ABSTRACT
This study evaluated the changes that occurred in the serum levels of calcium and phosphorus in laying hens infected with velogenic Newcastle disease (ND) virus (vNDV), and their relationship to the decrease in egg production usually associated with ND. Two hundred and forty laying hens (32 weeks old) were randomly assigned into four groups of 60 each viz: VAI – vaccinated with ND vaccines and intramuscularly inoculated with vNDV, VAU – vaccinated uninfected, UNI – unvaccinated infected and UNU – unvaccinated uninfected. At weekly intervals blood was collected from six randomly selected hens in each group for serum calcium and phosphorus assays. Groups VAI and UNI showed a significant (p < .05) drop in egg production. Serum phosphorus levels of groups VAI and UNI were significantly (p < .05) lower than those of groups VAU and UNU. There was a highly positive correlation between serum phosphorus levels and egg production which was highly significant (r = .74; p < .01). The changes in serum calcium levels of infected groups were only slight, and the relationship between serum calcium levels and egg production was low, positive and not significant (r = .26; p > .05). Drop in egg production that occurred in the ND-infected laying hens was positively strongly correlated with the drop in serum phosphorus levels.
1. Introduction
Newcastle disease (ND) is a viral disease of poultry caused by a single-stranded, non-segmented, negative-sense RNA virus known as avian paramyxovirus serotype 1, belonging to the genus Avulavirus, subfamily Paramyxovirinae within the family Paramyxoviridae, order Mononegavirales (Mayo Citation2002; Lamb et al. Citation2005). The disease is worldwide in distribution (Alexander and Senne Citation2008) and is regarded throughout the world as one of the most important poultry diseases. It causes high flock morbidity and mortality in susceptible birds, leading to serious economic losses (Alexander and Senne Citation2008). In countries with intensive poultry farming all over the world, outbreaks of ND pose a risk for the sufficient supply to humans with valuable protein. Additionally, ND outbreaks can cause massive economic damage through control efforts and trade losses (Alexander Citation2001; Alexander and Senne Citation2008). ND belongs to the notifiable diseases of list A of the World Organisation for Animal Health (OIE Citation2013).
Infection with vNDV has been associated with severe systemic disease, accompanied by high morbidity and mortality in poultry (Okoye et al. Citation2000; Igwe et al. Citation2014). It causes drop in egg production and egg quality and a mortality from 0% to 50% in layers in natural outbreaks (Rao et al. Citation2002; Alexander Citation2003; Miller and Koch Citation2013) and affects the reproductive organs of chicken (Biswal and Morrill Citation1954; Rao et al. Citation2002). Drop in egg production and quality/and or poor shell quality are issues that affect producers of high-performing egg layers all around the world. These issues most commonly arise from deficiency, imbalance or malabsorption of calcium, phosphorus or vitamin D3 or due to diseases (Harrison and McDonald Citation2006; Bohn Citation2012). Such disorders may primarily affect the anterior pituitary or hypothalamus, thus interfering with normal luteinizing hormone and follicle-stimulating hormone production (Bentley Citation1998; Dacke Citation2000).
Calcium and phosphorus are considered the main minerals for laying birds, due to their expressive participation in the metabolism, skeletal structure and metabolism, maintenance of production, and in the quality of the egg shell (Dacke Citation2000). During the laying process in hens, the calcium levels can become extremely high, reaching levels of 30 mg/dl (Johnson Citation2000). The involvement of calcium in egg shell formation has been reported to lead to increased intestinal and bone mobilization of calcium which are needed to constantly replenish blood calcium for maximum egg production, quality and hatchability (Johnson Citation2000). In addition, calcium is required for the production of hard-shelled eggs and the rapid growth rate in young birds (Hurwitz Citation1989). Serum calcium is essential for bone homeostasis, muscle and nerve conduction, blood coagulation and the control of hormone secretion, particularly vitamin D3 and parathyroid hormone (Stanford Citation2006). Phosphorus is an essential nutrient for laying hens because of its role in egg shell formation and metabolism (Said et al. Citation1984). Inorganic phosphorus is derived from the diet. It is a major constituent of bone and a vital cellular component, playing important roles in the storage, release, and transfer of energy and in acid–base metabolism. Phosphorus may affect more biological systems than any other element. It is an important element in many body functions including bone formation, acid–base balance, metabolism of fat, carbohydrates, proteins and lipids, and in egg formation (Wideman Citation1987; Pastore et al. Citation2012). In poultry, it has been reported that calcium and phosphorus can be affected by poultry vaccination and diseases (Fernandez et al. Citation1994; Talebi Citation2006).
