ABSTRACT
Serum lipid profile, hormones and liver enzymes were studied to determine the health status in moulted layers supplemented with protein, probiotics and symbiotics. Four equal groups (n = 50 each) as control (CONT; CP16% diet), high protein (HP; CP18% diet), symbiotic (SYM; CP16% diet, symbiotic @ 85 mg/L orally) and probiotic (PRO; CP16% diet, probiotic @ 85 mg/L orally) were studied. Fifteen birds were slaughtered at 5%, peak and end of post-moult production periods from each group to collect blood. A two-factorial completely randomized design along with the DMR test was applied. The serum was analysed to determine various parameters by commercially available kits. The LDL-cholesterol (LDL-C; mg/dL) and cortisol (µg/dL) concentration in HP, SYM and PRO and thyroxin (T4; µg/dL) in PRO and SYM were significantly reduced as compared to CONT. Triiodothyronine (T3; ng/mL) concentration in HP, SYM, PRO; HDL-cholesterol (HDL-C; mg/dL) in HP and AST (U/L) concentration in PRO were significantly increased (P ≤ .05) as compared to CONT. The decrease (P ≤ .01) in LDL-C and cortisol levels and increase in HDL-C level indicate the reducing effect of these supplementations on stress, whereas an increase in T3 in supplemented birds (HP and PRO) signifies its metabolic boosting impact in post-moult laying hens.
Introduction
During the last decade, many researches have been focusing on poultry nutrition, health, management and productivity in order to maximize the economic potential of the poultry industry. In most of the agriculture-based countries, as in Asia, animal production is the back bone of the economic infrastructure. The moulted laying hens are economically beneficial particularly to avoid the culling of birds at the end of their first production cycle and rearing new flocks till the age of maturity (20–22 weeks).
The current investigations focus on exploring the efficiency of supplementation with protein, probiotics and symbiotics on the health status of spent moulted layers. The previous research by Anwar et al. (Citation2012a) has shown the efficacy of protein, probiotic and symbiotic supplementation in the moulted layers on overall health status. According to their report, the supplementation with protein, probiotic and symbiotic had a potential role in lowering the oxidative stress, particularly protein supplementation enhanced the antioxidant enzyme activity which ultimately proved to be a beneficial health-promoting supplementation. It has also been observed that the protein requirements of laying hens were increased as a result of rearing specialized hens because they produce more frequently (Johnson and Lohman, Citation2003). The high protein (HP) diet in the chicken causes the increase in cell mitosis, hypertrophy of intestinal villus height and absorptive capacity of epithelial cells (Yamauchi, Citation2007). So, protein supplementation plays a significant role in the dynamics of serum biomarkers. Probiotics are the microbial cell preparations which contain yeast/bacteria or both that enhances the colonization and development of beneficial microorganisms in the gut of the host (e.g. moulted egg-laying hens) and are known to have a beneficial impact on the health of the host by enhancing their overall immune response (Anwar et al. Citation2015). Symbiotic is a combination of fermentable nutrients, vitamins and minerals with microbial culture. The microbial cell preparations are proven to be a good substitution of the antibiotics after the emerging issue of antibiotic resistance (Patterson and Burkholder, Citation2003). Use of prebiotics and probiotics in humans and rodents has been associated with the reduction in pathogen colonization, alteration of gut microflora and reduction of serum triglycerides and cholesterol (Simmering and Blaut, Citation2001). The importance of feeding microbial cultures for the serum lipid profile has been shown by Kalavathy et al. (Citation2008), who reported a decrease in the triglyceride (TG) and LDL-C in chickens who were fed with Lactobacillus culture. Metabolic hormones and corticosterone are imperative markers in the moulted birds as thyroxine (T4) and triiodothyronine (T3) are the principal metabolic hormones involved in the growth, egg production and particularly in the process of moulting in the layers (Renden et al. Citation1994). The supplementation with protein, probiotic and symbiotic shows a dynamic impact on the cellular morphology of endocrine cells of the anterior pituitary including growth and prolactin hormone-producing cells (Anwar et al. Citation2012b). The increase in serum cortisol level was observed in the post-moulted layers as compared to pre-moulting cortisol level, which was attributed to the stress of the moulting process (Sandhu et al. Citation2007). Despite extensive research in the field, there is a paucity of published literature regarding the study of the relationship of protein, probiotic and symbiotic supplementation with the lipid profile, metabolic hormones, cortisol and liver enzymes in moulted layers. The present study was, therefore, designed to evaluate the effectiveness of supplementations with protein, probiotics and symbiotics on these selected biochemical parameters in the moulted layers.
