ABSTRACT
A total of 360 Hy-Line W-36 hens at 30 wk of age were assigned to 6 dietary treatments that consisted of 6 different (11, 15, 19, 23, 27 and 31) digestible Trp:Lys ratios. Hens were fed the experimental diets from 30 to 36 wk of age. Productive traits were recorded every wk and egg quality was measured at 36 wk of age. Total IgM, IgG, anti-sheep red blood cell antibody titres, plasma corticosterone, serotonine and glucose concentrations and heterophil to lymphocyte ratio (H:L) were determined at wk 6 of the experiment. Results showed a significant improvement (P < .0001) in egg production, egg mass, feed intake, feed conversion ratio and body weight by increasing the standardized ileal digestible (SID) Trp:Lys ratio from 11 to 19. Relative weights of shell (P < .05) and shell strength (P < .06) were affected by dietary treatments. Hens fed graded levels of SID Trp responded by decreasing the H:L ratio in a linear or curvilinear manner (P < .05). The study reported here indicated that the ideal SID Trp:Lys ratio for laying hens ranged from17.5 to 29 based on the response criteria and regression models.
Introduction
Tryptophan is one of the essential amino acids mainly used for protein synthesis. In addition, it has numerous metabolic roles such as the conversion to niacin and the precursor of serotonin and melatonin. Because its concentration is the lowest of all amino acids in organisms, it can easily play a rate-limiting role in protein synthesis (Corzo et al. Citation2005). The specific inclusion rates of the plant origin protein sources can indicate Trp degree of limitation. The daily total tryptophan recommendation for laying hens varies from 117 to 239 mg/hen per d and is necessary to optimize laying hens’ performance (Ishibashi Citation1985; Othani et al. Citation1989; Jensen et al. Citation1990; Harms & Russell Citation1999). Based on the recent estimation by Bregendahl et al. (Citation2008), Trp requirement for true digestible Trp was 120 mg/d, which was similar to the 122 mg/d of digestible Trp reported by Coon and Zhang (Citation1999) for 33–49-wk-old laying hens and the 149 mg/d of total Trp reported by Harms and Russell (Citation2000) for 30–36-wk-old hens. However, the NRC (Citation1994) recommendation (160 mg/d total Trp) is greater than that observed by Bregendahl et al. (Citation2008). Nevertheless, the existing database regarding the tryptophan requirement for laying hens is inconsistent and mostly reported for performance rather than other response criteria such as stress or immunity (NRC Citation1994; CVB Citation1996; Coon & Zhang, Citation1999; Bregendahl et al. Citation2008; Rostagno et al. Citation2011). The solution to obtaining reliable amino acid requirements is not to use the absolute amino acid recommendations, but rather to use the ideal amino acid profile for laying hens (Bregendhal et al. Citation2008.). The ideal amino acid profile employs the concept that, while absolute amino acid requirements change broadly due to genetic or environmental factors, the ratios among them are only slightly affected. Thus, once the ideal amino acid profile has been determined, the requirement for a single amino acid (e.g. lysine) can be determined experimentally or modelled for a given field situation and the requirement for all the other amino acids calculated using the individual ideal amino acid ratios in the profile. Such an approach has been adopted with success by the swine industry (NRC, Nutrient Requirements of Swine, Citation1998) and is finding use in the broiler industry as well (Mack et al. Citation1999; Baker Citation2003; Dari et al. Citation2005). Although feed-grade synthetic form l-Trp is currently available for dietary formulation, the Trp requirement of laying hens is typically met by intact protein sources. However, results of dose-response studies addressing the Trp need in laying hens are variable. It is suggested that 0.2–0.4 g/kg of dietary Trp supplementation has beneficial effects on shell strength, serum IgM and superoxide dismutase concentration of brown-egg laying hens under high temperature and humidity conditions (Dong et al. Citation2012). In the de Lima et al. (Citation2012) research, increases in the digestible Trp to Lys promoted morphological characteristics of the digestive and reproductive systems of laying hens, in addition to performance parameters. This study was, therefore, conducted to estimate digestible Trp to Lys ratio for maximum performance, immunity and welfare of Hy-Line W-36 hens using different non-linear regression models.
