ABSTRACT
The cardiovascular system in newborns has unique features; the system differs from that of foetuses due to the onset of pulmonary respiration and the closure of shunts and differs from that of adults due to heart and autonomic system immaturity. Twenty Ile de France lambs were evaluated during the first 35 days of life to describe changes in the electrical conduction of the heart and in the sympathetic and parasympathetic system during the neonatal period. Electrocardiographic evaluation and the sympathovagal balance was assessed by heart rate variability (HRV) were performed, and ambulatory electrocardiography was performed with a Holter system from birth and at 7, 14, 21, 28 and 35 days of age. There was a significant difference in the duration of the PR and QT intervals and the T wave, as well as a decrease in the amplitude of the P, R and T waves for the evaluated moments. The heart rate and total QRS decreased progressively until 35 days, whereas the HRV indexes increased during the same period. The neonatal period requires care and attention, as several adaptations for neonate survival in the extrauterine environment occur during this period.
Abbreviations: HRV: heart rate variability; PR, QT: electrocardiographic intervals; P, R and T: electrocardiographic waves; QRS: electrocardiographic complexes; I, II, III, aVL, aVF and aVL: electrocardiographic member derivations; FP: frontal plane of electrocardiogram; HR: heart rate; RR or NN: interval between two R waves in Holter evaluation; NNmed: the average value of all normal cycles measured during the Holter evaluation; RMSSD: square root of the average of successive differences squared between normal RR intervals measured during the Holter evaluation; pNN50: successive differences between the percentage of RR intervals greater than 50 ms in Holter evaluation
Introduction
The electrocardiogram (ECG) is a supplementary examination. It is non-invasive and low cost, enabling the detection of changes in the electrical conduction of the heart chambers (arrhythmias) and axis in the frontal plane (Tilley Citation1992; Camacho et al. Citation2014; Mendes Netto Citation2014; Torad et al. Citation2017). In addition, it is useful to study heart rate variability (HRV) and autonomic balance, an indicative marker of reduced vagal activity that is protective against the induction of ventricular arrhythmias (Sosa et al. Citation1995; Lorga Filho et al. Citation2013).
Neonatal period in human accounts for the first 28 completed days of life (World Health Organization Citation2018), however, no official definition of the neonate has been provided in animals. The first week of life is taken into consideration in studies on neonatal mortality in cattle (Azzam et al. Citation1993), but other definitions encompass much larger periods (e.g. until 45 days of age (Wittum et al. Citation1994)). Similarly, in small ruminants, the entire period from birth until weaning (at about 30–45 days) maybe be named as neonatal (Mellado et al. Citation1998; Awemu et al. Citation1999; Nowak and Poindron Citation2006). During this period, colostrum intake plays an important role in the physiology of neonatal development, as it is composed of a complex mixture of fat, lactose, vitamins, minerals, antimicrobials and anti-inflammatory agents that will contribute to the development of the neonate immunology, circulatory and gastrointestinal system (Hernandez-Castellano et al. Citation2014).
During the neonatal phase, the cardiocirculatory physiology differs in blood pressure, peripheral vascular resistance, cardiac output, the relationship between the left and right ventricular mass, left ventricular anatomical conformation and autonomic nervous system innervation (Hines Citation2013). This innervation varies frequently during the course of the day, such as respiratory movements that alter the vagal tone or sympathetic activity, causing sinus cycles that are not the same length (variation in RR intervals); this is called HRV. This index can be calculated using an ambulatory Holter system electrocardiographic or based on the measurement of RR intervals individually and by comparing RRs. For parasympathetic stimulation, there is a quick response of short duration and amendment in the first heartbeat, i.e. consecutive RRs reflecting the vagal tone with increasing HRV (Brito Citation2009; Pereira Citation2011).
The study of HRV in sheep is of great interest because of the similarity of the sheep heart to the human heart, including the size of the heart chambers, heart rate, autonomic innervation and cardiac output. This similarity justifies research interests, particularly during the foetal and neonatal period (Markovitz et al. Citation1989; Von Borell et al. Citation2007). One advantage of using sheep in research includes their availability, size, low maintenance cost and rapid gestation (Ali et al. Citation1996; Dixon and Spinale Citation2009).
Because of the importance of electrocardiographic studies on sheep and the lack of literature regarding this subject, this study aimed to evaluate and characterize potential and development in lambs during the neonatal period using HRV to describe adjustments during the transitional period and propose benchmarks for the age group and species studied.