Materials and methods
A total 200 white leghorns (Gallus gallus domesticus) at the age of 70 weeks (i.e. ending of their first production cycle) were procured from a commercial layer farm and were brought to the poultry research station equipped with caged housing system (one bird/cage) at the Institute of Pharmacy, Physiology and Pharmacology, University of Agriculture, Faisalabad. During the acclimatization period of 1 week feed (CP 16%, Energy 2795 kcal) @ 100 g/bird/day, water ad libitum and16 h of light were provided. The birds were initially weighed and moulting was started according to the schedule (), till the birds lose 35% of body weight from their initial body weight. Thereafter, birds were randomly allocated to four groups (50 birds each) and the respective treatments were started as follows: CONT as control (CP 16% diet; no other supplement), HP (CP 18% diet, no other treatment), SYM (CP 16% diet; Symbiotic in drinking water @ 85 mg L−1) and PRO (CP 16% diet; Probiotic @ 85 mg L−1in drinking water daily). The temperature of the poultry shed was maintained at 25°C ± 2°C throughout the experiment. The detailed lightening schedule throughout the experiment has been mentioned in . The composition of feed, probiotic and symbiotic is presented in . The probiotic (Protexin®) was provided by Probiotics UK International, England and symbiotic (Perfectin®) was supplied by Diasham, Singapore.
Table 1. High dietary zinc (3 g/kg)-induced moulting schedule of spent white leghorn.
Table 2. Composition of feed, symbiotic (perfectin) and probiotic (protexin) offered to different groups.
Serum biochemistry
During the experiment, 15 birds were randomly selected from each group at 5% (5P), peak (PP) and at the production end (EP) and were decapitated to collect the blood in sterilized test tubes which was a procedure of 1–2 min. Members of the Institutional Review Board and Ethical Committee for the Use of Animals in Research, University of Agriculture, Faisalabad, have evaluated the current research proposal and provided their consent to conduct the experiment. To harvest the serum, test tubes with clotted blood without anticoagulant were centrifuged at 277 × g and 4°C in a refrigerated centrifuge (Beckman TJ-6, USA) machine for 10 min. The harvested serum was stored in serum cups at −20°C till the analysis. Analytical kits (Fluitest, Biocon® Diagnosemittel GmbH & Co., Germany) were used to estimate the total serum TG, total cholesterol (T-Chol), LDL-cholesterol (LDL-C) and HDL-cholesterol (HDL-C) by semiautoanalyzer Biosystem Inc., BTS-330, S.A., Costa Brava, Barcelona, Spain (Pisani et al. Citation1995). The detectable ranges in these assays were from 3 to 800 mg/dL. Serum thyroid hormones, triiodothyronine (T3) and thyroxin (T4) were assessed by the ELISA kits of JD Biotech (HsinYi Road, Taipei, Taiwan). The sensitivity of this assay was estimated to be 0.2 ng mL−1 for T3 and was 0.4 μg dL−1 for T4. Serum cortisol concentration was determined by Accu-Bind ELISA antibody-coated wells with enzyme reagents and corticosteroid standards provided by Monobind Inc., Lake Forest, CA, USA. The detection spectrum of the assay ranged from 0.4 μg to 95 μg dL−1, the regression coefficient was 0.98 and the sensitivity was 0.25 μg dL−1. The ELISA readings were taken on an ELISA plate reader (URIT Medical Electronic Co., Ltd. Guangxi, China). The within-assay precision coefficient of variance (%) was kept under 10 for the hormonal ELISA tests. The liver enzymes (AST and ALT) were estimated with the kits procured from Randox Laboratories Ltd., Ardmore, Crumlin Co., UK. The readings were taken on a semi autoanalyzer (Biosystem, BTS-330, Barcelona, Spain). The linearity was maintained when absorbance of the sample was under 0.17 for AST and 0.5 for ALT. In case the absorbance exceeded the limit, the sample was diluted to 10-fold and final results were multiplied with 10 to get the precise concentration.
