ABSTRACT
This study was conducted to evaluate the effect of zinc-methionine (ZnM) on growth performance, nutrient utilization, antioxidant status and immune response in broiler chickens reared at high ambient temperature. A total of 480 one-day-old chicks were randomly distributed into 24 floor pens (20 chicks/pen) and were given either a control diet, 0 ZnM (G0) or 25, 50 and 100 mg/kg ZnM (G1, G2 and G3, respectively). The growth performance was significantly affected by the treatments, ZnM supplementation increased body weight gain and improved feed conversion (p < .05) in broilers. Protein utilization was improved by feeding ZnM (p < .05). Plasma total cholesterol was decreased, while plasma HDL-cholesterol was tending to be increased. Interestingly, an increase in ZnM supplementation enhanced Zn concentrations (p < .05) in breast muscle along with a reduction in malondialdehyde concentration and saturated fatty acids (p < .05) and an augmentation in unsaturated fatty acids (p < .01). Dietary ZnM supplementation resulted in a significant increase in serum glutathione peroxidase concentration which accompanied with an improving in humoral immune response. It could be concluded that dietary organic Zn supplementation improved growth performance, nutrient digestibility, Zn content in raw meat, antioxidative properties and humoral immunity and reduced meat lipid peroxidation in broilers under high ambient temperature.
1. Introduction
In majority of tropical countries, heat stress is one of the most important stressors negatively affecting poultry industry leading to a great economic loss each year. Higher ambient temperature is detrimental to live weight gain, feed intake (FI), feed efficiency, nutrient utilization, protein digestibility, total mineral retention and immune response of chicken broilers (Sahin and Kucuk Citation2003; Sahin et al. Citation2009; Saleh, Hayashi, et al. Citation2013, Citation2014). Moreover, heat stress can potentially promote formation of excess quantities of reactive oxygen species (ROS), which damages cell phospholipid membranes and other vital macromolecules causing lipid peroxidation, that consequently associated with disorders, such as apoptosis, various diseases and impairing muscle membrane integrity (Ebeid et al. Citation2013; Saleh Citation2014; El-Deep et al. Citation2016; Chand et al. Citation2017). Therefore, a balance between ROS production and the antioxidant system must be established to maintain immune function, health and productivity (Suraï Citation2002; Ebeid et al. Citation2011; Chand et al. Citation2016; Saleh et al. Citation2017).
Several methods are available to alleviate the negative effects of high ambient temperature on broiler performance. Because it is expensive to cool poultry buildings, such methods are mostly focused on dietary manipulation. In terms of reducing the negative effects of environmental stress, trace minerals and vitamins are used in the poultry diets to ameliorate the negative effects of stress. Dietary zinc (Zn) supplementation has also been reported to have a positive effect on the growth rate and feed efficiency of growing poultry under stress conditions (Sahin et al. Citation2005; Rao et al. Citation2016). Zinc is a fundamental element required for normal growth performance, bone development, feathering, skin quality, immunity, appetite regulation and structure and function of more than 300 enzymes associated with carbohydrate and energy metabolism, protein degradation and synthesis, nucleic acid synthesis, carbon dioxide transport and many other reactions (Prasad and Kucuk Citation2002; Sahin et al. Citation2009; Salim et al. Citation2011).
The NRC (Citation1994) specified 40 mg/kg of feed as Zn requirement of broilers. Zinc could be added to broiler’s diet as inorganic Zn or organic Zn (complexes with amino acids, proteins or carbohydrate). In recent years, organic Zn sources have been used progressively due to their potentially higher Zn bioavailability (Salim et al. Citation2011). Zinc-methionine (ZnM) is an organic Zn which is devoid of free divalent cations for chelation in the intestinal lumen with phytic acid. Therefore, it is metabolized in different methods which facilitate enhanced absorption of Zn (Burrell et al. Citation2004). In this context, ZnM could be advantageously incorporated in broiler’s diet at lower levels as compared to inorganic Zn for apprehending higher Zn bioavailability and lowering excretion of Zn to the environment (Sunder et al. Citation2013). It could, therefore, be hypothesized that heat stress increases Zn requirements of broilers and dietary organic Zn might enhance immune response by maintaining the oxidative balance. The objective of the present study was to inspect the effects of ZnM on growth performance, nutrient utilization, antioxidant status and immune response in broilers reared at high ambient temperature.
