ABSTRACT
The Integral Traceability System for tracking and tracing the milk samples used in quality control was checked for one year while monitoring 526 milk samples from sheep's, goats’ and cows’. This system includes a customized automated cooler for carrying samples with a smart sensor inside to store the data collected during the process, and a dongle to transfer the collected data to a computer to be further analysed. The technologies combined to record and trace milk samples on trips from farms to the laboratory (e.g. microcontrollers, sensors, radio frequency identification and global positioning system) were linked. This system allowed us to objectively know the duration of the sampling route and the temperature and time conditions of samples travelled in until they were analysed in an official dairy laboratory. These conditions ensured that the baseline milk quality was preserved, and was therefore adequate according to both European regulations and the price set to be paid for quality. Hardware and software prototypes worked successfully under the real study conditions, and this system may be proposed to become a reference method in the dairy sector.
KEYWORDS:
1. Introduction
Milk is a fragile substance, thus preserving its quality right from milking until it is processed in the dairy industry has always been a challenge and a permanent concern. To achieve this, it is essential to obtain good-quality milk from primary production and to maintain it until it is processed in the dairy industry by keeping any possible disruption to a minimum (Pirisi et al. Citation2007; Zeng et al. Citation2007; Franciosi et al. Citation2011). In order to avoid risks, and to ensure hygiene-sanitary quality and raw cows’, sheep's and goats’ milk safety, in its Regulations (EC) Nos. Citation852/Citation2004 and Citation853/Citation2004 European legislation lays down general food hygiene rules and specific ones for food of animal origin. It also sets out aspects relating to mandatory controls (EC) No. Citation853/Citation2004 on raw milk production on farms, and in dairy centres and laboratories. Raw milk has to be tested for not only its physico-chemical composition, but also for its hygienic characteristics, such as microbiology, somatic cell count (SCC) and absence of drug residues (EC No. Citation853/Citation2004).
Minimizing health risks from milk and dairy products requires a system of continuous preventive measures, starting with animal feed suppliers, moving on to farmers and on-farm controls (including the prudent use of veterinary drugs), to milk processors and the application of good hygiene practices and food safety management systems throughout the chain (Voulodimos et al. Citation2010; Charlebois and Haratifar Citation2014).
In the European Union, Regulation EC No. 178/2002 requires mandatory traceability for all food, feed, food producing animals at all stages of production, processing and distribution within its territory. Furthermore, several laws have been published in Spain to ensure the hygienic-sanitary quality and safety of raw milk by enforcing European law. It establishes a computer traceability management system based on barcodes named LeTrA Q. One aspect to which little importance has been attached is the traceability of milk samples collected for official laboratory analysis. In fact, we sometimes forget that the quality of the milk contained in the farm bulk tank depends basically on this sample and so, therefore, does the price that will be paid for it to farmers (De Garnica et al. Citation2011; Malacarne et al. Citation2013).
The traceability systems used in the dairy industry should, therefore, reliably ensure and inform the inalterability of milk samples taken from farms to labs. In fact, food safety traceability may be used as a tool to manage the risks related to food safety and animal health issues, and to improve the quality and processes of products by identifying non-compliances (Charlebois and Haratifar Citation2014).
Thus, it would be very interesting for the dairy sector to have a system available that traces the collection and transport conditions of the milk samples destined to milk quality control, preserves their quality and also ensures their inviolability and traceability (Wang et al. Citation2010; De las Morenas et al. Citation2014; Sun et al. Citation2016).
Current procedures for the traceability of samples are based on barcodes, but many limitations for them have been shown, e.g. to offer only a simple reading at the same time, less data capacity and non-sustainability against moisture and dirt (Charlebois et al. Citation2014). Radiofrequency systems (radiofrequency identification (RFID)) are being imposed in the agro-food sector (Voulodimos et al. Citation2010; Wang et al. Citation2010; Charlebois et al. Citation2014) as they offer versatility, robustness and easy use (Wang et al. Citation2010; De las Morenas et al. Citation2014). Good examples of using this technology are the development of systems that track livestock management (Voulodimos et al. Citation2010), those that monitor the raw milk transportation system (Sun et al. Citation2016). Active RFID tags are the most cutting-edge technology for supply-chain integrity and traceability, and can automatically capture a range of information about product origin, properties and data (Wang et al. Citation2010; Charlebois et al., 2015).