Materials and methods
Ethics statement
The study was conducted at Medical Clinic for Large Animals of the School of Veterinary Medicine and Animal Sciences, UNESP, in Botucatu, São Paulo, Brazil. This study was approved by the Ethics Committee on the use of Animals – CEUA, Protocol n° 230/2012.
Electrocardiography
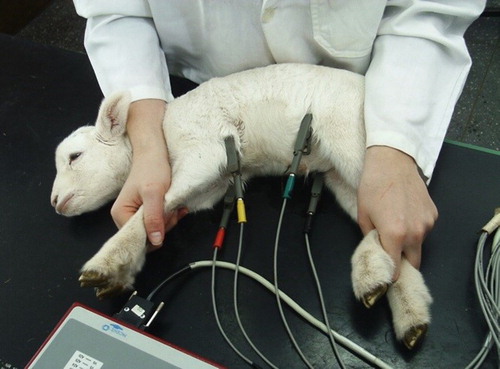
Twenty healthy Ile de France lambs of both sexes were assessed from birth (24 h) and on the 7th, 14th, 21st, 28th, and 35th days of age. The exams were performed using the computerized electrocardiogram (TEB®, São Paulo, Brazil), lambs were submitted to electrocardiographic examination weekly at the same time, with six member derivations (I, II, III, aVR, aVL, and aVF), for 25 mm/s and sensitivity 1 cm = 1 mV, during 90 s in the frontal plane (FP). The animals were placed in right lateral decubitus on a table covered with rubber to avoid interference and contained manually without sedation or anaesthesia.
For the derivation in the FP, four ‘alligator’-type electrodes were placed on both forelimbs in the radio-ulnar humeral articulation (yellow on the left, and red on the right) and another two electrodes were placed in the lower limbs in the femur-ankle joint patella region (black on the right and green on the left). Positioning electrode regions were previously moistened with alcohol for better electrical conduction. After placement of the electrodes, the animals were given 5 min to calm down and acclimate to the electrodes such that the exam could be performed under nearly ideal conditions. The interpretation of the electrocardiographic tracings was based on the DII analysed as follows: duration of P waves and QRS complex and T, PR and QT intervals in milliseconds (ms); amplitude of P, R and T in millivolts (mV); and polarity of T, pace, heart rate (HR) and cardiac electric axis ().
Ambulatory electrocardiography-Holter system
The recording was performed using a 24 h ECG system (Holter system – Cardios, São Paulo, Brazil) with continuous recording of three channels in pre-cordial leads with modified derivations (V1, V3 and V5) using a digital apparatus (Cardiolight, Cardios, São Paulo, SP) written on an electromagnetic card and analysed by computer decoding (CardioNet Software Client, Cardios, São Paulo, SP). The writer was directly tied to the backs of the lambs, offering freedom of movement, with protection of the device. The cables that were connected to the adhesive electrodes attached to the skin of the sheep were previously prepared (trichotomy and antisepsis). Two electrodes were positioned on the left, the red electrode was positioned near the sternum and the green electrode was positioned immediately above the red electrode. The other two electrodes were fixed on the right side of the chest, with the black electrode near the sternum and the white electrode immediately above the right scapula. After placing the recorder, the lambs were bandaged to protect and limit the movement of electrodes and signal loss.
The following parameters were analysed: percentage of artefacts, HR, number of QRS complexes in 24 h (QRSt), average of all RR intervals (NNmed), square root of the average of the successive differences squared between normal RR intervals (RMSSD), and adjacent and successive differences between the percentage of RR intervals greater than 50 ms (pNN50), with the last two referring to the heart rate variability (HRV).
Statistical analysis
Statistical analysis consisted of repeated measures models and ANOVA to compare the variables between the moments. A covariance structure auto-regressive function was used to model the correlation between repeated measures within the same animal. Tukey's test was used to adjust p-values resulting from multiple comparisons. For non-parametric variables, the Wilcoxon test was used for paired samples with Bonferroni correction to adjust the p-values resulting from multiple comparisons. The level of statistical significance was set at 0.05.