2. Materials and methods
2.1. Experimental design and dietary treatments
The experiment was conducted in accordance with the guidelines of the Department of Poultry Production, Faculty of Agriculture, Kafrelsheikh University, Egypt. All procedures were approved by the Animal Care and Welfare Committee of the Institute. A total of 480 one-day-old male broiler chicks (Cobb 500) were randomly assigned to 24 floor pens with 20 birds per pen (10 bird/m2). This study was conducted under hot climate conditions in Egypt (July and August). During the experimental period, the average daily temperature and relative humidity inside the house ranged from 33°C to 36°C and from 60% to 70%, respectively. Typical iso-energetic and iso-nitrogenous starter (0–2 week), grower (2–5 week) and finisher (5–6 week) diets, based on maize-soybean meal were formulated in mash form and offered ad libitum (). There is no Zn in the premix which used in all the diets. At one day, chicks received one of four dietary treatments: (i) G0 = control (0 ZnM), (ii) G2 = control + 25 mg/kg ZnM, (iii) G3 = control + 50 mg/kg ZnM and (iv) G 4 = control + 100 mg/kg ZnM. Broiler chicks were reared under similar managerial and hygienic conditions with a 24-h light programme. ZnM complex was obtained from Ibex Company, Egypt and the purity of ZnM complex was 98%.
Table 1. Ingredients and nutrient composition of the basal diet.
2.2. Growth performance and carcass parts
Feed intake (FI) and body weight (BW) were recorded weekly by pen, the average daily gain (ADG) and feed conversion ratio (FCR) were computed. Mortality was checked daily and weights of dead birds were used to adjust FCR. At the conclusion of the trial, birds were selected. After euthanasia, feathers, heads, necks and shanks were removed, and the remaining carcasses were dissected. The yield percentage of breast, liver and abdominal fat were calculated based on dressed weight.
2.3. Chemical analysis
At 40 d, excreta were collected from 10 birds per group. Excreta samples were weighed, dried in an oven at 60°C for 24 h, homogenized and finely ground to determine dry matter and crude protein digestibilities according to AOAC (Citation2000). Crude protein content in diet and excreta was measured to determine nitrogen retention using the Kjeldahl method. The calculations were as follows;
Nitrogen retention (%) = (total nitrogen intake – total nitrogen excreted)/ total Nitrogen intake × 100.
2.4. Blood samples and plasma biochemical analysis
At the end of the experimental period (42 d), blood samples were collected in heparinized test tubes and then centrifuged (3000 rpm for 20 min) to separate the plasma. Plasma and meat samples were stored at −30°C and −10°C, respectively, until further analysis. Total concentrations of total cholesterol, HDL-cholesterol, LDL-cholesterol, glucose, total protein, albumin and globulin were measured calorimetrically using commercial kits (Diamond Diagnostics, Egypt) according to the procedures outlined by the manufacturer. Serum glutathione peroxidase (GSH-Px) activity was determined by the method of Levander et al. (Citation1983) and glutamic oxalacetic transaminase (GOT) and glutamate pyruvate transaminase (GPT) activities were determined by the method of Reitman and Frankel (Citation1957). Concentration of muscle malondialdehyde (MDA) was measured by the method of Ohkawa et al. (Citation1979).
2.5. Assay of serum antibody titres
Serum antibody titres against Newcastle disease (ND) and Avian Influenza (H9N1) were determined by means of hemagglutination inhibition (HI) test using standard methods described in OIE (Citation2009). Antibody titre to infectious bursal disease virus was determined by commercial ELISA kits (Synbiotics Laboratories, USA), according to manufacturer’s instructions.
2.6. Meat analysis
Breast muscle tissue was used to measure muscle Zn concentration and fatty acids profile according to the method described by Saleh (Citation2013). Lipids were extracted from muscle by a mixture of chloroform-methanol (2:1) at the ratio of (1:5) in separator funnel and shaken carefully for 1 h. The extract was allowed to separate; the organic layer was taken, then passed through a glass funnel containing anhydrous sodium sulfate and finally evaporated to near dryness by a stream of nitrogen. A sample of total lipids (50 mg) was transferred into a screw-cap vial, and 2 ml benzene and 10 ml 1%H2SO4 in absolute methanol was added. The vial was covered under a stream of nitrogen before heating in an oven at 90°C for 90 min. Ten millilitres of distilled water were added to the cooled vial and the methyl esters in each vial were extracted. Ether extracts were combined and concentrated to its minimum volume by using a stream of nitrogen.