Calcium and phosphorus metabolism has been well researched in production in laying hens and pet birds for economic reasons (Harrison and McDonald Citation2006; Stanford Citation2006, Citation2007; Käppeli et al. Citation2011). There is however a dearth of reports on serum calcium and phosphorus in laying birds in viral diseased states. Knowing that ND affects most body systems, including the nervous, reproductive, renal and digestive systems which are directly involved in both calcium and phosphorus metabolism and egg formation, there is the need to investigate the changes that occur in serum calcium and phosphorus levels of laying hens infected with ND virus (NDV). The objective of this study was therefore to evaluate changes in serum levels of calcium and phosphorus in laying hens infected with vNDV, and correlate these changes with the drop in egg production that occurs in ND. Furthermore, the importance of calcium and phosphorus for egg production in layers has been described in several previous studies. However, just few documents present studies on layers, and under field conditions just like this document.
2. Materials and methods
This study was scrutinized and approved by the University Committee on Medical and Scientific Research Ethics.
2.1. Hens
Two hundred and forty Isa-Brown pullets obtained from Zartech Farms, Nigeria, were used for the study. They were randomly assigned into four groups of 60 each viz: VAI – vaccinated with ND vaccines and inoculated with vNDV, VAU – vaccinated uninoculated, UNI – unvaccinated inoculated and UNU – unvaccinated uninoculated. Pullets in groups VAI and VAU were given Hitchner B1 vaccine intraocularly as day-old chicks, 4 weeks later LaSota was given orally in drinking water, at 9 and 16 weeks of age Komarov was administered intramuscularly (IM), and oil-emulsion-inactivated vaccine IM was given according to National Veterinary Research Institute, Vom, Plateau State, Nigeria and Biovac®, Israel. Brooding of all the pullets was done on deep litter. Each of the groups was brooded separately under the same environmental conditions. Feed and water were provided ad libitum. The hens were kept in isolation in the Poultry Experimental Unit of the Department under strict biosecurity measures. The daily minimum and maximum temperatures were 24.28°C and 32.19°C, with a mean of 28.24°C, and a relative humidity of about 70% during the rainy season that falls to about 20% during the dry season.
General care of the birds was provided in accordance with the Institutional Animal Care and Use Committee, as outlined in the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching.
2.2. Velogenic NDV inoculum
The virus used was the vNDV strain, duck/Nigeria/903/KUDU–113/1992, which belongs to genotype XV11 (Shittu et al. Citation2016). The virus was isolated in Kuru, Plateau State of Nigeria from an apparently healthy duck and characterized biologically by Echeonwu et al. (Citation1993). The inoculum had a median embryo infective dose (EID50) of 106.46 per ml. It is a velogenic strain of NDV and this pathotype is enzootic in Nigeria and other African countries, Middle and Far East and America where it causes severe outbreaks of ND in commercial farms and indigenous Nigerian chicken (Echeonwu et al. Citation1993; Solomon et al. Citation2012).
2.3. NDV challenge
At the peak of egg production, 32 weeks of age, each hen in groups VAI and UNI was inoculated IM with 0.2 ml of the inoculum on day 0 post infection (PI). Each hen in groups VAU and UNU was inoculated IM with 0.2 ml of phosphate buffered saline (uninfected groups) as placebo.
2.4. Clinical signs
The hens were observed twice daily for clinical signs of ND from days 0 to 49 PI when the last egg samples for the study were collected. The daily and weekly percentage (%) egg production for each group and the egg shell quality parameters were recorded during this period.