Results
Electrocardiography
The results of the electrocardiographic parameters are displayed as the mean ± standard deviation and are presented in and . The predominant cardiac rhythm was sinus with a progressive decrease in HR between birth and 35 days of age (37.81 ± 211 and 149.35 ± 33.51 bpm). The T wave showed positive polarity in 80% of the strokes. The heart electrical axis of the lambs was variable at all times, varying between −173° and + 180°. For the moments, there was no difference, with an increase in the PR interval duration and QT interval from the 14th day and in T wave from the 7th day. There was a decrease in the amplitude of the P, R and T waves over the 35 days. The P-wave amplitude and duration of the QRS complex showed no significant difference (p > 0.05).
Figure 2. Electrocardiographic tracing of lamb 20, deriving DII, frontal, and birth (24 h) at 35 days of age (25 mmseg, N).
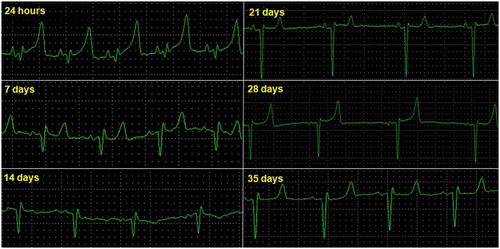
Table 1. Mean and standard deviation of variables obtained by ECG in the frontal plane at the second moment of evaluation.
Ambulatory electrocardiography-Holter system
The results obtained for each parameter in the time domain using the Holter system electrocardiographic tracings are presented in and are separated by moments of evaluation. Monitoring is considered satisfactory because the monitors were placed for 24 h (90% of recordings). The shortest evaluation was 22 h. The highest percentage of artefacts was found at 35 days and did not alter the results of the study. HR and total QRS decreased significantly between birth and 35 days of age (p < 0.05). NNmed indices and pNN50 increased progressively until the end of the neonatal period (p < 0.05). The RMSSD index increased from the 14th day to the 21st day and decreased further to the 28th day, ending with an increase to 35 days of age (p < 0.05).
Table 2. Mean and standard error* of variables obtained by 24-h ECG using a Holter system for the second moment of evaluation.
Discussion
The HR fell progressively and significantly over the study period but maintained sinus tachycardia compared to adults (70–80 bpm). HR and the number of QRS complexes are closely related because the frequency is based on the number of beats, which in turn is counted by the quantity of QRSs present at the circuit (r2 = 0.948). There is an inversely proportional relationship between age and HR, with a high rate at birth (170 bpm) until 30 days of age (120 bpm) (Radostits et al. Citation2007). The HR reached 115 bpm at 60 days of age (Atmaca et al. Citation2014). The sinoatrial node undergoes (in)direct influence of the parasympathetic system that has not yet developed and cannot act to decrease the frequency immediately after childbirth, allowing the sympathetic system to cause physiological tachycardia. The baroreceptors are immature and require high concentrations of norepinephrine to be sensitized for a heart pumping effect that reduces HR and blood pressure. The high frequency is also maintained by a high concentration of circulating plasma catecholamines arising from the stress of confinement and may be 30 times greater than in adults because of the inability to metabolize these molecules (Tudbury and Atkinson Citation1950; Torío et al. Citation1997; Dönmez and Çinar Citation2003; Ker and Webb Citation2005). Tachycardia also cooperates in maintaining cardiac output because the ejection volume is significantly lower than in adults (Chan et al. Citation2008).
In the present study, the prevailing rhythm was sinus (95.7%; 155/162 strokes) with the presence of some sinus arrhythmia (4.3%; 7/162 strokes) that followed a constant pattern and similar respiratory movements, suggesting that these respiratory sinus arrhythmias are due to vagal stimulation (Radostits et al. Citation2007). In Angora goats, 84% sinus rhythm and 18% sinus arrhythmia were found between one and two years of age. The authors also suggested a positive relationship (Atmaca et al. Citation2014). Gallega sheep exhibit sinus rhythm (66.7%) and respiratory sinus arrhythmia (23.3%) between two and seven years, demonstrating that older animals maintain a rhythmic pattern but with a slow HR (Torío et al. Citation1997).
