newts Neurergus crocatus imported from Iraq into Germany. Dis Aquat Organ. 103:185–189. doi: 10.3354/dao02556
- Voyles J, Vredenburg VT, Tunstall TS, Parker JM, Briggs CJ, Rosenblum EB. 2012. Pathophysiology in mountain yellow-legged frogs (Rana muscosa) during a chytridiomycosis outbreak. PLoS ONE. 7:e35374. doi: 10.1371/journal.pone.0035374
- Westfall PJ, Patterson JC, Chen RE, Thorner J. 2008. Stress resistance and signal fidelity independent of nuclear MAPK function. Proc Natl Acad Sci USA. 105(34):12212–12217. doi: 10.1073/pnas.0805797105