2.5. Estimation of biochemical blood parameters
Two millilitres of blood was collected from six randomly selected laying hens in each group on day 0 PI and at weekly intervals for the 49 days PI. Serum samples were harvested and used immediately for determination of serum calcium and phosphorus levels, following standard procedures using Quimica Clinica Applica test kits. The determination of serum calcium was based on the ortho-cresolphthalein direct method (Connerty and Briggs Citation1966), while that of phosphorus was based on the Fiske-SubbaRow method (Fiske and SubbaRow Citation1925; Godwin Citation1970).
2.6. Enzyme linked immunosorbent assay
Blood samples were collected from 10 hens in each group on days 0, 10, 15 and 21 PI. The separated serum was stored at −20°C until used for enzyme linked immunosorbent assay (ELISA) test. ELISA was performed on all sera at a 1:500 final working dilution using commercial ND antibody test kits that were purchased from IDEXX Laboratories Inc. and graciously provided by Southeast Poultry Research Laboratory, Athens, Georgia. Duplicate titres were obtained and calculated using XCHEK software (IDEXX Laboratories Inc). An optical density of 650 nm wavelength was used to detect the colour change using an Emax reader (Molecular Devices, Sunnyvale, CA).
2.7. Statistical analyses
Data generated for the study were subjected to one-way analysis of variance. Variant means were separated post hoc using the least significant difference method (Okafor Citation1992). Probabilities less than or each at 0.05 were accepted as significant.
3. Results
3.1. Clinical signs
There was a drastic drop in egg production in both infected groups (VAI and VAU) of hens beginning at week 1 PI, with the egg production of these groups being significantly (p < .05) lower than those of groups VAU and UNU (). The egg production in group UNI was significantly (p < .05) lower than those of groups VAU and UNU all through the experiment even when there was improvement from their very low week 2 PI values from week 4 PI onwards. The egg production in group VAI was also significantly (p < .05) lower than those of groups VAU and UNU all through except on week 4 PI, and their recovery to almost normal production levels was better than that of group UNI (). Only in group UNI hens produced white-coloured (bleached) soft-shelled and cracked eggs by day 6 PI. Misshapen and ridged eggs with different shades of colour (bleached and brown) were observed by day 10 PI in hens that survived, and persisted to the end of the experiment (). There was no change in egg shell colour and quality in group VAI (). Torticollis and paralysis of the wings and limbs were observed in group UNI on day 6–9 PI and persisted to days 18–21 PI. The lesions and the results of the immunohistochemical studies will be presented in another publication.
Figure 1. Bleached, misshapen and ridged eggs laid between days 10 and 21 PI by group UNI laying hens compared with group UNU.
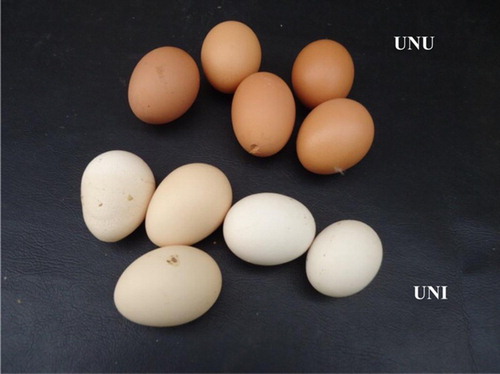
Figure 2. No appreciable change in egg shell colour and quality of eggs laid by VAI laying hens compared with VAU throughout the experimental period.
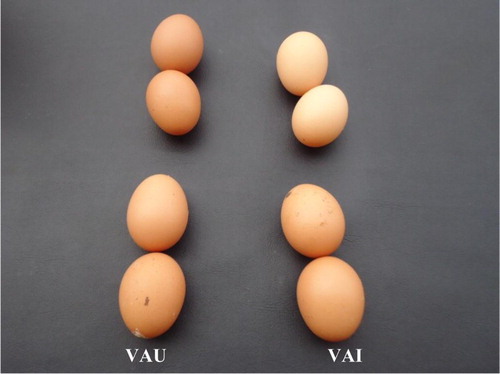
Table 1. Means weekly percentage egg production ± standard error mean of laying hens experimentally infected with vNDV, compared with uninfected groups.