The values for the duration of P are higher than those found in sheep without trace set at three years of age (0.02 s) (Dönmez and Çinar Citation2003) but are consistent with Gallega sheep (0.039 s) 23 adults and Dorper lambs, for which a value of 0.064 s was obtained using the conventional frontal plane technique (Ker and Webb Citation2005). The magnitude was similar to that of sheep at three years (0.1 mV) but greater than the values for adults and lambs (0.118 and 0.09 mV, respectively) (Torío et al. Citation1997; Dönmez and Çinar Citation2003; Ker and Webb Citation2005). The heart of newborns is relatively larger as the circulation is from right to left due to blood deviations (foramen ovale and arterial trunk duct) during the foetal period. At birth, pulmonary circulation and the left heart become dominant, mainly through an increase in the size of the ventricle, modifying the vector orientation (heart axis). The electrocardiographic pattern begins to introduce adult features, starting with the decreased amplitude of P (right atrial depolarization) (Tudbury and Atkinson Citation1950; Bright Citation1995; O’Connor et al. Citation2008).
The duration of the PR interval increased from birth to 35 days (0.079–0.093 s), similar to lambs (0.08 s) but less than adult sheep (0.102 s) (Torío et al. Citation1997; Ker and Webb Citation2005). The results indicated the immaturity of the parasympathetic system and performance of the sinoatrial and atrioventricular nodes as sympathetic because they suffer influence (in) directly. The atrioventricular node is sensitive to changes in sympathetic activity, leading to tachycardia and increased speed of electric conduction (Keunen et al. Citation2000). The delay in conduction of the electrical stimulation of the atria to the ventricles is also due to the lower myocardial mass in relation to adults (Chan et al. Citation2008; O’Connor et al. Citation2008).
Ventricular contraction is represented by the R wave amplitude and decreased between birth and 35 days of age (0.190–0.087 mV). This trend is likely explained by the maturation of the bundle of His and Purkinje cells, which leads to synchronized depolarization (Tilley Citation1992; Atmaca et al. Citation2014). Ambient temperature influences the electrocardiographic parameters, with R values near 0.160 mV in goats 60 days of age, similar to lambs at birth. Peripheral vascular resistance and positive compensatory inotropism occurs (Mendes et al. Citation2010). Lambs are born with increased resistance such that R wave decreases with maturity (Piccione et al. Citation2007).
The QRS complex showed a duration similar to that in adult animals (Torío et al. Citation1997; Dönmez and Çinar Citation2003; Ker and Webb Citation2005). This similarity may be due to the thoracic conformation ovine species and the technique that uses the front plane as the main electrocardiographic evaluation method (Nunes et al. Citation2014). The QRS complex represents ventricular depolarization that occurs in all directions of the ventricular endocardium in ruminants, enabling faster conduction due to the greater depth of the Purkinje fibres in the cardiac mass. In primates and carnivores, the cells penetrate only a quarter of the wall, making the dissipation of stimulus considerably slower (Hamlin Citation2007).
The present study observed an increase in QT intervals, whereas the frequency decreased over the course of weeks (0.168–0.209 s). Studies with lambs from two to five months and 10 to 12 months (Tudbury and Atkinson Citation1950; Bright Citation1995; Keunen et al. Citation2000; Mir et al. Citation2000; Ker and Webb Citation2005; Hamlin Citation2007; O’Connor et al. Citation2008; Mendes et al. Citation2010; Nunes et al. Citation2014) presented more QT intervals with lower frequencies (105 and 150 bpm), indicating a negative correlation. In adult animals, between one to two years (Atmaca et al. Citation2014) and two to seven years (Torío et al. Citation1997), the QT exhibits high values, with frequencies close to 100 bpm. The duration of the cardiac systole represents QT and is directly connected to the sympathetic nervous system, with a negative correlation with HR, i.e. the increase in the QT interval indicates a lower range between (Gonçalves et al. Citation2012).
The amplitude of the T wave (mV) also varied significantly; at birth, it was high and non-formed, with a significant decrease until the 28th day, when it again underwent a significant increase until the 35th day. The polarity of the T wave remained positive in derivation II in 86.25% of strokes in the frontal plane until day 35. The amplitude of the T wave (ventricular repolarisation) decreases significantly between the first and fourth days after birth, similar to the results obtained in this study (Tovar et al. Citation1985). In adult Gallega sheep, the T wave was positive in 72% of strokes and biphasic in 28%, without practical value for diagnostics, and can have any polarity without pathological features (Torío et al. Citation1997). The same trend is observed in children, who present a positive T wave or negative biphasic wave at birth. After birth, a negative pattern often occurs, and a T wave greater than the average newborn indicates of hypoxia (Chan et al. Citation2008).