Analysis of fatty acids in meat was carried out by gas liquid chromatography using Shimadzu gas chromatograph GC-4 CM (PFE) equipped with flame ionization detector. A standard mixture of methyl esters was analysed under identical conditions prior to running the samples. The retention times of the unknown sample of methyl esters were compared with those of the standard. The concentration of methyl esters were calculated by the triangulation method.
2.7. Statistical analysis
Data were evaluated by analysis of variance (ANOVA) for a complete randomized block design using the general linear models procedure of SPSS Statistics 17.0 (SPSS Citation2008). When the ANOVA showed significant differences, Tukey’s multiple comparison test was applied. The overall level for statistical significance was set at p < .05. All values were expressed as means ± standard error of the mean.
3. Results
The effects of dietary ZnM supplementation on body weight gain (BWG), FI, FCR, breast muscle weight (BMW), liver and abdominal fat weights in broilers during heat stress condition are summarized in . Final BW was significantly increased (p < .05) in G2 compared with control group (G0); however, no significant differences were observed among G0, G1 and G3 (p > .05). Chicks received G2 and G3 had the highest significant ADG (51.45 and 51.24 g, respectively) as compared to those that had received G0 (47.60 g). Chicks received G1 had intermediate ADL (49.81 g) but it was not different from G0 (p > .05). FI and FCR were significantly decreased (p < .05) in birds received G2 and G3 as compared to those received G0. Breast muscle weight (pectoral superficial muscle) was significantly increased (p < .01) by the dietary supplementation of 50 and 100 mg ZnM/kg (G2 and G3, respectively) as compared to those which had received 0 or 25 mg ZnM/kg (G0 and G1, respectively). On the other hand, abdominal fat weight was decreased in groups G2 and G3 as compared to groups G0 or G1 while, liver weight was not influenced by treatment.
Table 2. Effect of dietary zinc-methionine supplementation on growth performance in broilers under high ambient temperature.
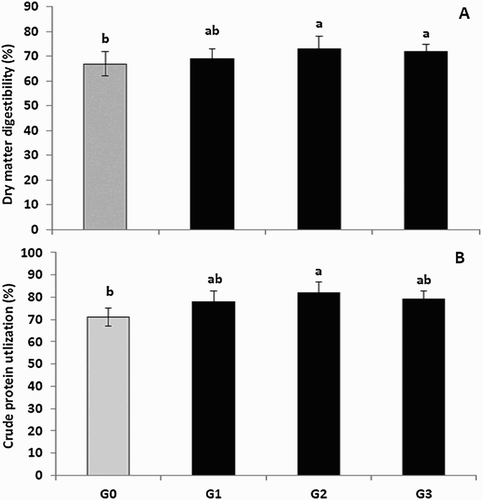
The dry matter digestibility and crude protein utilization results showed a significant differences because of treatment (p < .05, (A,B)). Dry matter digestibility was high for birds that received G2 and G3, intermediate for birds that received G1 and low for the G0 group (p < .05). On the other hand, crude protein utilization was the best for chicks that received G2, while it was intermediate for G1 and G3 and it was the lowest for G0 (p < .05).
Figure 1. Effect of feeding Zinc-methionine supplementation on dry matter digestibility (A) and crude protein utilization (B). Values are means represented by vertical bars. a,b: mean values with unlike letters were significantly different (p < .05).
The effects of ZnM on fatty acids profile in beast muscle are summarized in . Palmitic acid, palmitioleic acid and stearic acid were decreased significantly by feeding ZnM (p < .05), while, oleic acid, vaccenic acid and linoleic acid were significantly increased (p < .01).
Table 3. Effect of dietary zinc-methionine supplementation on fatty acids profile in breast muscle in broilers.
With respect to the effect of dietary ZnM supplementation on antioxidative properties including GSH-Px activity as well as lipid peroxidation index in blood plasma ((A)), it could be observed that dietary 50 and 100 mg ZnM/kg significantly enhanced GSH-Px activity in comparison with control.
Figure 2. Effect of feeding zinc-methionine supplementation on plasma GSH-Px (A), muscle MDA (B) and muscle Zn concentration (C). Values are means represented by vertical bar. a,b,c,d: mean values with unlike letters were significantly different (p < .05).
