ABSTRACT
This study compared the pathologic effects of velogenic Newcastle disease (ND) virus in broilers and pullets using the normal pathogenic dose of the virus following an earlier report that challenge of the two types of chickens with a low dose of the virus caused mortalities in broilers but none in pullets. One hundred and twenty chickens (10 weeks old) were randomly assigned into four groups (n = 30 each): inoculated intramuscularly with velogenic viscerotropic ND virus (vvNDV) pullets – IP, uninfected pullets – UP, infected broilers – IB and uninfected broilers – UB. Anorexia, depression, ruffled feathers, green diarrhoea, tucking of the head under their wings, droopy wings and prostration were observed on days 2 and 3 post inoculation (PI) in groups IP and IB respectively. Mortality in IP and IB was 100%. Weight loss was significant (P < 0.05) by day 4 PI in both IP and IB when compared with their controls. Percentage weight loss in IP and IB were 20.26% and 38.66%, respectively. Proventricular, intestinal and caecal tonsil haemorrhages were significantly (p < 0.05) more severe in group IB than IP. Histopathology showed more severe necrosis and depletion of the lymphocytes in the bursa of Fabricius, thymus and spleen of group IB than IP. The above observations show that the lesions of vvNDV infection may be more severe in IB than IP.
Introduction
Newcastle disease (ND) is a viral disease caused by Newcaastle disease virus (NDV) which is an avian paramyxovirus belonging to the genus Avulavirus in the family Paramyxoviridae and order Mononegavirales (Lamb et al. Citation2005). It infects a wide range of birds including poultry, cage and wild birds (Kaleta and Baldauf Citation1988). Chickens are the most susceptible (Eze et al. Citation2014; Igwe et al. Citation2014; Okoroafor et al. Citation2018), being the only avian species that show severe gastrointestinal lesions of ND. The velogenic pathotype of ND is the most severe form of ND with the velogenic viscerotropic pathotype being the most common and enzootic in Africa, Middle and Far East and some countries of Central and South America (Spradbrow Citation1988; Echeonwu et al.Citation1993; Liu et al. Citation2003; Solomon et al. Citation2012) It is a disease of severe economic importance due to high mortalities in chickens, loss of egg production due to significant reduction in serum phosphorus levels (Igwe et al. Citation2018), loss of international trade on poultry and poultry products due to embargo by the international community and the expensive stamping out policy used in the control of the disease in those countries where velogenic Newcastle disease (vND) is exotic. Control of ND is mainly by vaccination and biosecurity. However, it has been reported that vaccination could protect against the clinical signs but not against the multiplication and shedding of the vvNDV, atrophy and depletion of the lymphocytes in the lymphoid organs ((Miller et al. Citation2007, Citation2013; Ezema et al. Citation2009, Citation2016; Cattoli et al. Citation2011; Bwala et al. Citation2012; Okwor et al. Citation2016; Sa e Silva et al. Citation2016; Okoroafor et al. Citation2018).
The pathology and pathogenesis of ND have been well studied in chickens but there is limited information on the effect of variation in types and breeds of chickens on the severity of the disease. This paper describes a comparative study of the severity of vvNDV infection in broilers and pullets.
Materials and methods
Chickens
One hundred and twenty 1 day- old chicks comprising of 60 white Marshal broilers and 60 Isa Brown pullets, obtained from a reputable commercial local hatchery were used for this study. They were randomly assigned into four groups (n = 30 each): inoculated intramuscularly with vvNDV pullets – IP, uninfected pullets – UP, infected broilers – IB and uninfected broilers – UB. Brooding of each of the groups was on deep litter in isolation in the Poultry Ex perimental Facility of the Department under strict biosecurity measures (if the measures were applied!?). Feed and water were provided ad libitum. Each of the groups was brooded separately under the same environmental conditions. General care of the birds was provided in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendment.
Velogenic Newcastle disease virus inoculum
A Nigerian strain of velogenic viscerotropic NDV (vvNDV) known as duck/Nigeria/903/KUDU–113/1992 was used. It was isolated from apparently healthy duck, purified and characterized by Echeonwu et al. (Citation1993). The strain belongs to NDV class II, genotype XVII (Shittu et al. Citation2016). The inoculum had a medium embryo infective dose (EID 50 ) of 106.4 per ml.
Newcastle disease virus challenge
At ten weeks of age, the pullets and broilers were serologically negative for NDV antibodies. intramuscularly (IM) inoculated with 0.1 ml of the inoculums chickens received the same volume of PBS IM as placebo The groups were housed and reared on deep litre in separate locations.