Material and methods
A total of 360 Hy-Line (W-36) hens at 30 wk of age were allocated to 6 treatments. Each dietary treatment was assigned to six replicates in a randomized complete block design. Each experimental unit consisted of 10 hens that were housed in groups of 5 in 2 adjacent cages. The experiment was conducted from 30 to 36 wk of age. In a 9-d pre-experimental period, means of daily feed intake was recorded. Based on the 90 g daily feed intake, a basal diet was formulated to meet or exceed nutrient recommendations by the Hy-Line W-36 nutritional guidelines (Citation2009), except for the Trp, Lys and crude protein (CP) (). The CP content of basal diet was reduced and the diet also contained corn gluten meal in order to obtain a low level of Trp. Crystalline feed-grade amino acids (AAs) were added to the basal diet to meet minimum AA ratio requirements. In order to prevent excessive Lys supply, digestible Lys (0.75%; 675 mg/d) in basal diet was calculated as 90% of Hy-Line recommendation. Graded levels of l-Trp (0.03% increments) were added to the basal diet (substituted by corn starch) to create dietary standardized ileal digestible (SID) Trp:Lys ratios 11, 15, 19, 23, 27 and 31 (0.08%, 0.11%, 0.14%, 0.17%, 0.20% and 0.23% SID Trp). In addition, a commercial control diet was formulated similar to that of the AA-deficient basal diet, and formulated to meet or exceed nutrient recommendations by the Hy-Line W-36 nutritional guidelines (Citation2009). Diets were formulated based on the SID amino acids using the Hy-Line W-36 nutritional guidelines.
Table 1. Ingredient and calculated nutrient.
All protein-containing feedstuff were analysed for protein, total amino acid and dry matter prior to formulation. Digestible amino acid content of feed ingredients was calculated using digestibility coefficients recommended by Evonik (AminoDat4.0). Feed (in mash form) and water were supplied ad libitum to the hens. Egg production was recorded daily; feed consumption and egg weight were recorded weekly. Egg mass and feed conversion (g feed/g egg) were calculated from egg production, egg weight and feed consumption. Body weight changes were obtained by weighing all hens per replicate at the beginning and end of the experiment. Two eggs from each replicate (12 eggs/treatment) were collected at 6th wk of the experiment for measuring the egg components (albumen, yolk and shell percentages), Haugh units and shell quality. Shell breaking strength and shell thickness were measured using an egg shell force gauge (model no. 55R1123, Instron Corp., Canton, MA) and Karl Deutsch D-56 (wuppertalechometer 1061), respectively.
On wk 5 of the experiment, six birds per treatment were injected intravenously via the wing with 0.5 mL of 7% sheep red blood cell (SRBC) suspension. Blood samples were collected from each bird at 12 d after SRBC challenge. The total, mercaptoethanol-sensitive (MES, presumably IgM) and mercaptoethanol-resistant (MER, presumably IgG) anti-SRBC antibody titres were determined using a microheamagglutination technique as described by Yamamoto and Glick (Citation1982) and Dix and Taylor (Citation1996). The antibody data were expressed as the log2 of the reciprocal of the highest dilution giving visible agglutination.
At 4 wk of experiment, birds were vaccinated against Lasota Newcastle disease (ND) virus by spray method. Ten days after vaccination, one bird from each replicate were randomly selected to collect blood samples for antibody titre analysis. Serum antibody titres against Newcastle disease virus were measured by the hemagglutination inhibition test (HI), and HI antibodies were then converted into log. On wk 6 of experimental period, blood samples were collected (6 birds/treatment) using heparinized syringes. Plasma corticosterone concentrations were determined using an enzyme immunoassay kit. Glucose and serotonine were determined using an auto-analyser.
Statistical analysis
Data were analysed by using the Generalized linear models procedure of SAS software in a randomized complete block design (SAS Citation2003). Differences among treatments means were compared using Duncan’s multiple range tests. Various broken-line, quadratic and exponential regression models were fitted to data for estimating the Trp:Lys ratio for various responses. The SAS NLIN procedure was used to fit linear plateau (LPL), quadratic plateau (QPL), two-slope broken-line, two-slope broken-line with quadratic function at values of x < R and a straight line at values of x > R, where x = tryptophan levels below or higher than the requirement point, two straight-line, one-breakpoint model that included a random component for the slope of the curve below the requirement (Robbins et al. Citation2006). In the exponential model, the dietary SID Trp:Lys ratio was calculated that was required to achieve 95% of the maximum of the parameter considered.