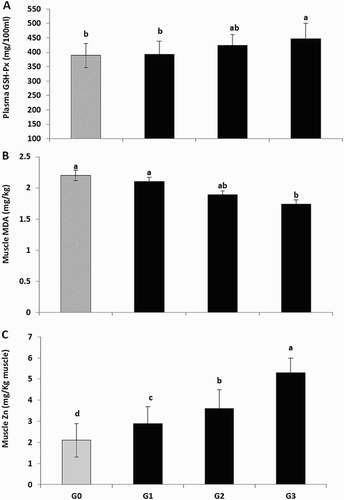
Breast muscle Zn concentration was increased linearly as the level of ZnM increased in the diet (p < .05, (C)). The influences of dietary ZnM supplementation on lipid oxidation in breast muscle of broilers reared in high ambient temperature are graphically presented in (B). Using MDA as an index of lipid oxidation, it could be observed that dietary treatments decreased MDA values in meat (p < .05). In addition, the 100 mg ZnM/kg treatment was the most effective inhibitor of lipid oxidation, followed by 50 mg ZnM/kg compared with control.
Results of immune response, presented in , show that different levels of dietary organic Zn had a positive effect on humoral immunity as measured by antibody titres against ND when compared with the control diet (p < .05) under heat stress condition. The highest scores of antibody titres against Proflok IBD were attained by broilers fed 50 and 100 mg ZnM/kg compared with those of 0 or 25 mg ZnM/kg. Although, no significant differences were detected in antibody titres against avian influenza H9N1, there is a numerical increase due to dietary ZnM supplementation under heat stress condition.
The results for plasma TAG, total cholesterol, HDL-cholesterol, GOT, glucose concentrations, muscle Zn concentration are shown in . Plasma TAG, total cholesterol concentrations were significantly lower in the ZnM groups (G1, G2 and G3) as compared to the control (G0) (). Plasma HDL-cholesterol, GOT and glucose were not affected by treatment (p > .05, ). The main effect of dietary ZnM supplementation on plasma total protein, albumin and globulin in broilers reared in high ambient temperature are presented in . It is clear that no significant differences between treatments were observed.
Table 4. Effect of dietary zinc-methionine supplementation on blood plasma biochemical constituents.
4. Discussion
Results of the present study indicated that dietary ZnM supplementation (50 or 100 mg/kg) increased BWG and BMW, improved FCR and lowered abdominal fat weight under high ambient temperature (). These results are in coincident with Rao et al. (Citation2016) who indicated that supplementation of organic Zn increased (p < .05) body mass gain and FI compared to those fed the control in broilers reared in cyclic heat-stressed condition. Also, Sahin et al. (Citation2005) postulated that Zn picolinate supplementation (30 or 60 mg/kg) improved growth performance and carcass quality in quails exposed to heat stress. One possible explanation for such improvements might be related to the fact that Zn is considered of critical importance in maintaining the structure of metalloproteins such as insulin, growth hormone and insulin-like growth factor-I (Macdonald Citation2000; Saleh, Eid, Ohtuska, et al. Citation2012; Khan et al. Citation2014; Midilli et al. Citation2014). Furthermore, Zn positively affects feed utilization through participating in the metabolism of carbohydrates, lipids and proteins (Macdonald Citation2000) which finally translated into improvements in growth performance.
As shown in , BMW was increased when ZnM was added at the rate of 50 and 100 mg/kg, and this improvement might be related to Zn requirements for normal protein synthesis and metabolism (Midilli et al. Citation2014). Dietary organic Zn decreased abdominal fat in the present study (). These results are in accordance with Kucuk et al. (Citation2003) who documented that abdominal fat decreased (p < .05) upon dietary zinc and vitamin A supplementation.
In the present study, feeding ZnM-supplemented diet improved dry matter digestibility and crude protein utilization. These results are in harmony with other studies (Sahin and Kucuk Citation2003; Sahin et al. Citation2009) which noted that increasing supplemental Zn (0, 30 and 60 mg/kg) linearly increased digestibility of dry matter, organic matter, crude protein and ether extract. It is well known that Zn has a protective role on pancreatic tissue against oxidative damage, it might help the pancreas to function properly including secretions of digestive enzymes, thus improving digestibility of nutrients. Also, Zn is required for the activity of over 300 enzymes and participates in many enzymatic and metabolic functions in the body (Prasad and Kucuk Citation2002). Therefore, it could be assumed that dietary organic Zn might play a vital role in enhancing the digestion and absorption of nutrients in the gastrointestinal tract during heat stress conditions which consequently might participate in improving the growth performance in the present study.