Statistical analysis
The two-way ANOVA completely randomized experimental design (two-factorial) was applied to seek the difference in significance between the treatment groups at different production stages (Steel et al. Citation1997). The obtained data were tested by the Duncan multiple range test to calculate the mean ± SE among different groups (Duncan Citation1955).
Results
Overall mean TG concentration was found highly significant in the supplemented groups (HP, SYM and PRO) compared to CONT (control), being highest (P ≤ .01) in HP irrespective of the production stage (). A significant increase in TG concentration was observed in HP versus CONT at 5P, and it was even higher (P ≤ .01) in HP compared to other groups (SYM and PRO) including CONT at PP (). Overall mean T-Chol concentration was significantly higher in HP versus SYM and PRO () irrespective of the production period studied. The mean T-Chol increased significantly (P ≤ .01) in HP at PP compared to CONT and other supplemented groups, whereas it decreased (P ≤ .01) in PRO at EP versus CONT (). The overall mean HDL-C concentration was higher (P ≤ .01) in HP compared to CONT excluding the stage of production (). The HDL-C increased (P ≤ .01) in HP at PP and EP as compared to CONT. Overall, mean LDL-C concentration, regardless of the production phase, decreased (P ≤ .01) in SYM and PRO as in CONT (). A significant decrease in the LDL-C was observed in all the supplemented groups of HP, SYM and PRO at EP in comparison to CONT (). The overall mean cortisol concentration decreased significantly in all the supplemented groups of HP, SYM and PRO when compared with CONT, regardless of the stage of production (). The mean cortisol level decreased (P ≤ .01) in SYM as in CONT at PP, which was eventually found to be decreased (P ≤ .01) at EP in all the supplemented groups of HP, SYM and PRO compared to CONT (). In metabolic hormones, overall triiodothyronine (T3) concentration, irrespective of the production span, increased (P ≤ .01) in HP, SYM and PRO; however, in the SYM group, T3 was even higher than in HP (). The overall mean thyroxine (T4) concentration excluding the production stage decreased (P ≤ .01) in SYM and PRO than in CONT (). The mean T4 concentration significantly decreased in SYM and PRO at EP when compared to CONT and HP (). Overall, regardless of the stage of production, the AST concentration significantly increased in PRO compared to CONT (). The mean AST concentration significantly increased in SYM and PRO versus CONT at EP ().
Figure 1. Overall mean serum lipid profile of moulted hens in different treatment groups irrespective of production stage. ABC similar alphabets on overall mean ± SE bars do not differ significantly at P ≤ .01. CONT: Control CP 16% E = 2795 kcal, no supplement; HP: CP 18% Diet E = 2800 kcal; SYM: CP 16%, E = 2795 kcal, symbiotic in daily drinking water (85 mg L−1); PRO: CP 16%, probiotic in daily drinking water (85 mg L−1).
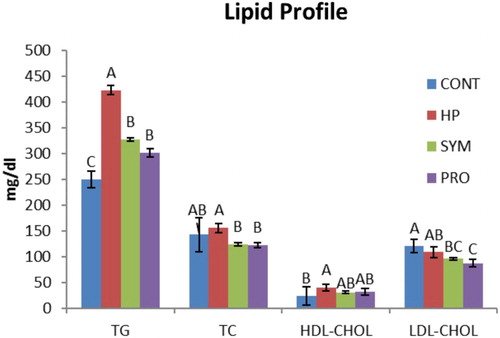
Figure 2. Overall mean serum cortisol level of moulted hens in different treatment groups irrespective of production stage. AB similar alphabets on overall mean ± SE bars do not differ significantly at P ≤ .01. CONT: Control CP 16% E = 2795 kcal, no supplement; HP: CP 18% Diet E = 2800 kcal; SYM: CP 16%, E = 2795 kcal, symbiotic in daily drinking water (85 mg L−1); PRO: CP 16%, probiotic in daily drinking water (85 mg L−1).