The cardiac axis ranged throughout the period. It is not possible to compare it to the literature. The heart of an adult sheep moves from left to right resulting in an axis in the third quadrant, left-facing (+90° to +130°) (Dönmez and Çinar Citation2003). The axis of Gallega sheep is left-facing (−165° to −137°) in the first quadrant, which is explained by the placement of electrodes in relation to the heart and ventricular depolarization that occurs from the apex to the base of the left ventricle to the right ventricle (Torío et al. Citation1997). There is no consensus in the literature regarding the heart axis in sheep, in either adults or newborns. Many factors can change the axis, such as the electrode positioning, technique used, size of the animal, breed and age. In children, the cardiac axis is situated between 0° and +90° due to the increased right cardiac mass; with cardiovascular adaptation and an increase in the left ventricle, the heart axis shifts to a left position between −30° and +90° (Chan et al. Citation2008; O’Connor et al. Citation2008).
The NNmed index increased significantly during the first 35th days of life. This trend indicates the influence of the sympathetic nervous system on the sinoatrial node, which increases the frequency, making the RR intervals smaller (Mohr et al. Citation2002; Azhibekov et al. Citation2014). Maturation of the autonomic nervous system begins during foetal life and ends during the neonatal period. An indication of this development is a decrease associated with the increase in HRV. This predominance in children occurs due to excess stress, which inhibits the actions of the parasympathetic system (De Rogalski Landrot et al. Citation2007).
The animals are subjected to many stress factors that activate the sympathetic system, increasing the HR. In this case, there is physiological neonatal tachycardia. Without vagal tone, there is a decrease because there is no variance between the RR intervals (Mohr et al. Citation2002; Faria et al. Citation2009). This is also related to the beginning of maturation of the parasympathetic tone, which causes a slow HR, increasing the distance between the RR. This trend is related to a reduction in stress generated by the adaptation to physical and environmental influences. This decrease also contributes to the increase in the indices because the place of origin was the same used for the tests (Faria et al. Citation2009). Until the eighth week of life, lambs have autonomic control by the sympathetic system, with modification and vagal dominance only until three months of age (Despres et al. Citation2003).
The parasympathetic system can be observed by RMSSD and pNN50, which are influenced directly by vagal tone (Rassi Citation2000). There was decrease in RMSSD due to the high neonatal HR and immaturity of the parasympathetic system, leading to the maintenance of tachycardia and associated with a reduction in frequency over the study period. The parasympathetic system begins operating between 21 and 28 days, when it begins to increase the values of the index. The stress on the HRV in cows that the RMSSD reflects indicates that the vagal tone is directly decreased during peak moments of stress. Healthy animals should have a high HRV, showing good adaptation between the autonomic systems (Mohr et al. Citation2002).
The welfare of lambs is based on the HRV in lambs. Starting at two months, they were pet by humans during handling. The values of 30 ± 5 ms for the index system performance confirmed the outlying RMSSD during moments when the animal expressed positive emotions. The HR decreases, the parasympathetic predominates and RMSSD increases (Coulon et al. Citation2015). The values observed in this study are relatively low but lead to the same conclusion. With a decrease in HR and the beginning of the predominance of vagal tone, RMSSD tends to rise from birth to 35 days.
The pNN50 increased during the first 35 days of life. The index indicates how many RR intervals for pNN50 were longer 50 ms, and this situation is possible only because the parasympathetic system lowers the heart rate and increases the distance between the two waves (Rassi Citation2000). This trend was observed in the present study, and as the HR decreases, there is an increased intrinsic relationship with pNN50. The results obtained for RMSSD suggest that the parasympathetic system starts its activities between 14 and 21 days when it highly expresses its activities and exhibits a decreasing HR, indicating double modulation of the cardiac system and changes in the autonomic influence of the sinoatrial node. There is a gradual increase in parasympathetic mediation and greater volumetric capacity of the ventricles after the beginning of pulmonary circulation, which acts directly on the HRV and is intrinsically related to growth of the newborn (Finley and Nugent Citation1995; Koether et al. Citation2015).