Zinc concentration in breast muscle was increased in a dose dependent manner due to dietary ZnM supplementation ((C)). Several previous studies (Salim, et al. Citation2010; Kakhki et al. Citation2016) reported an increase in muscle Zn concentration using dietary Zn supplementation in broilers. Kakhki et al. (Citation2016) illustrated that muscle content of Zn in broilers has linear relationship with dietary Zn level. Interestingly, as shown in (B), using MDA as an index of lipid oxidation, it could be observed that dietary ZnM supplementation decreased MDA values in breast meat (p < .05). These findings suggested that dietary supplementation with organic Zn increased Zn concentration in breast meat which might be involved in improving the oxidative stability and alleviated the lipid oxidation of breast meat. This positive effect might be connected with the antioxidative properties of Zn (Sahin et al. Citation2009). It is well known that Zn plays a key role in suppression of free radicals because it is a cofactor of the main antioxidative enzyme Cu-Zn-superoxide dismutase which diminishes lipid peroxidation (Prasad and Kucuk Citation2002). This assumption was confirmed by Bartlett and Smith (Citation2003) who elucidated that dietary Zn reduced lipid oxidation of chicken thigh tissue in comparison with control group.
One of the most important findings of the present study is that dietary ZnM supplementation significantly enhanced plasma GSH-Px activity ((A)) in comparison with control in broilers exposed to high ambient temperature indicating that Zn might play an important role in alleviating the detrimental effects of heat stress. These results agree with previous studies (Kucuk et al. Citation2003; Sahin and Kucuk Citation2003; Sahin et al. Citation2005; Khan et al. Citation2012; Rao et al. Citation2016) which indicated that supplementation of organic Zn was reported to increase the activity of antioxidant enzymes and reduce lipid peroxidation in poultry under heat stress conditions. The enzyme GSH-Px is located primarily in the cytosol and has a general specificity in the detoxification of both lipid hydroperoxides and organic hydroperoxides (Suraï Citation2002; Khan et al. Citation2011; Saleh, Ohtuska, et al. Citation2013). Another mode of action proposed for Zn as an antioxidant is its prevention of lipid peroxidation via inhibiting glutathione depletion (Prasad Citation1997). Also, Zn induces production of Zn-metallothionein, which is an effective scavenger for hydroxyl radical and providing protection against immune-mediated free radical attack (Prasad and Kucuk Citation2002; Wang et al. Citation2011; Laudadio et al. Citation2012; Saleh, Eid, Ebeid, et al. Citation2012). Therefore, it might be mentioned that dietary organic Zn alleviated the negative effects of heat stress via maximizing the antioxidant defense system and minimizing lipid peroxidation in blood plasma, which probably translates into enhanced growth performance, muscle integrity, immunity and disease resistance in broiler chickens. Getting such beneficial results is vital in broiler production, especially under heat stress conditions.
The present study demonstrated that dietary ZnM positively affected the antibody titre against ND, IBD and H9N1 in broiler chickens under heat stress conditions (). This response is similar to previous observations (Bartlett and Smith Citation2003; Chand et al. Citation2014; Abudabos et al. Citation2017) which confirmed that Zn is an important element for all aspects of immunity under heat stress conditions. Bartlett and Smith (Citation2003) reported that dietary Zn supplementation improved lymphoid organ weights, primary and secondary antibody responses, phagocytic ability of macrophages, total IgM and IgG antibody titres in male broilers raised in high environmental temperature. These results are in accordance with those of Chand et al. (Citation2014) who demonstrated that antibody titre against ND, IBD and IB was increased significantly due to dietary Zn supplementation under heat stress conditions. Furthermore, by taking into account our results in lipid peroxidation and antioxidative properties, it could be concluded that dietary ZnM may enhance the immune responsiveness in broilers reared in high ambient temperature.
Table 5. Effect of dietray Zinc-methionine supplementation on antibody titre against Newcastle disease (ND), Proflok infectious bursal disease (IBD) and Avian Influenza (H9N1) virus in broilers under high ambient temperature.