3.2. Effect on serum calcium levels
The serum calcium levels of group UNI were significantly (p < .05) higher than that of group VAU on day 7 PI (). On day 14 PI, the serum calcium levels of hens in group VAI were significantly (p < .05) lower than those of groups VAU and UNU (), while on day 21 PI, that of group UNI was significantly (p < .05) lower than those of hens in groups VAU and UNU (). There were no significant (p > .05) variations in serum calcium levels on days 28 and 35 PI. On day 42 PI, the serum calcium level of group VAI was significantly (p < .05) lower than those of hens in groups VAU, UNI and UNU, while on day 49 PI, that of group UNI was significantly (p < .05) lower than those of groups VAU and UNU (). The correlation between serum calcium levels and egg production was positive, direct and low, and not significant (r = .26; p > .05).
Table 2. Means levels of serum calcium (mg/dl) ± standard error mean of laying hens experimentally infected with vNDV, compared with uninfected groups.
3.3. Effect on serum phosphorus levels
There was a decrease in serum phosphorus levels following infection (). On day 7 PI, the serum phosphorus levels of the hens in groups VAI and UNI were significantly (p < .05) lower than that of group UNU, but on day 14 PI that of group VAI were significantly (p < .05) lower than that of hens in groups VAU and UNU (). On day 21 PI, the serum phosphorus levels of the hens in groups VAI and UNI were significantly (p < .05) lower than those in groups VAU and UNU, while on day 28 PI that of group UNI was only significantly (p < .05) lower than that of group UNU (). On day 35 PI, the serum phosphorus levels of group UNI were significantly (p < .05) lower than that of group VAU, while on day 42 PI that of group UNI was significantly (p < .05) lower than that of group UNU (). On day 49 PI, the serum phosphorus levels of the hens in groups VAI and UNI were significantly (p < .05) lower and almost half of the levels in groups VAU and UNU (). The correlation between serum phosphorus and egg production was high, positive (direct) and significant (r = .74; p < .01).
Table 3. Means levels of serum phosphorus levels (mg/dl) ± standard error mean of laying hens experimentally infected with vNDV, compared with uninfected groups.
3.4. Enzyme linked immunosorbent assay
All IDEXX ELISA titres of ≤396 are negative for NDV antibodies. The antibody titres were negative at day 0 PI in the unvaccinated groups while vaccinated laying hens had high average titres of between 1473 and 1484 (). After challenge, sero conversion occurred and the titres rose in both groups VAI and UNI at days 10, 15 and 21 PI. Throughout the experiment, titres of the hens of the UNU group remained negative while that of the VAU fluctuated from days 10–21 PI, but still maintained above titre 396.
Table 4. Means levels of serum ELISA antibody titres (log10) ± standard error mean of laying hens experimentally infected with vNDV, compared with uninfected groups.
4. Discussion
The findings in this present study of significantly lower serum phosphorus levels in the infected groups and its high, positive (direct) and significant correlation with drastic drop in egg production showed a significant adverse effect of ND on serum phosphorus concentrations. This corresponds to the findings in psittacine birds (Stanford Citation2006) and with several diseases in pet birds (Hochleithner Citation1994) in which dietary phosphate deficiency in egg laying birds reduces both egg numbers and fertility rates in psittacine birds (Stanford Citation2006) and in which changes in inorganic phosphorus concentration can occur with several diseases in pet birds (Hochleithner Citation1994). In laying hens and young birds inorganic phosphate could be affected by poultry vaccinations and diseases (Fernandez et al. Citation1994; Talebi Citation2006). Lloyd and Gibson (Citation2006) observed a decrease in the level of serum calcium and phosphorus with moderate and severe Spironucleus-infected pheasants. Similarly, Fernandez et al. (Citation1994) recorded a decrease in calcium and phosphorus concentrations in laying hens fed aflatoxin-containing feed. Inorganic phosphorus is derived from the diet and is important in egg shell formation, acid–base metabolism, muscle and nerve conduction, a major constituent of bone, and a vital cellular component, playing important roles in the storage, release and transfer of energy. Drop in egg production, egg abnormalities and significantly lower serum phosphorus levels were found to be strongly related in this study. Decreased serum inorganic phosphate levels may occur from hypovitaminosis D due to reduced production of metabolite 1,25(OH)2D3 in the degenerated kidneys observed in this study. ND has been reported to cause degeneration and necrosis of kidneys (Brown et al. Citation1999; Okoye et al. Citation2000; Igwe et al. Citation2013, Citation2014). Significantly decreased serum phosphorus levels in the infected groups (VAI and UNI) of laying hens were also found to be correlated to the period of drop in egg production and quality and has several practical implications, apart from drop in egg production, because serum phosphorus is important in egg shell formation, acid–base metabolism and muscle and nerve conduction. Neurological signs were observed in unvaccinated infected group during the infection, while ND in chicken caused severe gastrointestinal, kidney and reproductive lesions which could have affected calcium and phosphorus metabolism and homeostasis and the active form of vitamin D3 involved in the biosyntheses of Ca-binding protein. In birds, this protein is involved in active transport of Ca across the intestinal and uterine wall for bone and egg shell formation (Keshavarz Citation2003; Bohn Citation2012; Campbell Citation2012). This might have led to reduction in egg production in infected groups, and egg quality in unvaccinated infected group observed in the present study.