Results and discussion
The effects of Trp:Lys ratios on performance, egg quality, H/L ratio and plasma glucose, serotonin and corticosterone concentrations and antibody titres against SRBC and ND are shown in –. The basal diet was clearly deficient in Trp. Considerable improvement (P < .001) in egg production, egg mass, feed intake, feed conversion ratio and body weight changes were observed when the SID Trp:Lys was increased from 11 to 19 (). There were no significant increases in egg weight as dietary Trp:Lys increased from 11 to 31. Hens fed the control diet had greater egg weight compared to those fed the titration diets (P < .05). In agreement with the current experiment, Harms and Russell (Citation2000) reported that egg production, egg weight and egg content were significantly increased by dietary supplementation of Trp (0.12–0.20%) to the basal diet. Jensen et al. (Citation1990) reported that rate of egg production was significantly improved by tryptophan supplementation in diet containing various levels of protein, and the requirement increased as the protein level increased. Pan et al. (Citation2013) reported that Trp supplementation promoting the balance of amino acids may be also an important reason in increased bird performance. Increasing SID Trp:Lys did not affect egg yolk, albumen content and albumen quality (). Shell percentage, strength and thickness were increased quadratically by increasing the Trp level in diets. Except for the heterophil:lymphocyte (H:L) ratio, variables such as plasma serotonine, corticosterone, glucose and also immune response parameters failed to respond to SID Trp:Lys (). Hens fed the graded levels of Trp generally showed decreased H:L ratio. The effects of tryptophan on the reproductive and digestive systems’ development, immunity and stress responses of birds have been studied. In a recent study de Lima et al (Citation2012) concluded that in addition to performance criteria, increase in the digestible Trp to Lys promoted morphological characteristics of the digestive and reproductive system of laying hens. These parameters were not evaluated in the current study. Dong et al (Citation2012) suggested that 0.2–0.4 g/kg of dietary Trp supplementation has beneficial effects on shell strength, serum IgM and superoxide dismutase concentration of brown laying hens under high temperature and humidity conditions.
Table 2. The effects of different Trp:Lys ratios on laying hens’ performance (30–36 wk of age) and body weight change.
Table 3. The effects of different Trp:Lys ratios on egg components and quality.
Table 4. The effects of different Trp:Lys ratios on H/L ratio and plasma glucose, serotonine, corticostron concentration and antibody titre against SRBC.
fDifferent regression equations from laying hens receiving progressive dietary Trp:Lys ratios for different response criteria are shown in . The minimal break point on egg production () and egg mass occurred at an SID Trp:Lys ratio of 17.5, with a maximum quadratic requirement of 25.5 SID Trp:Lys (). Estimates for the optimal SID Trp:Lys ratio based on feed conversion ratio (FCR) are shown in . The break point occurred at an SID Trp:Lys ratio of 15.9, with the maximum quadratic at 24.9 SID Trp:Lys. Researches on the Trp requirement of the laying hens are scare, and the reported literature shows large differences. NRC (Citation1994) have proposed a requirement of 0.16% of Trp in diets for the commercial laying hens. Bray (Citation1969) calculated the Trp requirement of 117 mg/hen per d for a flock of laying hens with body weight of 1.99 kg and maximum egg output of 46 g/hen per d. Valuable research on the Trp requirement for different strains of laying pullets has been done by Morris and Wethli (Citation1978). They produced a table of prediction for optimum Trp intake. These researchers estimated the optimum Trp intake of Bray’s flock to be 129 mg/hen per d by using coefficients derived from Bray’s data of 172 mg/hen per d from their table.
Figure 1. Fitted broken-line and quadratic plot of the percentage of the maximal egg production as a function of SID Trp:Lys in 30–36 wk Hy-Line white-laying hens.
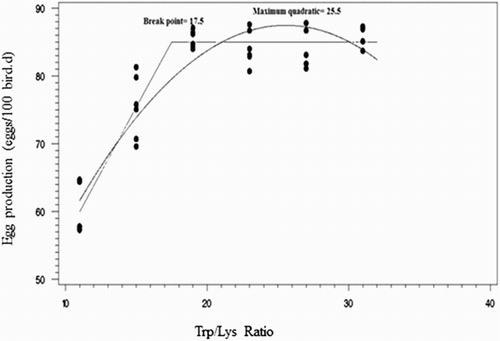
Table 5. Various regression equations from laying hens receiving progressive dietary Trp:Lys ratios for different response criteria.