Results of the current study showed that feeding ZnM-supplemented diet decreased plasma TAG and total cholesterol, while plasma GOT and glucose were not influenced. These results are in agreement with Herzig et al. (Citation2009) who demonstrated that there was a significant decrease of plasma cholesterol when high amounts of Zn were administered to broiler chickens. Similarly, Kucuk et al. (Citation2003) reported that supplemental Zn decreased serum cholesterol concentration in heat-stressed broiler chickens. Aksu et al. (Citation2011) also reported the decrease of total cholesterol and LDL-C, combined with the increase in HDL-cholesterol, in the plasma of chickens when the feed mixtures were supplemented with organic complexes of Zn. The decrease in plasma cholesterol due to Zn was explained by the fact that Zn is involved in lipid metabolism (Midilli et al. Citation2014). Abd-El-Samee et al. (Citation2013) reported that plasma total lipids and cholesterol were decreased by feeding Zn in broiler diets. Uyanik et al. (Citation2001) indicated that Zn supplementation decreased serum cholesterol concentration of broilers, this may be due to ZnM prevent cholesterol from absorption in gastro intestinal tract (Tizard et al. Citation1989; Wang et al. Citation2011) and can promote the growth and activity of lactic acid bacteria (Gibson and Roberfroid Citation1995), which reduces the cholesterol level by producing enzymes disintegrating bile salts and making them un-conjugated. Results of the present study revealed that plasma glucose concentration was not significantly affected by dietary ZnM supplementation. However, Kucuk et al. (Citation2003) documented that supplemental Zn decreased serum glucose concentration in heat-stressed broiler chickens. In general, plasma levels of GOT and GPT were considered as liver indices for liver damage. Therefore, results of the present study may provide evidences for occurrence of no toxicity of feeding ZnM in broiler chickens.
5. Conclusion
Based on the data presented above and taking into account the antioxidative properties, it is possible to suggest that supplemental dietary organic Zn might be involved in enhancing growth performance, nutrient digestibility, Zn content in raw meat, meat oxidative stability, plasma antioxidative status and immune responsiveness in broilers subjected to heat stress.
Acknowledgement
The authors wishes to acknowledge the helpful suggestions and helps of Department of Poultry Production, Faculty of Agriculture, Kafrelsheikh University, Egypt.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abd-El-Samee DL, El-Wardany I, Nematallah GA, Abo-El-Azab OM. 2013. Effect of dietary organic Zn and prebiotic on productive performance and immune response of growing quails. Iranian J Applied Anim Sci. 3:761–767.
- Abudabos AM, Alyemni AH, Dafalla YM, Khan RU. 2017. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J Appl Anim Res. 45:538–542. doi: 10.1080/09712119.2016.1219665
- Aksu T, Aksu MI, Yoruk MA, Karaoglu M. 2011. Effects of organically-complexed minerals on meat quality in chickens. Br Poult Sci. 52:558–563. doi: 10.1080/00071668.2011.606800
- AOAC [Association of Official Analytical Chemists]. 2000. Official methods of analysis. 17th ed. Washington (DC): AOAC.
- Bartlett JR, Smith MO. 2003. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci. 82:1580–1588. doi: 10.1093/ps/82.10.1580
- Burrell AL, Dozierrd WA, Davis AJ, Complon MM, Freeman ME, Vendrell PF, Ward TL. 2004. Responses of broilers to dietary zinc concentrations and sources in relation to environmental implications. Br Poult Sci. 45:225–263. doi: 10.1080/00071660410001715867
- Chand N, Muhammad S, Khan RU, Alhidary IA, Rahman Zia ur. 2016. Ameliorative effect of synthetic γ-aminobutyric acid (GABA) on performance traits, antioxidant status and immune response in broiler exposed to cyclic heat stress. Environ Sci and Pollut Res. 23:23930–23935. doi: 10.1007/s11356-016-7604-2
- Chand N, Naz S, Maris H, Khan RU, Khan S, Qureshi MS. 2017. Effect of betaine supplementation on the performance and immune response of heat stressed broilers. Pakistan J Zool. 49:1857–1862. doi: 10.17582/journal.pjz/2017.49.5.1857.1862
- Chand NS, Naz Khan A, Khan S, Khan RU. 2014. Performance traits and immune response of broiler chicks treated with zinc and ascorbic acid supplementation during cyclic heat stress. Int J Biometeorol. 58:2153–2157. doi: 10.1007/s00484-014-0815-7
- Ebeid T, Fayoud A, Abou El-Soud S, Eid Y, El-Habbak M. 2011. The effect of omega-3 enriched meat production on lipid peroxidation, antioxidative status, immune response and tibia bone characteristics in Japanese quail. Czech J Anim Sci. 56:314–324.