It would also be very interesting to be able to automate the identification and monitoring of milk samples from farms to the dairy laboratory, and such a prototype traceability system for the dairy industry (TRAZALE) has been designed (De las Morenas et al. Citation2014), which offers high-quality tracking of collected milk samples by controlling certain critical parameters, such as temperature, transport time and the path route.
Therefore, the aim of this work consists in testing a traceability system through RFID, the TRAZALE system, on dairy commercial farms of sheep, goats and cows during a one-year study in a southeast area of Spain called Castilla-La Mancha.
2. Material and methods
2.1. Milk samples
Over a one-year period (August 2015–July 2016), and in the different seasons (summer, autumn, winter and spring), a follow-up was done of the milk collection routes on sheep, goat and cow farms in southeast Spain, which covers approximately 85,000 km2 in what is the Spanish Castilla-la Mancha Region. Collection was organized on 40 routes (10 routes in each season), tracing 194 farms: 103 sheep, 60 goats and 31 cow farms, which represent approximately 5% of the dairy farms in this region. During sampling, process monitoring was carried out using the TRAZALE system that had been set up on a tanker, and constantly controlled that temperature (Tª) set out by regulations (0–8°C, LeTra Q Citation2017). The system also recorded the time when samples were collected, the location of the farm and transport time (t). When each route finished, samples were stored at the dairy plant, where they were kept under controlled conditions of Tª and t until sent to the regional Dairy Laboratory (LILCAM), where they were analysed.
The system proposed to maintain the quality of the milk samples from their collection point begins with sample collection on the farm itself. While collecting milk from a farming area, a lorry visits each farm and stores milk in its tank. When milk is collected from each producer, a 40-mL milk sample is taken, preserved with Azidiol at a concentration of 3.3 μL/ml (i.e. 133 μL/40 ml) (LeTra Q Citation2017), which is stored in a cooler that is kept refrigerated at 4°C until it can be analysed. According to De Garnica et al. (Citation2011), only one AZ-preserved milk sample could be sufficient for composition, SCC and bacterial count analyses from the bulk tank or silo milk.
In order to collect samples, containers with radiofrequency tags (RFID) were used which identified them individually. During the collection route, samples were placed inside refrigerators equipped with portable RFID readers, which had to identify them before authorizing their opening and storage. When the route had finished, all the collected samples were sent to LILCAM for their physico-chemical and hygiene-sanitary analysis, whose results report on compliance with European and Spanish legislation, and determine the price that the farmer is paid for supplying milk.
2.2. Laboratory analyses
A total of 526 bulk milk samples were analysed at LILCAM. The milk samples’ composition (total solids (TS), fat, protein, lactose and non-fat solids) were determined in FT120 equipment (Foss Electric, Hillerød, Denmark), subjected to quality controls and inter-comparative trials. The means values ± SD for fat, total protein and lactose were expressed as g/100 g. SCC were determined by the level of somatic cells, calculated by flow cytometry in a Fossomatic FC (Foss Electric, Hillerød, Denmark), where the results appear as SCC/ml and geometric mean (×103 cells/ml). The total bacterial count (TBC) was analysed in a BactoScan FC (Foss Electric, Hillerød, Denmark), and the results were expressed as log cfu/ml and geometric mean (×103 cfu/ml).
2.3. The TRAZALE system
The proposed system (De las Morenas et al. Citation2014; Garcia et al. Citation2015) is divided into four basic components: (1) sample tubes provided by RFID tags; (2) the electronic tube that records all the information, which the authors call a ‘smart sensor’; (3) the cooler that hosts the GPS and RFID units, and is capable of writing information inside the electronic tube through a radio link and (4) the USB dongle for downloading the information stored in the electronic tube to a computer in the lab trough the radio link.
2.4. Statistical analysis
In order to study whether the effect of species or season on raw milk composition and hygienic parameters was significant, linear models were investigated. As several farms were visited more than once, linear mixed effects models were fitted, including the farm as random effect.
Significance tests for the factor effects were performed. Furthermore, Tukey contrasts were used to make pairwise comparisons between level factors.
All the tests were performed using the R statistical software version 3.4.1 (R Core Team Citation2017). The linear mixed models were fitted via the ‘lme4’ package, version 1.1.13 (Bates et al. Citation2015), while the Tukey contrasts were done with the ‘multcomp’ package, version 1.4-7 (Hothorn et al. Citation2008).