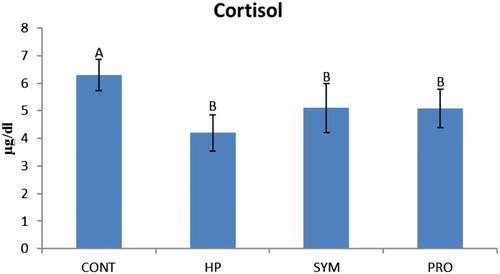
Figure 3. Overall mean serum metabolic hormones level of moulted hens in different treatment groups irrespective of production stage. ABC similar alphabets on overall mean ± SE bars do not differ significantly at P ≤ .01. CONT: Control CP 16% E = 2795 kcal, no supplement; HP: CP 18% Diet E = 2800 kcal, SYM: CP 16%, E = 2795 kcal, symbiotic in daily drinking water (85 mg L−1); PRO: CP 16%, probiotic in daily drinking water (85 mg L−1).
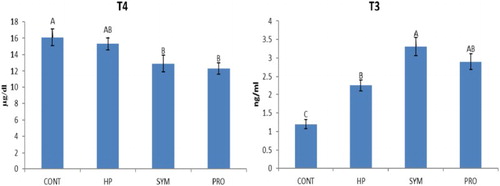
Figure 4. Overall mean serum liver enzymes level of moulted hens in different treatment groups irrespective of production stage. AB similar alphabets on overall mean ± SE bars do not differ significantly at P ≤ .01. CONT: Control CP 16% E = 2795 kcal, no supplement; HP: CP 18% Diet E = 2800 kcal; SYM: CP 16%, E = 2795 kcal, Symbiotic in daily drinking water (85 mg L−1), PRO: CP 16%, probiotic in daily drinking water (85 mg L−1).
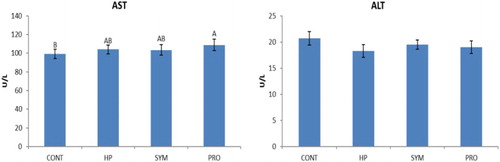
Table 3. Serum lipid profile of moulted hens in different treatment groups at various production stages.
Table 4. Serum hormonal profile and liver enzymes of moulted hens in different treatment groups at various production stages.
Discussion
The regulation of HDL metabolism is mediated by various cellular and plasma factors and membrane proteins (Eckardstein et al. Citation2001; Tol Citation2002). Amongst these mediators, the phospholipid transfer protein (PLTP) is most significant, as it transfers the lipolytic remnants from LDL and chylomicron 193 to HDL (Qin et al. Citation2000). The PLTP and CETP activities are increased in HP and could be a potential reason for the enhanced level of T.Chol and HDL-C. Acylation-stimulating protein (ASP), an adipokine, is connected with the lipid metabolism (Sniderman et al. Citation2000). Cianflone et al. (Citation2004) reported an increase in the synthesis of TG and its storage in adipocytes by ASP stimulation of diacylglycerol acyltransferase which is a rate-limiting enzyme responsible for the synthesis of TG. It has been previously reported that the reversible decrease in ASP level was due to fasting and weight loss (Cianflone et al. Citation1995), which might have played a contributory role in increased TG concentration in the PRO group that was observed in the current study. Reportedly, protein and probiotic supplementation plays a role in reducing the lipid peroxidation and increasing the antioxidant capacity of the body in moulted egg-laying hens (Anwar et al. Citation2012a). The increased TG level was estimated for its role in the formation of plaque and oxidative stress, but these are the slightest lipid sub-fraction of the arteriosclerotic plaque, which neglects it from the risk elements (Marinello et al. Citation2003); however, the LDL-C shows a strong relationship with the development of atherosclerotic plaques and oxidative stress (Gardener et al. Citation2009). In a previous report by Anwar et al. (Citation2012a), protein and probiotic supplementation in the moulted birds did decrease the total oxidant status with a counter increase in the antioxidant enzymes of the body. The use of yeast (Saccharomyces cerevisiae) as probiotic supplementation in the diet of broilers has been shown to have a reducing effect on the serum TG and cholesterol concentration (Shareef and Al-Dabbagh, Citation2009). Cavallini et al. (Citation2009) have also reported similar findings in cholesterol-fed rabbits and demonstrated that the addition of Enterococcus faecium did decrease the TG concentration significantly. A 22–33% decrease in the cholesterol level was observed in humans after using oral probiotics (Pereira and Gibson, Citation2002) and it had anti-hypercholesterolaemia effects in mice in experimental animals fed with high-fat diet (Taranto et al. Citation2000). The assimilation of cholesterol by gut microbes or binding of cholesterol by cell wall bacteria particularly (Lactobacillus) could be the possible mechanism behind this phenomenon (Liong and Shah, Citation2005).