- Ebeid TA, Zeweil HS, Basyony MM, Dosoky WM, Badry H. 2013. Fortification of rabbit diets with vitamin E or selenium affects growth performance, lipid peroxidation, oxidative status and immune response in growing rabbits. Livestock Sci. 155:323–331. doi: 10.1016/j.livsci.2013.05.011
- El-Deep M, Ijiri HD, Ebeid TA, Ohtsuka A. 2016. Effects of dietary nano-selenium supplementation on growth performance, antioxidative status, and immunity in broiler chickens under thermoneutral and high ambient temperature conditions. J Poult Sci. 43:255–265.
- Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota. Introducing the concept of prebiotics. J Nutrit. 125:1401–1412.
- Herzig I, Navratilova M, Totusek J, Suchy P, Vecerek V, Blahova J, Zraly Z. 2009. The effect of humic acid on zinc accumulation in chicken broiler tissues. Czech J Anim Sci. 54:121–127.
- Kakhki RAM, Bakhshalinejad R, Shafiee M. 2016. Effect of dietary zinc and α-tocopheryl acetate on broiler performance, immune responses, antioxidant enzyme activities, minerals and vitamin concentration in blood and tissues of broilers. Anim Feed Sci and Technol. 221:12–26. doi: 10.1016/j.anifeedsci.2016.08.016
- Khan RU, Naz S, Dhama K. 2014. Chromium: pharmacological applications in heat stressed poultry. Internation J Pharm. 10:213–217. doi: 10.3923/ijp.2014.213.217
- Khan RU, Naz S, Nikousefat Z, Selvaggi M, Laudadio V, Tufarelli V. 2012. Effect of ascorbic acid in heat-stressed poultry. World’s Poult Sci J. 68(3):477–490. doi: 10.1017/S004393391200058X
- Khan RU, Naz S, Nikousefat Z, Tufarelli V, Javdani M, Rana N, Laudadio V. 2011. Effect of vitamin E in heat-stressed poultry. World’s Poult Sci J. 67(3):469–478. doi: 10.1017/S0043933911000511
- Kucuk O, Nurhan S, Kazim S. 2003. Supplemental zinc and vitamin A can alleviate negative effects of heat stress in broiler chickens. J Biological Trace Element Res. 94:225–236. doi: 10.1385/BTER:94:3:225
- Laudadio V, Dambrosio A, Normanno G, Khan RU, Naz S, Rowghani E, Tufarelli V. 2012. Effect of reducing dietary protein level on performance responses and some microbiological aspects of broiler chickens under summer environmental conditions. Avian Biology Res. 5(2):88–92. doi: 10.3184/175815512X13350180713553
- Levander OA, Deloach DP, Morris VC, Moser PB. 1983. Platelet glutathione peroxidase activity as an index of selenium status in rats. J Nutrit. 113:55–63.
- Macdonald RS. 2000. The role of zinc in growth and cell prolifereation. J Nutrit. 130:1500–1508.
- Midilli M, Salman M, Muglali OH, Ögretmen T, Cenesiz S, Ormanci N. 2014. The effects of organic or inorganic zinc and microbial phytase, alone or in combination, on the performance, biochemical parameters and nutrient utilization of broilers fed a diet low in available phosphorus. In J Food Agricult Vet. 8: 461–467.
- NRC. 1994. Nutrient requirements of domestic animals. 9th ed. Washington (DC): National Academy Press.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358. doi: 10.1016/0003-2697(79)90738-3
- OIE. 2009. Manual of diagnostic tests and vaccines for terrestrial animals: mammals, birds and bees, 5th ed, vol 1, part 2, chapter 2.3.14, p 576-589. Biological standrads commission, world organization for Animal Health Paris, France.
- Prasad AS. 1997. The role of zinc in brain and nerve functions. In: A. Connor, editor. Metals and oxidative damage in neurological disorders. New York (NY): Plenum Press; p. 95–111.
- Prasad AS, Kucuk O. 2002. Zinc in cancer prevention. Cancer Metast Rev. 21:291–295. doi: 10.1023/A:1021215111729
- Rao RS, Prakash VB, Raju MVL, Panda NAK, Kumari RK, Reddy EPK. 2016. Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer. J Biological Trace Element Res. 172:511–520. doi: 10.1007/s12011-015-0587-x
- Reitman S, Frankel S. 1957. A colorimetric method for deterxmination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. American J Pathol. 26:1–13.
- Sahin K, Kucuk O. 2003. Zinc supplementation alleviates heat stress in laying Japanese quail. J Nutrit. 33:2808–2811.