3. Results and discussion
3.1. Milk traceability
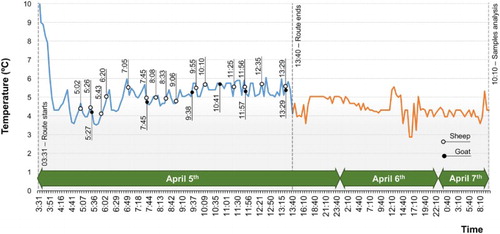
Apart from analysing the milk samples’ composition at LILCAM, after each route had finished all the information stored in the record sensor (device number 2 ‘smart sensor’ in Section 2.3) was downloaded by the data management software. This software can graphically represent the stored information () or can export it to a text file to be processed in a spreadsheet. After exporting the information, the device can be reset to be reused on successive routes (De las Morenas et al. Citation2014).
The mean Tª and t values, and the number of collection points, on the different studied routes (10 per season) over a one-year period (a total of 40 routes) appear in . Here we see wide variability according to the route's characteristics; i.e. if the milk comes from a single species; the number of farms where milk is collected (from six farms with only sheep or cow to 25 sheep and goat farms), or the furthest or shortest distance to the Dairy Plant, or even the distance to LILCAM.
Table 1. Total number of samples routes per year, season, and temperature and analysis time (mean ± standard deviation).
The mean t value that elapses since the first milk sample was collected on each route until its analysis is finally recorded at LILCAM was 46.99 h, time period during which they were kept under refrigerated conditions (4.67°C) thanks to the TRAZALE system cooler feature that ensures that regulations are met (PMO Citation2015; LeTra Q Citation2017). The refrigerated conditions, as well as preservative Azidiol being added, ensure minimum milk spoilage, and favour more accurate results (De Garnica et al. Citation2011). Thus, milk characteristics will be similar to the onset ones on the respective farm and, therefore, the inalterability that the system provides will help farmers be paid the fairest price for the milk they produce (Pirisi et al. Citation2007). At the same time, the milk with better raw milk baseline quality will provide a longer shelf life if it is thermally treated; e.g. pasteurization (Barbano et al. Citation2006). Factors such as Tª and t, along with the milk's bacteriology, are those that most strongly influence the quality of milk during its collection and transport until it reaches the dairy industry (Guinot-Thomas et al. Citation1995; Franciosi et al. Citation2011).
Likewise, microbial growth can be controlled by maintaining milk at a Tª below 10°C (Malacarne et al. Citation2013; PMO Citation2015) and with preservative Azidiol because there were no differences between the baseline raw milk and the refrigerated one as the microbial population is similar in cows’ milk. Nevertheless, at the same time there was a risk of psychrotolerant microorganism growth proliferating under these conditions (refrigeration time longer than 24 h), which can be a major cause of milk spoilage (Quigley et al. Citation2013). If storage under refrigeration conditions goes from 25 to 48 h, it can lead to a marked increase in the total flora of small ruminants’ milk (Zeng et al. Citation2007; Yamazi et al. Citation2013), particularly if the milk sample is not preserved with Azidiol (De Garnica et al. Citation2011). This is because Tª and t periods need to be monitored since a period longer than 24 h at 8–10°C can lead to marked milk spoilage, and also to an impaired rennet coagulation aptitude when making cheese (De Garnica et al. Citation2011; Malacarne et al. Citation2013).
During the four seasons, six samples were sampled from the shortest route, while 25 samples were sampled during the second and the third seasons (the longest), with barely any variability noted. Milk production is quite stable all year long, basically with cows, and the same can be stated for goats and sheep, but to a lesser extent. This has been made possible, thanks to the appearance of management techniques in farming systems that are increasingly more intensive, and control reproduction, feeding and milking, among other aspects such as residues in milk (Pirisi et al. Citation2007; Beltrán et al. Citation2015).
The use of RFID elements to carry out follow-ups of the samples obtained during milk collection processes on sheep, goat and cow farms in Castilla-La Mancha allows one to monitor and follow-up important variables while they are transported by means of a wireless sensorial network fitted to sample containers (De las Morenas et al. Citation2014).