There was a gradual continual increase in serum calcium in the two uninfected groups (groups VAU and UNU) from week 01 and throughout the period of the experiment. This is in agreement with reports of increase in plasma Ca levels at the beginning of laying period of hens and subsequent gradual increase reported by Pavlik et al. (Citation2009) and Käppeli et al. (Citation2011).
The slightly higher serum calcium levels of unvaccinated infected hens recorded in the present study could be a result of dehydration which was one the clinical signs of ND which could have been caused by reduced feed and water consumption and diarrhoea observed in this group within this period. This corresponds to the findings where significant increase in serum calcium levels has been reported with dehydration and disease states affecting the digestive tract in pet birds/avian species (Hochleithner Citation1994; Harr Citation2009). The slightly higher serum calcium levels in unvaccinated infected hens at day 7 PI may also be closely related to severe inflammatory changes, severe morphological damage caused by vNDV in reproductive tract of laying hens notably the shell gland affecting its functional role of calcium absorption, with resultant effect of calcium accumulation in the blood, and not depletion of calcium stores due to ND. The slight increase in the serum calcium level at day 7 PI was followed by a varying pattern of decreased serum calcium levels in the infected groups (VAI and UNI) throughout the experimental period. Campbell (Citation2012) reported that calcium for egg formation is derived from intestinal absorption and bone mobilization. Decreased serum calcium levels have been found to be associated with malabsorption or maldigestion disease process in animals (Bohn Citation2012) and this malabsorption could interfere with the intestinal and uterine absorption of calcium across the intestinal and uterine wall for bone and eggshell formation (Keshavarz Citation2003; Bohn Citation2012; Campbell Citation2012).
Vaccination is used as a control measure and to improve productivity; however, the present study showed that vaccination may not fully protect hens against drop in egg production due to vNDV infection. This may suggest that vaccination was however not effective in preventing damage of the organs and tissues that are directly or indirectly related to egg laying which could account for slight decrease in calcium and significant decrease in phosphorus levels in the serum.
5. Conclusion
Based on the results of this study, it was concluded that NDV infection in laying hens is associated with significant decrease in serum phosphorus levels which were strongly positively correlated with the drop in egg production.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alexander DJ. 2001. Newcastle disease. Br Poult Sci. 42:5–22. doi: 10.1080/713655022
- Alexander DJ. 2003. Newcastle disease and other avian paramyxoviridae infections. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of poultry. 11th ed. Ames (IA): Iowa State University Press; p. 63–87.
- Alexander DJ, Senne DA. 2008. Newcastle disease, other avian paramyxoviruses, and pneumovirus infections. In: Saif YM, Fadly FA, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. Diseases of poultry. 12th ed. Ames (IA): Blackwell; p. 75–93.
- Bentley PJ. 1998. Hormones and calcium metabolism. In: Bentley PJ, editor. Comparative vertebrate endocrinology. Cambridge (UK): Cambridge University Press; p. 269–301.