Akram et al. (Citation2002) have reported the fasting resulted in an increase in the circulatory cortisol in the induced moulted layers and attributed it to the influence of stress due to fasting. It is shown in numerous studies that exposure to stress increases corticoid concentrations (Davis Citation1996). It has also been reported that the cortisol level was significantly raised in induced moulted laying hens (zinc-induced and fast-induced) as compared to the cortisol level prior to moulting, which clearly demonstrates the effect of moulting on the circulatory cortisol. The cortisol concentration in the present investigation was high at 5P in all the groups showing the after-effect of moulting. Sandhu et al. (Citation2007) have also reported the decline in the cortisol level in the moulted birds at PP which was significantly high just after the moulting at 5% production. Increased corticotrophin-releasing hormone was seen during the stress which depicts the stimulation of the hypothalmo-pituitary axis (Eutamene and Bueno Citation2007). In the current study, cortisol concentration was decreased irrespective of the stage of production (P ≤ .01) in all the supplemented groups as compared to the control. Supplementation of Lactobacillus has also shown promising results in reducing the high level of cortisol in neonatal rat pups (Gareau et al. Citation2007).
Anwar et al. (Citation2012b) have reported the efficacy supplementation with probiotic and protein on the morphological dynamics of anterior pituitary growth hormone-producing cells in the moulted laying hens, which was responsible for better growth and production potential of the supplemented group. More recently Anwar et al. (Citation2015) have reported an increase in the gonadotrophs cell and nuclear size and area which are ultimately responsible for the change in hormonal dynamics of metabolic hormones as well in the supplemented moulted birds in the current study. It has been reported that the circulatory cortisol is also responsible for a subsequent decrease in thyroid hormones (Garriga et al. Citation2006). Sohail et al. (Citation2010) reported the supplementation with lactobacilli-based probiotic, in broilers birds kept under heat-stress environment, caused an increase in T4 level with a corresponding reduction in serum cortisol. A suggested plausible mechanism could be that the afferent nerves of gastrointestinal tract are stimulated in a reaction of change in the gut flora to normalize the proinflammatory cytokines IL2/IL10; thus probiotics may incidentally decrease the levels of systemic corticosterone and adrenocorticotropic hormone by affecting the level of HPA (Eutamene and Bueno, Citation2007). The major glucocorticoid in the avian species is corticosterone but in some conditions like chronic stress in the egg-laying white leghorns cortisol can also be measured. Likewise, Lonkar et al. (Citation2016) have reported improvement in the performance of egg-laying hens during summer stress by depicting the cortisol index.
Moreover, it has been reported that the AST level differs with genomic and climatic variation (Hassan et al. Citation2009). Guyton and Hall (Citation Citation2011) have reported a direct relationship between altered protein metabolism and liver transaminase. In the current study, the protein metabolism was increased in HP and PRO groups and could be attributed to the higher level of AST in these groups. The increase of AST and ALT levels in the early production stage (5P) just after the moulting in all the groups could very well be related to the corticosterone stimulation of the liver as stress level was high till 5P. This assumption is substantiated by the earlier findings regarding the direct correlation of adrenal activity with the concentration of ALT (Gildersleeve et al. Citation1983).