- Sahin K, Sahin N, Kucuk O, Hayirli A, Prasad AS. 2009. Role of dietary zinc in heat-stressed poultry: a review. Poult Sci. 88:2176–2183. doi: 10.3382/ps.2008-00560
- Sahin K, Smith M O, Onderci M, Sahin N, Gursu MF, Kucuk O. 2005. Supplementation of zinc from organic or inorganic source improves performance and antioxidant status of heat-distressed quail. Poult Sci. 84:882–887. doi: 10.1093/ps/84.6.882
- Saleh AA. 2013. Effects of fish oil on the production performances, polyunsaturated fatty acids and cholesterol levels of yolk in hens. Emirates J Food Agri. 25: 605–661. doi: 10.9755/ejfa.v25i8.14005
- Saleh AA. 2014. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Anim Sci Papers and Reports. 32:65–79.
- Saleh AA, Eid YZ, Ebeid TA, Ohtsuka A, Hayashi K. 2012. The modification of the muscle fatty acid profile by dietary supplementation with Aspergillus awamori in broiler chickens. Br J of Nutrit. 108:1596–1602. doi: 10.1017/S0007114511007069
- Saleh AA, Eid YZ, Ohtsuka A, Hayashi K. 2012. Feeding Aspergillus awamori reduces skeletal muscle protein breakdown and stimulates growth in broilers. Animal Sci J. 83:594–598. doi: 10.1111/j.1740-0929.2011.00999.x
- Saleh AA, Gálik B, Arpášová H, Capcarová M, Kalafová A, Šimko M, Juráček M, Rolinec MB, Bíro D, Abudabos AMA. 2017. Synergistic effect of feeding Aspergillus awamori and lactic acid bacteria on performance, egg traits, egg yolk cholesterol and fatty acid profile in laying hens. Italian J Animl Sci. 16: 132–139. doi: 10.1080/1828051X.2016.1269300
- Saleh AA, Hayashi K, Ijiri D, Ohtsuka A. 2014. Beneficial effects of Aspergillus awamori in broiler nutrition. World’s Poult Sci J. 70:857–864. doi: 10.1017/S0043933914000907
- Saleh AA, Hayashi K, Ohtsuka A. 2013. Synergistic effect of feeding Aspergillus Awamori and Saccharomyces Cerevisiae on growth performance in broiler chickens; promotion of protein metabolism and modification of fatty acid profile in the muscle. J Poult Sci. 50:242–250. doi: 10.2141/jpsa.0120153
- Saleh AA, Ohtsuka A, Yamamoto M, Hayashi K. 2013. Aspergillus awamori feeding modifies lipid metabolism in rats. BioMed Res Int. Article ID 594393:7. doi:10.1155/2013/594393.
- Salim H, Lee HR, Jo C, Lee SK, Lee BD. 2010. Effect of sources and levels of zinc on the tissue mineral concentration and carcass quality of broilers. Avian Biol Res. 3:23–29. doi: 10.3184/175815510X12636595095213
- Salim HM, Lee HR, Jo C, Lee SK, Lee BD. 2011. Supplementation of graded levels of organic zinc in the diets of female broilers: effects on performance and carcass quality. Br Poult Sci. 52:606–612. doi: 10.1080/00071668.2011.616485
- SPSS [Statistical Packages for the Social Sciences]. 2008. Statistical software for windows version 17.0. Chicago (IL): Microsoft.
- Sunder GS, Kumar CV, Panda AK, Raju MLN, Rao SVR. 2013. Effect of supplemental organic Zn and Mn on broiler performance, bone measures, tissue mineral uptake and immune response at 35 days of age. Current Res in Poult Sci. 3:1–11. doi: 10.3923/crpsaj.2013.1.11
- Suraï PF. 2002. Selenium in poultry nutrition 2. Reproduction, egg and meat quality and practical applications. World’s Poult Sci J. 58:431–450. doi: 10.1079/WPS20020032
- Tizard IR, Carpenter RH, McAnalley BH, Kemp MC. 1989. The biological activities of mannans and related’ complex carbohydrates. Mol Biother. 1:290–296.
- Uyanik F, Eren M, Atasever A, Tunçoku G, Kolsuz AH. 2001. Changes in some biochemical parameters and organs of broilers exposed to cadmium and effect of zinc on cadmium induced alterations. Israel J Vet Med. 56:128–134.
- Wang JH, Wu CC, Feng J. 2011. Effect of dietary antibacterial peptide and zinc-methionine on performance and serum biochemical parameters in piglets. Czech J Anim Sci. 56:30–36.