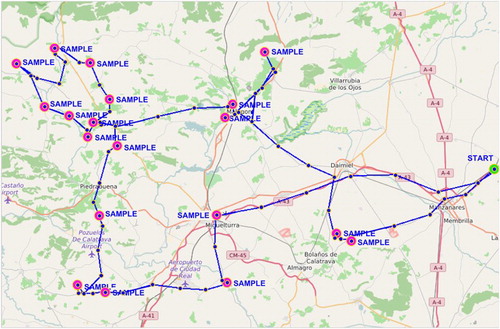
By way of example, the graphical representation of the route and transport conditions, which the samples obtained from monitored dairy ruminant farms are submitted to, are presented in the . In the course of the route, location maps to trace the route that passes through the farms where milk is collected at (). The route corresponds to sheep and goat farms, and the figure provides us with information about when it started (3.31 am) and when the sample was collected on the first farm (5.02 am), which was placed inside a TRAZALE system refrigerator, at a Tª of around 5°C. Collection of milk samples continued and lasted 10 h, approximately. In all, 21 milk samples were collected (15 sheep and 6 goat samples), but 17 farms were visited. This was because there was sheep's and goats’ milk on four farms, but in separate tanks and with individualized treatments. The GPS device that is built in the system allowed the milk collection route to be traced on a map, and pointed out the point at which each sample had been collected (). It can be considered one of the longest route, not only because of the number of farms involved, but also due to the distance among them, and it was possible to detect any problem that could occur during the route. When the route finished (1.40 pm), the samples remained with the sensor at the dairy industry, where their Tª and t conditions continued to be monitored until they were taken to LILCAM two days after, at 8.10 am. In general, the analyses of all samples were done in an interval of 44.40–53.10 h. After the first milk sample had been collected, milk samples were kept at a Tª that neither exceeded 6°C nor went below 2°C, such conditions ensure the control of total flora developing (Zeng et al. Citation2007; De Garnica et al. Citation2011; Quigley et al. Citation2013), and also prevents the risk of psychrotrophs developing or other technological parameters being altered (Malacarne et al. Citation2013). Furthermore, it is considered that Azidiol-preserved sheep's milk kept at 4°C is suitable for maintaining the initial concentration for all the studied bacterial groups and species, particularly for mesophilic, psychotropic and coliform organisms for 96 h (De Garnica et al. Citation2011).
At the end of each route and at the Lab, tags can be removed from container tubes, and can be reset and used again even though they are not expensive. The system also allows a lot more information to be included in a tag than in a bar code (De las Morenas et al. Citation2014; Sun et al. Citation2016), which ensures the possibility of transporting samples over long distances and quality, and requires efficient traceability techniques. There are other benefits offered by applying efficient traceability systems in the dairy industry; for example, dairy farmers benefit from improved reliability for preserving sample quality as traceability methods would help avoid the possibility of their high-quality supply being affected by lower quality supplies from other farmers (Sun et al. Citation2016). The laboratory personnel will be able to identify any problem and allocate responsibility to a specific farm. Finally, consumers will benefit from a higher quality product (De las Morenas et al. Citation2014).
3.2. Milk composition
The mean chemical composition values (TS, fat, protein, lactose and non-fat solids) of the 527 analysed tanked milk samples were distinguished according to species ().
Table 2. Chemical composition (g/100 g) of sheep, goat and cow bulk milk in collected samples.
Evidently the mean values of each parameter significantly differed (P < .01–.001) because species is one of the main factors of variation in milk composition (Pirisi et al. Citation2007; Sanz Ceballos et al. Citation2009). According to the TS parameter, the bibliography describes that sheep's milk is much richer (∼19.2%), followed by goats’ milk with 14.5%, which is slightly higher than cows’ milk (12.64%).
With small ruminants (sheep and goats), the TS values were higher than those reported in the reviews by Park et al. (Citation2007) and by Balthazar et al. (Citation2017), or than those indicated by other authors who have worked with milk of these species (Sánchez et al. Citation2005; Sanz Ceballos et al. Citation2009; De Garnica et al. Citation2011). This may be due to the characteristics of the farming systems employed in the study area, and specifically to the autochthonous breeds used there, these being sheep breed Manchega and goat breed Murciano-Granadina, present in the majority of flocks in Castilla-La Mancha. These breeds are characterized by their high fat content (7.8 and 5.4 g/100 g, respectively) and for their protein content (5.9 and 3.8 g/100 g, respectively), which make them a very suitable raw material to be processed and turned into dairy by-products, particularly traditional high-quality cheeses and fermented milks (Pirisi et al. Citation2007; Sanz Ceballos et al. Citation2009; Beltrán et al. Citation2015).