According to the broken-line, quadratic and exponential models, the dietary Trp:Lys to reach the optimum (100% of maximum in the broken-line and quadratic models; 95% of maximum in the exponential model) of shell thickness () and strength and H:L ratio () was higher than those for egg production, egg mass, feed intake and FCR (). Broken-line, curvilinear (quadratic) and exponential responses to dietary Trp/Lys level were observed for H:L ratio.
Figure 2. Fitted broken-line and quadratic plot of the percentage of the maximal egg shell thickness (mm) as a function of SID Trp:Lys in 30–36 wk Hy-Line white-laying hens.
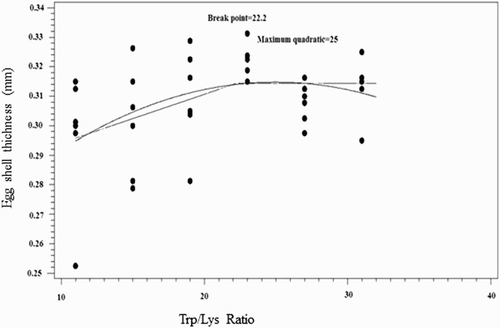
Figure 3. Fitted broken-line and quadratic plot of the H:L as a function of SID Trp:Lys in 30–36 wk Hy-Line white-laying hens.
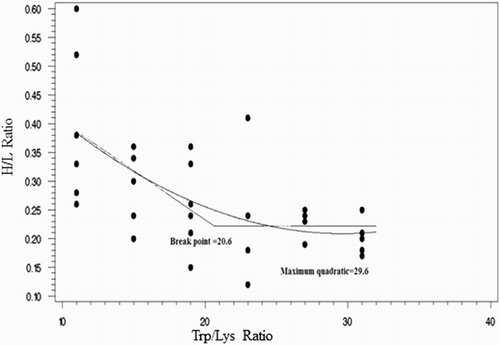
Bregendahl et al. (Citation2008) published an extended study on the ideal protein profile of modern laying hens, but there are several other references for this topic including Jais et al. (Citation1995), Coon and Zhang (Citation1999), Rostagno (Citation2011) and Leeson and Summer (Citation2005). Regarding Trp:Lys the ratio provided by Jais et al. (Citation1995) was substantially lower compared to the other works. These data demonstrated that the minimum SID Trp:Lys ratio from 30 to 36 wk for performance criteria is 17.5, and for maximizing the shell quality parameters and H:L ratio is 20 and 30, respectively.
Bregendahl et al. (Citation2008) by using broken-line models reported that the optimum tryptophan to lysine ratio is 22 for performance traits in laying hens. Jais et al (Citation1995) and CVB (Citation1996) of the Netherlands suggested a ratio to Lys of 16 and 19, respectively. For performance criteria, except for Jais et al. (Citation1995) other researchers (NRC (Citation1994), 23; CVB (Citation1996), 19; Coon and Zhang, (Citation1999), 20; Leeson and Summers, (Citation2005), 21; Bregendahl et al. (Citation2008), 22; Rostagno et al. (Citation2011), 23, de Lima et al (Citation2012), 24.5) reported Trp to Lys ratio higher than the current study.
In summary, the study reported here indicated that the minimum requirement of SID Trp:Lys ratio for laying hens (30–36 wk) ranged from 17.5 to 29 based on the response criteria and regression model.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Baker DH. 2003. Ideal amino acid patterns for broiler chicks. In: JFP D’Mello, editor. Amino acids in animal nutrition. Oxon: CABI; p. 223–235.
- Bray DJ. 1969. Studies with corn-soya laying diets. 8. Requirements for limiting amino acids—the basal diet and the requirements for isoleucine, lysine and tryptophan. Poult Sci. 48:674–684.
- Bregendahl K, Roberts SA, Kerr B, Hoehler D. 2008. Ideal ratios of isoleucine, methionine, methionine plus cystine, threonine, tryptophan, and valine relative to lysine for white leghorn-type laying hens of twenty-eight to thirty-four weeks of age. Poult Sci. 87:744–758.