Conclusions
Neonatal lambs, and the majority of newborn mammals, present unique features on electrocardiographs. They display dynamics due to the immaturity of the cardiovascular system, such as a high-amplitude, T wave, physiological tachycardia and great variation in electrical conduction. Heart receptors are not yet fully adapted to life outside the womb, and the pattern does not represent the neonatal physiological pattern. The indices directly related to HRV (RMSSD and pNN50) were high until 35 days of age, indicating autonomic control of the heart. It is not possible to confirm the maturity of the vagal tone because the values obtained are not consistent with the literature, suggesting that adaptation can be completed after the neonatal period. In humans, this maturation process takes years to complete. There is a need for further studies after 35 days to characterize the complete cardiac autonomic and parasympathetic maturation. In addition, many other autonomic processes are involved, such as endocrine regulation and kidney function. This complexity and delay in maturity makes the neonate vulnerable to several factors, pathological or not. The results of this study demonstrate the influence of age and growth on cardiac parameters, and it is likely that the dynamics of cardiovascular development in sheep occur beyond the neonatal period until the standard of the age group is reached. The values described can be used in both veterinary clinics and experimental models of medicine.
Ethics approval
This study was approved by the Ethics Committee on the use of Animals – CEUA, Protocol n° 230/2012.
Availability of data and material
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Carla Maria Vela Ulian http://orcid.org/0000-0001-6868-0223
Carlos Roberto Padovani http://orcid.org/0000-0002-7719-9682
Bianca Paola Santarosa http://orcid.org/0000-0003-0937-1919
Maria Lúcia Gomes Lourenço http://orcid.org/0000-0002-8337-4168
Additional information
Funding
References
- Ali ML, Kumar SP, Bjornstad K, Duran CM. 1996. The sheep as an animal model for heart valve research. Cardiovasc Surg. 4(4):543–549. doi:10.1016/0967-2109(95)00142-5.
- Atmaca N, Şimşek O, Emre B. 2014. Some electrocardiographic values of Angora goats. Nurgül Ank Univ Vet Fak Derg. 61:15–19. doi:10.1501/Vetfak_0000002599.
- Awemu EM, Nwakalor LN, Abubakar BY. 1999. Environmental influences on preweaning mortality and reproductive performance of Red Sokoto does. Small Rumin Res. 34(2):161–165. doi: 10.1016/S0921-4488(99)00058-9
- Azhibekov T, Noori S, Soleymani S, Seri I. 2014. Transitional cardiovascular physiology and comprehensive hemodynamic monitoring in the neonate: relevance to research and clinical care. Semin Fetal Neonat Med. 19(1):45–53. doi:10.1016/j.siny.2013.09.009.
- Azzam SM, Kinder JE, Nielsen MK, Werth LA, Gregory KE, Cundiff LV, Koch RM. 1993. Environmental effects on neonatal mortality of beef calves. J Anim Sci. 71(2):282–290. doi: 10.2527/1993.712282x
- Bright JM. 1995. The cardiovascular system. In: Hoskins JD, editor. Veterinary pediatrics: dogs and cats from birth to six months. Philadelphia, PA: W.B. Saunders; p. 95–123.
- Brito FS. 2009. Eletrocardiografia ambulatorial: sistema Holter. Arq Bras Cardiol. 93:e179–ee264.
- Camacho AA, Mucha CJ, Circulatório S, Seção B. 2014. Semiologia do sistema cardiocirculatório de cães e gatos. In: Feitosa FL, editor. Semiologia Veterinária – A arte do diagnóstico. 3rd ed. São Paulo: Zentrum Verlagsgesellschaft; p. 241–262.
- Chan TC, Sharieff GQ, Brady WJ. 2008. Electrocardiographic manifestations: pediatric ECG. J Emerg Med. 35(4):421–430. doi:10.1016/j.jemermed.2007.09.039.
- Coulon M, Nowak R, Peyrat J, Chandèze H, Boissy A, Boivin X, Frasch MG. 2015. Do lambs perceive regular human stroking as pleasant? Behavior and heart rate variability analyses. PLOS One. 10(2):e0118617. doi:10.1371/journal.pone.0118617.
- De Rogalski Landrot IR, Roche F, Pichot V, Teyssier G, Gaspoz JM, Barthelemy JC, Patural H. 2007. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Auton Neurosci Basic Clin. 136(1-2):105–109. doi:10.1016/j.autneu.2007.04.008.
- Despres G, Boissy A, Desire L, Le Neindre P, Veissier I. 2003. Validation of measure of sympatho-vagal effect in lambs through autonomic blockades and heart rate variability indexes. J Vet Adv. 2:615–619.
- Dixon JA, Spinale FG. 2009. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2(3):262–271. doi:10.1161/CIRCHEARTFAILURE.108.814459.