For cows’ milk composition parameters, the mean values of each parameter fell within the normal range for this species (3.84 g/100 g for fat and 3.40 g/100 g for protein), where the herd milk breed (Holstein Friesian) and farming conditions were highly standardized (use of stables and feed with unifeed) compared to other studies carried out with refrigerated samples (Franciosi et al. Citation2011; Malacarne et al. Citation2013; Balthazar et al. Citation2017).
Lactose was the most stable component, although significant differences were found depending on species (P < .01) as the highest and different values were obtained for cows compared to small ruminants’ milk.
Regarding the time of year when milk samples were collected, the fat and protein values were significantly higher during end of autumn and winter, which coincided with the end of milking, and with lower temperatures when milk production lowered and became enriched.
Bulk tank SCC and TBC () studies are principal tools used by technicians and farmers to evaluate udder health, as well as the efficiency of production processes and cleaning and sanitation practices (Gonzalo et al. Citation2005, Citation2010; De Garnica et al. Citation2011).
Table 3. Least square means, standard errors, and geometric means of SCC and TBC of sheep, goat and cow bulk milk in collected samples.
The level of somatic cells in small ruminants’ milk resulted in log SCC values of 6.01–6.31 (geometric mean: 1.032–2.064 × 103 cells/ml), and were similar to those indicated by other authors (Raynal-Ljutovac et al. Citation2007), or were slightly higher (Martínez et al. Citation2003; Sánchez et al. Citation2005), but in this case, this took place in individual samples. Such a high value could be associated with lactation status (Raynal-Ljutovac et al. Citation2007; Zeng et al. Citation2007), particularly in goats in the last three milking months, when the risk of this parameter increasing is higher. And without being necessarily related to mammary infection, since many non-infectious factors cause considerable variation in SCC (Raynal-Ljutovac et al. Citation2007). This very useful indicator of udder health status in dairy cows (Barbano et al. Citation2006) has been related to intra-mammary infections and to milk yield losses (Gonzalo et al. Citation2005, Citation2010) in dairy sheep. However, it is still a matter of debate in sheep and goats, and could be one of the reasons why its levels have still not been included in European legislation (EC No. Citation853/Citation2004). In any case, the obtained values do not adapt to, for instance, the regulations of those countries with legislation on this matter, such as the USA (PMO Citation2015), which sets a maximum limit of 750 × 103 cells/ml in sheep's milk and one of 1500 × 103 cells/ml in goat's milk. An increase of SCC is related to proteolytic and lipolytic activity that might modify milk composition (Baudry et al. Citation1997; Raynal-Ljutovac et al. Citation2007; Gonzalo et al. Citation2010), which would result in losses of cheese-making aptitudes and in developing sensorial defects (Barbano et al. Citation2006; Raynal-Ljutovac et al. 2017).
The higher counts in goats’ milk evidence the specific circumstances related to this species (Baudry et al. Citation1997; Sánchez et al. Citation2005) even though SCC is an important milk component as regards quality, hygiene and mastitis control aspects (Martínez et al. Citation2003).
Nevertheless, it is common to find higher counts in physiological terms (Gonzalo et al. Citation2005, Citation2010; Raynal-Ljutovac et al. Citation2007) due to the fact that in the goat's herds (and also in the sheep's) several breeding females exist all year long, and post-partum and dry milk periods successively occur.
Mastitis control programmes based on continuous dry therapies could lower SCC, and could also show reduced antibiotic residues (AR) occurrence as mastitis treatment is probably the most frequent reason for a higher risk of AR occurrence (Gonzalo et al. Citation2010; Beltrán et al. Citation2015).
A different perspective was observed on cow dairy farms with cows’ milk because lower counts were obtained with log SCC values of 5.45 (geometric mean 279 × 103 cells/ml), which statistically differed from the values obtained for sheep and goats. These values are within the limits set by legislation for cows’ raw milk (log SCC 5.60; geometric mean 400 × 103 cells/ml) (EC No. Citation853/Citation2004). This parameter has progressively lowered in the last decade for dairy cow herds given its direct effect on loss of the nutritional value in pasteurized milk and on the milk fluid shelf life (Barbano et al. Citation2006).