Conclusion
Increase in the HDL-C and metabolic hormones and reduction in the LDL-C and cortisol levels in the supplemented groups were noticed depicting the significant role of supplementation in reducing the lipid peroxidation which is a preliminary step in the development of oxidative stress. The results demonstrate the beneficial impact of protein, probiotic and symbiotic supplementation in the moulted laying hens as an early stress reliever after the induced moulting which is helpful to enhance the second phase production potential of the moulted birds.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Akram M, Rahman ZU, Na CS, Kim SH, Ryu KS. 2002. Effect of induced molting on the relative weights and hormone levels of thyroid, ovary and adrenal glands in spent laying hens. Korean J Poult Sci. 29:243–247.
- Anwar H, Rahman ZU, Javed I, Muhammad F. 2012a. Effect of protein, probiotic, and symbiotic supplementation on serum biological health markers of molted layers. Poult Sci. 91:2606–2613. doi: 10.3382/ps.2012-02172
- Anwar H, Rahman ZU, Javed I, Muhammad F. 2012b. Immunohistochemical localization and morphometry of somatotrophs and lactotrophs in protein, probiotic and symbiotic supplemented molted layers. Europ J Histochem. 56:173–178. doi: 10.4081/ejh.2012.e28
- Anwar H, Rahman ZU, Javed I, Muhammad F. 2015. Immune potentiating role of protein, probiotic and symbiotic supplementation in molted white leghorn hens. Avian Biol Res. 8:25–34. doi: 10.3184/175815515X14217780084082
- Cavallini DCU, Bedani R, Bomdespacho LQ, Vendramini RC, Rossi EA. 2009. Effects of probiotic bacteria, isoflavones and simvastatin on lipid profile and atherosclerosis in cholesterol-fed rabbits: a randomized double-blind study lipids. Health Dis. 8:1–8. doi: 10.1186/1476-511X-8-1
- Cianflone K, Roncari DAK, Maslowska M, Baldo A, Forden J, Sniderman AD. 2004. The adipsin/acylation stimulating protein system in human adipocytes: regulation of triacylglycerol synthesis. Biochemistry. 33:9489–9495. doi: 10.1021/bi00198a014
- Cianflone K, Sniderman AD, Kalant D, Marliss EB, Gougeon R. 1995. Response of plasma ASP to a prolonged fast. Int J Obesity. 19:604–609.
- Davis GS. 1996. The effects of a direct-fed microbial on body weights and feed efficiency in bobwhite quail. Gamebird Bull. 29(2):1–6.
- Duncan DB. 1955. Multiple range and multiple F-test. Biometries. 11:1–42. doi: 10.2307/3001478
- Eckardstein VA, Nofer JR, Assmann G. 2001. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscl Throm Vas. 21:13–27. doi: 10.1161/01.ATV.21.1.13
- Eutamene H, Bueno L. 2007. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut. 56:1495–1497. doi: 10.1136/gut.2007.124040
- Gardener H, Morte DD, Mitchell SV, Elkind Sacco LR, Rundek T. 2009. Lipids and carotid plaque in the northern Manhattan study (NOMAS). Cardiovasc Disorders. 9:55–50. doi: 10.1186/1471-2261-9-55
- Gareau MG, Jury J, MacWueen G, Sherman PM, Perdue MH. 2007. Probiotic treatment of rat pups normalizes corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 56:1522–1528. doi: 10.1136/gut.2006.117176
- Garriga C, Hunter RR, Amat C, Planas JM, Mitchell MA, Moreto M. 2006. Heat stress increases apical glucose transport in the chicken jejunum. Am J Physiol Regul Integr Comp Physiol. 290:195–201. doi: 10.1152/ajpregu.00393.2005
- Gildersleeve RP, Satterlee DG, Johnson WA, Scott TR. 1983. The effects of forced molt treatment on blood biochemicals in hens. Poult Sci. 62:755–762. doi: 10.3382/ps.0620755
- Guyton AC, Hall JE. 2011. Guyton and hall textbook of medical physiology. 13th ed. Philadelphia, United States of America: Elsevier Saunders.
- Hassan SF, Abdel-Fattah SA, Elsalmoney AE, Hassan MSH. 2009. Relationship between some serum enzyme activities, liver functions and body weight in growing local chickens. Int J Poult Sci. 8:700–705. doi: 10.3923/ijps.2009.700.705
- Johnson K, Lohman S. 2003. The effects of different diets on hen egg production. Innisfail: Innisfail Jr./Sr. High School Chinooks, Edge School Division.