- Dönmez N, Çinar A. 2003. Effects of chronic fluorosis on electrocardiogram in sheep. Biol Trace Elem Res. 92(2):115–122. doi:10.1385/BTER:92:2:115.
- Faria EG, Nogueira SSS, Sousa MG. 2009. Avaliação da variabilidade da frequência cardíaca não espectral em cães e gatos neonatos. MedVep Revista Científica de Medicina Veterinária de Pequenos Animais e Animais de Estimação. 7:354–356.
- Finley JP, Nugent ST. 1995. Heart rate variability in infants, children and young adults. J Auton Nerv Syst. 51(2):103–108. doi:10.1016/0165-1838(94)00117-3.
- Gonçalves AC, Gebrim EM, Monteiro ML. 2012. Imaging studies for diagnosing Graves’ orbitopathy and dysthyroid optic neuropathy. Clinics. 67(11):1327–1334. doi:10.6061/clinics/2012(11)18.
- Hamlin RL. 2007. Animal models of ventricular arrhythmias. Pharmacol Ther. 113(2):276–295. doi:10.1016/j.pharmthera.2006.08.006.
- Hernandez-Castellano LE, Almeida AM, Castro N, Arguello A. 2014. The colostrum proteome, ruminant nutrition and immunity: a review. Curr Protein Pept Sci. 15(1):64–74. doi: 10.2174/1389203715666140221124622
- Hines MH. 2013. Neonatal cardiovascular physiology. Semin Pediatr Surg. 22(4):174–178. doi:10.1053/j.sempedsurg.2013.10.004.
- Ker J, Webb EC. 2005. Electrocardiographic surrogates of structural myocardial alterations in the Dorper sheep heart. Onderstepoort J Vet Res. 72(4):273–277. doi:10.4102/ojvr.v72i4.182 doi: 10.4102/ojvr.v72i4.174
- Keunen H, Van Wijngaarden WJ, Sahota DS, Hasaart THM. 2000. The PR interval-fetal heart rate relationship during repetitive umbilical cord occlusions in immature fetal sheep. Eur J Obstet Gynecol Reprod Biol. 89(1):69–74. doi:10.1016/S0301-2115(99)00160-8.
- Koether K, Ulian CM, Lourenço ML, Gonçalves RS, Sudano MJ, Cruz RK, da Silva Branchini N, Alfonso A, Chiacchio SB. 2015. The normal electrocardiograms in the conscious newborn lambs in neonatal period and its progression. BMC Physiol. 16:160. doi:10.1186/s12899-016-0020-5.
- Lorga Filho A, Cintra FD, Lorga A, Grupi CJ, Pinho C, Moreira DA, Sobral Filho DC, de Brito FS, Kruse JC, Sobral Neto J. 2013. Recommendations of the Brazilian society of cardiac arrhythmias for Holter monitoring services. Arq Bras Cardiol. 101(2):101–105. doi:10.5935/abc.20130164.
- Markovitz LJ, Savage EB, Ratcliffe MB, Bavaria JE, Kreiner G, Iozzo RV, Hargrove WC, Bogen DK, Edmunds LH. 1989. Large animal model of left ventricular aneurysm. Ann Thorac Surg. 48(6):838–845. doi:10.1016/0003-4975(89)90682-6.
- Mellado M, Del Angel E, Rebolloso O, García E. 1998. Immunoglobulin G concentration and neonatal survival of goat kids delivered in a pen or on open range. Prev Vet Med. 37(1–4):33–39. doi: 10.1016/S0167-5877(98)00107-X
- Mendes RS, Souza AP, Silva RMN, Sousa LVR, Dantas SBA, Mangueira JM, Souza BB. 2010. Influência da temperatura Ambiente sobre parâmetros eletrocardiográficos de caprinos criados no semi-árido paraibano. Revista Científica de Produção Animal. 12(2):129–132. doi:10.15528/2176-4158/rcpa.v12n2p129-132.
- Mendes Netto D. 2014. Sistema circulatório – seção A: semiologia do sistema cardiovascular de equinos e ruminantes. In: Feitosa FL, editor. Semiologia Veterinária – A arte do diagnóstico. 3rd ed. São Paulo: Zentrum Verlagsgesellschaft; p. 207–241.