In raw milk, the bacterial count according to species significantly differed (), with lower values for cows’ milk, but with much less marked differences than for SCC. Unlike SCC, this parameter is covered by EU legislation (EC No. Citation853/Citation2004), with different thresholds set according to the species and what milk is destined for. The limits for TBC lay down limit-related criteria for cows’ milk ≤ 100 × 103 cfu/ml, and for milk from species other than cow ≤ 500 × 103 cfu/ml, when the final destination of milk does not include heat treatment; or ≤1500 × 103 cfu/ml for heat-treated milk before processing. Demands are greater for cows’ milk where improved milking management conditions and milking techniques ensure better hygiene-sanitary quality (Franciosi et al. Citation2011). Even lower psychrotroph counts are obtained because keeping milk refrigerated on farms (8–12°C) all day long does not cause the mass growth of these microorganisms, which is why the collection frequency on the farms in the study area is one day. However, it is necessary to pay attention to avoid exceeding the limit as moderate increases in TBC have been reported when 24-h storage is exceeded or when preservation conditions go beyond 10°C (Malacarne et al. Citation2013; PMO Citation2015).
The hygiene values obtained for sheep's milk are suitable (log TBC 4.65) and could reflect that the milking management applied to dairy sheep flocks in the Castilla-La Mancha area is similar to those that follow a continuous dry milk therapy with log TBC of 4.92–5.04 (Gonzalo et al. Citation2010; De Garnica et al. Citation2011), and samples were analysed under similar preservation conditions (refrigeration at 4°C with preservative Azidiol). Hence, we can take the preservation conditions and the transport time offered from including the TRAZALE system as being suitable for today's milk sample monitoring procedures when considering how much to pay for quality.
Goat farms obtained slightly worse results (log TBC of 4.97), but they were well behind the value expected by law to prepare dairy by-products using raw milk. This value indicates that good hygiene practices are being used on farms, where the cooling system and the initial loading of milk condition count values (Zeng et al. Citation2007; Yamazi et al. Citation2013). Implementing hygiene rules is the most important into countries where goats’ milk production is being introduced because lack of care standards and specifications for cold storage of raw goats’ milk has a negative effect on its quality and results in a poor hygiene log TBC lower than 6.0 (Yamazi et al. Citation2013).
Excellent results were observed in goat flocks on day-one control when log TBC values of 4.16 were obtained, despite the major deterioration on successive on-farm preservation days. We must be aware that on-farm storage of goats’ milk for more than 4 days negatively impacts the quality of raw milk and any subsequent dairy products (Zeng et al. Citation2007).
We can state from the results obtained in the present study that the mean bacteriological value for all three species was lower than the maximum value permitted by EC Regulation No. Citation853/Citation2004 for products made with raw milk, which demonstrates the effective hygienic control exercised on the farms in our study area.
No significant differences in the milk's hygiene-sanitary quality were observed for the different times of the year (P > .05, data not shown), except for a slight increase in autumn–winter on dairy sheep farms, which had no influence as the TBC values were still well within the acceptable limits (log TBC of 4.95). The values of these parameters reflect the good udder health status of flocks/herds, as well as the correct cleanliness of facilities and equipment. The fact that no significant seasonal variation was noted indicates that the control of good farming practices being met is maintained all year round. As demonstrated, the SCC and TBC bulk tank milk variables can be used for monitoring mammary health, milk hygiene and safety in dairy animals over time (Gonzalo et al. Citation2010).
4. Conclusion
The TRAZALE traceability system not only offer a more accurate control of fulfilling the preservation conditions for milk samples set out in European and Spanish regulations, but also provides complete and objective information about the conditions in which the collection, transport and storage of milk samples are carried out until they are analysed. Under these conditions, monitoring the process also allows checks to be made of the samples’ physico-chemical and hygiene-sanitary quality up to 47 h after being collected. This delay did not affect the price set to be paid for quality.
Consequently, its inclusion in the LeTrA Q quality module could be considered by implementing the information recorded during the whole process.
After using the system to monitor more than 500 dairy farms over a one-year period, we conclude that indeed, according to De las Morenas et al. (Citation2014), this system may become a reference method in the dairy sector.