- Biswal G, Morrill CC. 1954. The pathology of the reproductive tract of laying pullets affected with Newcastle disease. Poult Sci. 33:880–897. doi: 10.3382/ps.0330880
- Bohn AA. 2012. Laboratory evaluation of electrolytes. In: Thrall MA, Weiser G, Allison R, Campbell TW, editors. Veterinary hematology and clinical chemistry. 2nd ed. Ames (IA): Wiley; p. 378–392.
- Brown C, King DJ, Seal BS. 1999. Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence. Vet Pathol. 36:125–132. doi: 10.1354/vp.36-2-125
- Campbell TW. 2012. Haematology of birds. In: Thrall MA, Weiser G, Allison R, editors. Veterinary hematology and clinical chemistry. 2nd ed. Ames, IA: Wiley; p. 238–276.
- Connerty HV, Briggs AR. 1966. Determination of serum calcium by means of ortho-cresolphthalein complexone. Am J Clin Pathol. 45:290–296. doi: 10.1093/ajcp/45.3.290
- Dacke GC. 2000. Parathyroids, calcitonin, and vitamin D. In: Whittow GC, editor. Sturkie’s avian physiology. 5th ed. London: Academic Press; p. 472–485.
- Echeonwu GON, Ireogbu CI, Emeruwa AC. 1993. Recovery of velogenic Newcastle disease virus from dead and healthy free roaming birds in Nigeria. Avian Pathol. 22:383–387. doi: 10.1080/03079459308418928
- Fernandez A, Verde MT, Gascon M, Ramos J, Gomez J, Luco DF, Chavez G. 1994. Variations of clinical biochemical parameters of laying hens and broiler chickens fed aflatoxin-containing feed. Avian Pathol. 23:37–47. doi: 10.1080/03079459408418973
- Fiske CH, SubbaRow Y. 1925. The colorimetric determination of phosphorus. J Biol Chem. 66:373–400.
- Godwin JF. 1970. Quantification of serum inorganic phosphorus, phosphatise and urinary phosphate without preliminary treatment. Clin Chem. 16:776–780.
- Harr KE. 2009. Diagnostic value of biochemistry. In: Harrison GJ, Lightfoot TL, editors. Clinical avian medicine. Ithaca (NY): International Veterinary Information Service, A3830.0909. http://www.ivis.org.
- Harrison GJ, McDonald D. 2006. Nutritional considerations – section II: nutritional disorders. In: Harrison GJ, Lightfoot TL, editors. Clinical avian medicine. Ithaca (NY): International Veterinary Information Service: A3810.0806, www.clinicalavianmedicine.com/. http://www.ivis.org.
- Hochleithner M. 1994. Biochemistries. In: Ritchie BW, Harrison GJ, Harrison LR, editors. Avian medicine principles and application. Lake Worth (FL): Wingers; p. 223–245.
- Hurwitz S. 1989. Calcium homeostasis in birds. Vitam Horm. 45:173–221. doi: 10.1016/S0083-6729(08)60395-7
- Igwe AO, Ezema WS, Eze DC, Okoye JOA. 2014. Experimental velogenic Newcastle disease can be very severe and viscerotropic in chickens but moderate and neurotropic in guinea fowls. Int J Poult Sci. 13:582–590. doi: 10.3923/ijps.2014.582.590
- Igwe AO, Ezema WS, Ibu JI, Eze JI, Okoye, JOA. 2013. Comparative study on the haematology and persistence of velogenic Newcastle disease virus in chickens and guinea fowls. Res Opin Anim Vet Sci. 3:136–142.
- Johnson AL. 2000. Reproduction in the female. In: Wittow GC, editor. Sturkie’s avian physiology. 5th ed. London: Academic Press; p. 568–591.
- Keshavarz K. 2003. A comparison between cholecalciferol and 25-OH-cholecalciferol on performance and eggshell quality of hens fed different levels of calcium and phosphorus. Poult Sci. 82:1415–1422. doi: 10.1093/ps/82.9.1415
- Käppeli S, Fröhlich E, Gebhardt-Henrich SG, Pfulg A, Schäublin H, Zweifel R, Wiedmer H, Stoffel MH. 2011. Effects of dietary supplementation with synthetic vitamin D3 and 25-hydroxycholecalciferol on blood calcium and phosphate levels and performance in laying hens. Arch Geflügelk. 75:179–184.