- Mir SA, Nazki AR, Raina R. 2000. Comparative electrocardiographic studies, and differing effects of pentazocine on ECG, heart and respiratory rates in young sheep and goats. Small Rumin Res. 37(1–2):13–17. doi:10.1016/s0921-4488(99)00123-6.
- Mohr E, Langbein J, Nürnberg G. 2002. Heart rate variability: a noninvasive approach to measure stress in calves and cows. Physiol Behav. 75(1–2):251–259. doi:10.1016/S0031-9384(01)00651-5.
- Nowak R, Poindron P. 2006. From birth to colostrum: early steps leading to lamb survival. Reprod Nutr Dev. 46(4):431–446. doi: 10.1051/rnd:2006023
- Nunes RB, Ferreira CFX, Aboin RM, Deus HG, Saito ME, Yonezawa LA. 2014. Parâmetros eletrocardiográficos de novilhas da raça, Jersey. AVS. 19(4):17–23. doi:10.5380/avs.v19i4.36124.
- O’Connor M, McDaniel N, Brady WJ. 2008. The pediatric electrocardiogram. Part I: age-related interpretation. Am J Emerg Med. 26(2):221–228. doi:10.1016/j.ajem.2007.08.003.
- Pereira EZ. 2011. Contribuição das eletrocardiografias convencional. Holter e de alta resolução no diagnóstico da cardiomiopatia arritmogênica do ventrículo direito dos cães da raça Boxer, p. 37. [dissertação] (Mestrado EM Clínica Médica Veterinária) – Faculdade de Ciências Agrárias e Veterinárias. Universidade Estadual Paulista, Jaboticabal.
- Piccione G, Borruso M, Fazio F, Giannetto C, Caola G. 2007. Physiological parameters in lambs during the first 30 days postpartum. Small Rumin Res. 72(1):57–60. doi:10.1016/j.smallrumres.2006.04.002.
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD. 2007. Diseases of the cardiovascular system. In: Hinchcliff KW, editor. Veterinary medicine: a text book of the diseases of cattle, horses, sheep, pigs and goats. Philadelphia, PA: Saunders Elsevier; p. 399–438.
- Rassi Jr A. 2000. Compreendendo melhor as medidas de análise da variabilidade da frequência cardíaca. J Diagn Cardiol. 14:8.
- Sosa EA, Terzi R, Gruppi C, Brito FS, de Paola AA, Pimenta J, Lorga AM, Maia IG, Gizzi JC, Solimene MC. 1995. [Consensus SOCESP-SBC on electrocardiography by Holter system]. Arq Bras Cardiol. 65(5):447–450.
- Tilley LP. 1992. Essentials of canine and feline electrocardiography interpretation and treatment, 3rd ed. Philadelphia, PA: LippincottWilliams & Wilkins.
- Torad FA, Amer MS, Shamaa AA, Elsherpieny EA. 2017. Echocardiographic measurements and indices in normal adult buffalo (Bubalus bubalis). J Appl Anim Res. 45(1):336–341. doi: 10.1080/09712119.2016.1190733
- Torío R, Cano M, Montes A, Prieto F, Benedito JL. 1997. Comparison of two methods for electrocardiographic analysis in Gallega sheep. Small Rumin Res. 24(3):239–246. doi:10.1016/S0921-4488(96)00951-0.
- Tovar P, Santisteban R, Porras A, Vivo R, Castejón FM. 1985. [Electrocardiographic analysis of auricular electric systole in the sheep]. Rev Esp Fisiol. 41(3):317–324.
- Tudbury PB, Atkinson DW. 1950. The electrocardiograms of one hundred normal infants and young children. J Pediatr. 36(4):466–481. doi:10.1016/S0022-3476(50)80290-1.
- Von Borell E, Langbein J, Després G, Hansen S, Leterrier C, Marchant-Forde J, Marchant-Forde R, Minero M, Mohr E, Prunier A, et al. 2007. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals – a review. Physiol Behav. 92(3):293–316. doi:10.1016/j.physbeh.2007.01.007.
- [WHO] World Health Organization. 2018. Healthy topics: infant, newborn; [accessed 2018 Jan 26]. http://www.who.int/topics/infant_newborn/en/.
- Wittum TE, Salman MD, King ME, Mortimer RG, Odde KG, Morris DL. 1994. Individual animal and maternal risk factors for morbidity and mortality of neonatal beef calves in Colorado, USA. Prev Vet Med. 19(1):1–13. doi: 10.1016/0167-5877(94)90010-8