Acknowledgements
The authors are grateful to Emilio Lopez Cano for advising on the data analysis, and also thank the farmers and dairy industries that participated in conducting this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Balthazar CF, Pimentel TC, Ferrão LL, Almada CN, Santillo A, Albenzio M, Mollakhalili N, Mortazavian AM, Nascimento JS, Silva MC, et al. 2017. Sheep milk: physicochemical characteristics and relevance for functional food development. Compr Rev Food Sci Food Saf. 16:247–262. doi: 10.1111/1541-4337.12250
- Barbano DM, Ma Y, Santos MV. 2006. Influence of raw milk quality on fluid milk shelf life. J Dairy Sci. 89(E. suppl.):E15–E19. doi: 10.3168/jds.S0022-0302(06)72360-8
- Bates D, Mäechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw. 67(1):1–48. doi: 10.18637/jss.v067.i01
- Baudry C, Cremoux R, Chartier C, Perrin G. 1997. Incidence de la concentration cellulaire du lait de chèvre sur sa production et sa composition. Vet Res. 28:277–286.
- Beltrán MC, Althaus RL, Molina A, Berruga MI, Molina MP. 2015. Analytical strategy for the detection of antibiotic residues in sheep and goat’s milk. Span J Agric Res. 13(1):e05–001. doi: 10.5424/sjar/2015131-6522
- Charlebois S, Haratifar S. 2014. The perceived value of dairy product traceability in modern society: an exploratory study. J Dairy Sci. 98:3514–3525 doi: 10.3168/jds.2014-9247
- Charlebois S, Sterling B, Haratifar S, Naing SK. 2014. Comparison of global food traceability regulations and requirements. Compr Rev Food Sci Food Saf. 13:1104–1123. doi: 10.1111/1541-4337.12101
- De Garnica ML, Santos JA, Gonzalo C. 2011. Influence of storage and preservation on microbial quality of silo ovine milk. J Dairy Sci. 94:1922–1927. doi: 10.3168/jds.2010-3787
- De las Morenas J, García A, Blanco J. 2014. Prototype traceability system for the dairy industry. Comput Electron Agric. 101:34–41. doi: 10.1016/j.compag.2013.12.011
- Franciosi E, Settanni L, Cologna N, Cavazza A, Poznanski E. 2011. Microbial analysis of raw milk cow’s milk used for cheese-making: influence of storage treatments on microbial composition and other technological traits. World J Microbiol Biotechnol. 27:171–180. doi: 10.1007/s11274-010-0443-2
- García A, Zangróniz R, De Las Morenas J, Blanco J. 2015. ‘An Automatic System to Monitor and Follow Up Samples’ TRAZALE. A patented invention presented by the UCLM at the Spanish Patent and Trademarks Office on 14 January 2013 with reference ES201300070 and application number P201300070. Awarded with publication no. ES2518615 and issued on 22/12/2015 (for a 20-year duration as of 14/01/2013).
- Gonzalo C, Carriedo JA, Blanco MA, Beneitez E, Juárez MT, De La Fuente LF, San Primitivo F. 2005. Factors of variation influencing bulk tank somatic cell count in dairy sheep. J Dairy Sci. 88:969–974. doi: 10.3168/jds.S0022-0302(05)72764-8
- Gonzalo C, Carriedo JA, García-Jimeno MC, Pérez-Bilbao M, De La Fuente LF, San Primitivo F. 2010. Factors influencing variation of bulk milk antibiotic residue occurrence, somatic cell count, and total bacterial count in dairy sheep flocks. J Dairy Sci. 93:1587–1595. doi: 10.3168/jds.2009-2838
- Guinot-Thomas P, Al Ammoury M, Laurent F. 1995. Effects of storage conditions on the composition of raw milk. Int Dairy Journal. 5:211–223. doi: 10.1016/0958-6946(95)92211-L
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom J. 50(3):346–363. doi: 10.1002/bimj.200810425
- LeTra Q. 2017. Milk quality. Spanish Ministry of Agriculture, Fisheries and Food. [accessed 2017 Jun 23]. http://www.mapama.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/Higiene-de-la-produccion-primaria-ganadera/calidad-de-la-leche-letra-q/default.aspx.