- Kalavathy R, Abdullah N, Jalaudin S, Wong CMVL, Ho YW. 2008. Effect of Lactobacillus cultures and oxy tetratcylcines on the growth performance and serum lipids of chickens. Int J Poul Sci. 7:385–389. doi: 10.3923/ijps.2008.385.389
- Liong MT, Shah NP. 2005. Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. Int Dairy J. 15:391–398. doi: 10.1016/j.idairyj.2004.08.007
- Lonkar VD, Kadam AS, Choudhary A, Maini S, Ravikanth K. 2016. Performance improvement in layer birds supplemented with herbal liver tonic and antistressor product during summer stress. Int J Pharm Sci. 8(7):129–132.
- Marinello E, Setacci C, Giubbolini M, Cinci G, Frosi B, Porcelli B, Terzuoli L. 2003. Lipid composition in atheromatous plaque: evaluation of the lipid three phase percentage. Life Sci. 72:2689–2694. doi: 10.1016/S0024-3205(03)00185-1
- Patterson JA, Burkholder KM. 2003. Application of prebiotics and probiotics in poultry production. Poult Sci. 82:627–631. doi: 10.1093/ps/82.4.627
- Pereira DI, Gibson GR. 2002. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Critical Rev Biochem Mol Biol. 37:259–281. doi: 10.1080/10409230290771519
- Pisani T, Gebski CP, Leary ET. 1995. Accurate direct determination of low density lipoprotein cholesterol using an immuno suppression reagent and enzymatic cholesterol assay. Arch Pathol Lab Med. 119:1127–1120.
- Qin S, Kawano K, Bruce C, Lin M, Bisgaier C, Tall AR, Jiang X. 2000. Phospholipid transfer protein gene knock-out mice have low high density lipoprotein levels, due to hyper catabolism, and accumulate apoA-IV-rich lamellarlipoproteins. J Lipid Res. 41:269–276.
- Renden JA, Lien RJ, Oates SS, Bilgili SF. 1994. Plasma concentrations of corticosterone and thyroid hormones in broilers provided various lighting schedules. Poult Sci. 73:186–193. doi: 10.3382/ps.0730186
- Sandhu MA, Rahman ZU, Rahman SU. 2007. Effects of induced molting on some immunological parameters in laying hens (Gallus domesticus). Arch Geflügelk. 71:110–116.
- Shareef AM, Al-Dabbagh ASA. 2009. Effect of probiotic (Saccharomyces cerevisiae) on performance of broiler chicks. Iraqi J Vet Sci. 23:23–29.
- Simmering R, Blaut M. 2001. Pro- and prebiotics – the tasty guardian angles. Appl Microbiol Biotechnol. 55:19–28. doi: 10.1007/s002530000512
- Sniderman AD, Maslowska M, Cianflone K. 2000. Of mice and men (and women)and the acylation-stimulating protein pathway. Curr Opin Lipid. 11:291–296. doi: 10.1097/00041433-200006000-00010
- Sohail MU, Ijaz A, Yousaf MS, Ashraf K, Zaneb H, Aleem M, Rehman H. 2010. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult Sci. 89:1934–1938. doi: 10.3382/ps.2010-00751
- Steel RGD, Torrie JH, Dieky DA. 1997. Principles and procedures of statistics, 3rd ed. New York (NY): McGraw Hill Book.
- Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. 2000. Effect of Lactobacillus reuteri on the prevention of hypercholesterolemia in mice. J Dairy Sci. 83:401–403. doi: 10.3168/jds.S0022-0302(00)74895-8
- Tol VA. 2002. Phospholipid transfer protein. Curr Opin Lipidol. 13:135–139. doi: 10.1097/00041433-200204000-00004
- Yamauchi KE. 2007. Review of a histological intestinal approach to assessing the intestinal function in chickens and pigs. Animal Sci Jo. 78:356–370. doi: 10.1111/j.1740-0929.2007.00448.x