- Lamb RA, Collins PL, Kolakofsky D, Melero, JA, Nagai Y, Oldstone MBA, Pringle CR, Rima BK. 2005. Paramyxoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy. Amsterdam: Elsevier; p. 655–668.
- Lloyd S, Gibson JS. 2006. Hematology and biochemistry in healthy young pheasants and red-legged partridges and effects of spironucleosis on these parameters. Avian Pathol. 35:335–340. doi: 10.1080/03079450600821794
- Mayo MA. 2002. A summary of the changes recently approved by ICTV. Arch Virol. 147:1655–1656. doi: 10.1007/s007050200039
- Miller PJ, Koch G. 2013. Newcastle disease, other avian paramyxoviruses and metapneumovirus infections. In: Swayne DE, Glisson JR, McDougald LR, editors. Diseases of poultry. 13th ed. Ames, Iowa, USA: Wiley; p. 89–138.
- [OIE] Office International des Epizooties. 2013. Old classification of disease notifiable to the OIE list A. The World Animal Health Information System. http://www.oie.int/en/animal-health-in-the-world/the-world-animal-health-information-system/old-classification-of-diseases-notifiable-to-the-oie-list-a/.
- Okafor LC. 1992. Biometry – basic principles and approaches. Geelink; p. 141–172.
- Okoye JOA, Agu AO, Chineme CN, Echeonwu GON. 2000. Pathological characterization in chickens of a velogenic Newcastle disease virus isolated from guinea fowl. Rev Elev Med Vet Pays Trop. 53:325–330. doi: 10.19182/remvt.9709
- Pastore SM, Gomes PC, Rostagno HS, Albino LFT, Calderano AA, Vellasco CR, da Silva Viana G, de Almeida RL. 2012. Calcium levels and calcium: available phosphorus ratios in diets for white egg layers from 42 to 58 weeks of age. Rev Bras Zootec. 41:2424–2432. doi: 10.1590/S1516-35982012001200007
- Pavlík A, Lichovníková M, Jelínek P. 2009. Blood plasma mineral profile and qualitative indicators of the eggshell in laying hens in different housing systems. Acta Vet Brno. 78:419–429. doi: 10.2754/avb200978030419
- Rao MS, Raj GD, Manohar BM. 2002. An in vitro and in vivo evaluation of the virulence of Newcastle disease virus and vaccines for the chicken reproductive tract. Avian Pathol. 31:507–513. doi: 10.1080/0307945021000005888
- Said NW, Sullivan TW, Sunde ML, Bird HR. 1984. Effect of dietary phosphorus level and source on productive performance and egg quality of two commercial strains of laying hens. Poult Sci. 63:2007–2019. doi: 10.3382/ps.0632007
- Shittu I, Sharma P, Joannis TM, Volkening JD, Odaibo GN, Olaleye, DO, Williams-Coplin, D, Solomon P, Abolnik C, Miller PJ, et al. 2016. Complete genome sequence of a genotype XVII Newcastle disease virus, isolated from an apparently healthy domestic duck in Nigeria. Genome Announc. 4(1):e01716–e01715.
- Solomon P, Aboinik C, Joannis TM, Bisschop S. 2012. Virulent Newcastle disease virus identification of a new clade of sub-lineage5f from live-bird markets. Virus Genes 44:98–103. doi: 10.1007/s11262-011-0678-5
- Stanford M. 2006. Calcium metabolism. In: Harrison GJ, Lightfoot TL, editors. Clinical avian medicine. Vol. 1. Palm Beach (FL): Spix; p. 141–151.
- Stanford M. 2007. Clinical pathology of hypocalcaemia in adult grey parrots (Psittacus e erithacus). Vet Rec. 161:456–457. doi: 10.1136/vr.161.13.456
- Talebi A. 2006. Biochemical parameters in broiler chickens vaccinated against ND, IB and IBD. Int J Poult Sci. 5:1151–1155. doi: 10.3923/ijps.2006.1151.1155
- Wideman RF. 1987. Renal regulation of avian calcium and phosphorus metabolism. J Nutr. 117:808–815.