- Malacarne M, Summer A, Franceschi P, Formaggioni P, Pecorari M, Panari G, Vecchia P, Sandri S, Fossa E, Scotti C, et al. 2013. Effects of storage conditions on physico-chemical characteristics, salt equilibria, processing properties and microbial development of raw milk. Int Dairy J. 29:36–41. doi: 10.1016/j.idairyj.2012.10.005
- Martínez JR, Gonzalo G, Carriedo JA, San Primitivo F. 2003. Effect of freezing on fossomatic cell counting in ewe milk. J Dairy Sci. 86:2583–2587. doi: 10.3168/jds.S0022-0302(03)73853-3
- Park YW, Juárez M, Ramos M, Haenlein GFW. 2007. Physico-chemical characteristics of goat and sheep milk. Small Rumin Res. 68(1–2):88–113. doi: 10.1016/j.smallrumres.2006.09.013
- Pirisi A, Lauret A, Dubeuf JP. 2007. Basic and incentive payments for goat and sheep milk in relation to quality. Small Rumin Res. 68:167–178. doi: 10.1016/j.smallrumres.2006.09.009
- PMO. 2015. Grade ‘A’ pasteurized milk ordinance. 2015 Revision. Washington (DC): U.S. Food and Drug Administration. EEUU. 447 pp.
- Quigley L, O’Sullivan O, Stanton C, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD. 2013. The complex microbiota of raw milk. FEMS Microbiol Rev. 37:664–698. doi: 10.1111/1574-6976.12030
- Raynal-Ljutovac K, Pirisi A, de Crémoux R, Gonzalo C. 2007. Somatic cells of goat and sheep milk: analytical, sanitary, productive and technological aspects. Small Rumin Res. 68:126–144. doi: 10.1016/j.smallrumres.2006.09.012
- R Core Team. 2017. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; [cited 2017 Sep 17]. Available from: https://www.R-project.org/
- Regulation (EC) N° 178/2002 of the European Parliament and of the council of 28 January2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Official Journal of the European Communities, L 31, 01/02/2002. p. 24
- Regulation (EC) N° 852/2004 of the European Parliament and of the council of 29 April 2004 laying down on the hygiene of food-stuffs. Official Journal of the European Communities, L 139, 30/04/2004. p. 23
- Regulation (EC) N° 853/2004 of the European Parliament and of the council of 29 April 2004 laying down specific hygiene rules for on the hygiene of food-stuffs. Official Journal of the European Communities, L 139, 30/04/2004. p. 151
- Sánchez A, Sierra D, Luengo C, Corrales JC, Morales CT, Contreras A, Gonzalo C. 2005. Influence of storage and preservation on fossomatic cell count and composition of goat milk. J Dairy Sci. 88:3095–3100. doi: 10.3168/jds.S0022-0302(05)72991-X
- Sanz Ceballos L, Ramos Morales E, de la Torre Adarve G, Díaz Castro J, Pérez Martínez L, Sanz Sampelayo MR. 2009. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. J Food Comp Anal. 22:322–329. doi: 10.1016/j.jfca.2008.10.020
- Sun H, Jiang QK, Kong Q, Chen Z, Li X. 2016. Design of real-time monitoring system on raw milk transport process. IJMUE. 11(4):335–342. doi: 10.14257/ijmue.2016.11.4.33
- Voulodimos AS, Patrikakis CZ, Sideridis AB, Ntafis VA, Xylouri EM. 2010. A complete farm management system based on animal identification using RFID technology. Comput Electron Agric. 70:380–388. doi: 10.1016/j.compag.2009.07.009
- Wang L, Kwok SK, Ip WH. 2010. A radio frequency identification and sensor-based system for the transportation of food. J Food Eng. 101:120–129. doi: 10.1016/j.jfoodeng.2010.06.020
- Yamazi AK, Moreira TS, Cavicchioli VQ, Burin RCK, Nero LA. 2013. Long cold storage influences the microbiological quality of raw goat milk. Small Rumin Res. 113:205–210. doi: 10.1016/j.smallrumres.2013.02.004
- Zeng SS, Chen SS, Bah B, Tesfai K. 2007. Effect of extended storage on microbiological quality, somatic cell count, and composition of raw goat milk on a farm. J Food Prot. 70:1281–1285. doi: 10.4315/0362-028X-70.5.1281