- Coon G, Zhang B. 1999. Ideal amino acid profile for layers examined. Feedstuffs. 71(14):13–15. 31.
- Corzo A, Moran TE, Hoehler D Jr, Lemme A. 2005. Dietary tryptophan need of broiler males from forty-two to fifty-six days of age. Poult Sci. 84:226–231.
- [CVB] CentraalVeevoederbureau. 1996. Aminozurenbehoefte van Leghennen en Vleeskuikens [Amino acid requirements for laying hens and broiler chickens]. Documentation Report nr. 18 (in Dutch). Lelystad, The Netherlands.
- Dari RL, Penz AM, Kessler AM Jr, Edwards HM, Emmert JL Jr, Webel DM. 2005. Use of digestible amino acids and the concept of ideal protein in feed formulation for broilers. J Appl Poult Res. 14:195–203.
- de Lima MR, Costa FGP, Guerra RR, da Silva JHV, Carlos Bôa-Viagem Rabello CBV, Miglino MA, Nogueira ET, Pinheiro SG. 2012. Digestible tryptophan:lysine ratio for laying hens. R Bras Zootec. 41:2203–2210.
- Dix MC, Taylor RL Jr. 1996. Differential antibody responses in B major histocompatibility (B) complex congenic chickens. Poult Sci. 75:203–207.
- Dong XY, Azzam MMM, Rao W, Yu DY, Zou XT. 2012. Evaluating the impact of excess dietary tryptophan on laying performance and immune function of laying hens reared under hot and humid summer conditions. Br Poult Sci. 53:491–496.
- Harms RH, Russell GB. 1999. Tryptophan requirement of the commercial hen. Poult Sci. 78:1283–1285.
- Harms RH, Russell GB. 2000. Evaluation on tryptophan requirement of the commercial layer by using a corn soybean meal basal diet. Poult Sci. 79:740–742.
- Hy-Line International. 2009. Hy-Line W-98 commercial management guide. West Des Moines, IA: Hy-Line Int.
- Ishibashi T. 1985. Tryptophan requirement of laying hens. Jpn Poult Sci. 22:256–263.
- Jais C, Roth FX, Kirchgessner M. 1995. The determination of the optimum ratio between the essential amino acids in laying hen diets. Arch Geflugelkd. 59:292–302.
- Jensen L, Calderon SVM, Mendonca CX. 1990. Response to tryptophan of laying hens fed practical diets varying in protein concentration. Poult Sci. 69:1956–1965.
- Leeson S, Summers JD. 2005. Commercial poultry production. 3rd ed. Guelph: University Books.
- Mack S, Bercovici D, Goote GD, Leclercq B, Lippens M, Pack M, Schutte JB, van Cauwenberghe S. 1999. Ideal amino acid profile and dietary lysine specification for broiler chickens of 20 to 40 days of age. Br Poult Sci. 40:257–265.
- Morris TR, Wethli E. 1978. The tryptophan requirement of young laying pullets. Br Poult Sci. 19:455–466.
- [NRC] National Research Council. 1994. Nutrient requirements of poultry. 9th rev. ed. Washington, DC: National Academy Press.
- [NRC] National Research Council 1998. Nutrient requirements of swine. 10th ed. Washington, DC: National Academy Press.
- Ohtani H, Saitoh S, Ohkawara H, Akiba Y, Takahashi K, Horiguchi M, Goto K. 1989. Research note: production performance of laying hens fed L-tryptophan. Poult Sci. 68:323–326.
- Pan X, Zongyou W, Hongrong W, Lihuai Y, Xianghuan L. 2013. Effects of dietary tryptophan on protein metabolism and related gene expression in Yangzhou goslings under different feeding regimens. Poult Sci. 92:3196–3204.
- Robbins KR, Saxton A, Southern L. 2006. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. 84:E155–E165.
- Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RFM, Lopes DC, Ferreira AS, Barreto SLT. 2011. Brazilian tables for poultry and swine-composition of feedstuffs and nutritional requirements, 3rd ed. Viçosa, MG, Brazil.
- SAS Institute. 2003. SAS user’s guide: statistics. Version 9.1. Cary, NC: SAS Institute, Inc.
- Yamamoto Y, Glick B. 1982. A comparison of the immune response between two lines of chickens selected for differences in the weight of the bursa of fabricius. Poult Sci. 61:2129–2132.