Clinical observation
The chickens were observed twice daily for clinical signs from days 0 to 5 PI Eight live chickens randomly selected collected in each group were weighed on days 0 and 4 PI and the mean percentage weight loss was calculated. Morbidity and mortality rates were recorded.
Pathological examination
Ten moribund and recently dead chickens in the IP and IB groups and normal chickens in the UP and UB groups were necropsied on day 4 PI and the haemorrhagic lesions in the gastrointestinal tracts (proventriculus, intestine and caecal tonsil) of each chicken were studied, scored and recorded as follows: no lesion = 0, mild lesion = 1, moderate lesion = 2 and severe lesion = 3.. Samples of the spleen, bursa of Fabricius, and thymus, were fixed in 10% buffered formal saline for minimum of 24 hr. They were processed before being embedded in paraffin wax. Sections (indicate the size) were stained with haematoxylin and eosin (HE) staining, according to the method of Suvarna et al. (Citation2008).
Virus isolation
Samples of the spleen, thymus, bursa of Fabricius and intestine (including contents) were collected aseptically on day 3 PI from 3 recently dead chickens in each group. The samples were kept in the freezer at −35°C until the organs of each group were pooled for virus isolation in embryonated chicken eggs.using the method of World Organisation for Animal Health (OIE) (Citation2012).
Statistical analysis
Mean values and significance of the differences of the mean body weight values were analysed using ANOVA. The mortality data were analysed using Chi square/Fisher’s exact test while the gross lesions were analysed using student’s t test. The significant means were separated using t-test for Equality of means and Levene’s test for Equality of Variance (Chatfield Citation1983). All tests were performed with a 5% level of significance.
Results
Clinical signs
There were no clinical signs in groups UB and UP. But the signs in groups IB and IP were severe. Clinical signs in the pullets were mainly depression, ruffled feathers, whitish diarrhoea, prostration, tucking of the head under the wings, paralysis of legs and wings and withdrawal from feed and were first observed on day 2 PI. In broilers, the signs included withdrawal from feed, depression, jerking of head, neck and head tremor, generalized spasm and recumbence, ruffled feathers and greenish diarrhoea and were first observed on day 3 PI. Weight loss was highly significant (P < 0.05) by day 4 PI in both IB and IP, with percentage weight loss of 38.66% and 20.26% respectively (). Morbidity was 100% for IB and IP.groups. All the mortalities occurred on days 3–5 PI (). There was no significant difference (p < 0.05) between the mortalities.
Table 1. Mean of body weights of control and VNDV-infected birds (g) ± SEM.
Table 2. Comparison of the cumulative mortality rate of broiler and pullets challenged with vvNDV.
Gross pathology
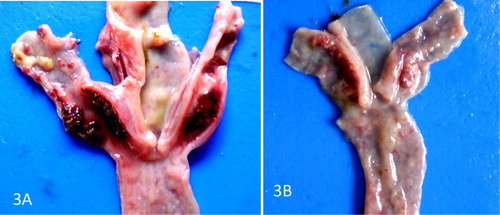
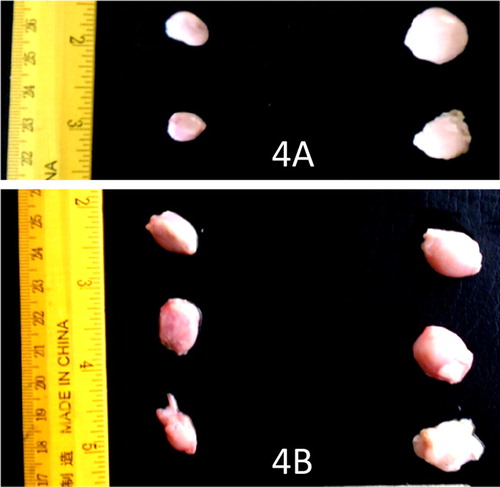
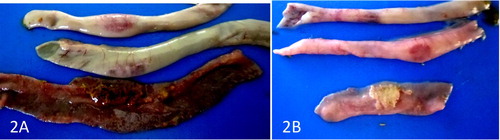
There were no gross lesions in the UB and UP groups but the lesions in the IB and IP groups were severe. The infected pullets showed congestion of the breast, thigh, and leg muscles and proventricular haemorrhage on day 3–5 PI and proventricular haemorrhage was more severe in broilers days 4 and 5 PI ((A and B)). The score of the lesion was significantly higher in broilers than pullets on day 4 PI (). Catarrhal and haemorrhagic enteritis were observed in the intestine on day 3 PI which progressed to sharply demarcated ulcers on days 4 and 5 PI in both types of chickens. The lesion score for intestinal ulcers was significantly higher (p < 0.05) in broilers than pullets on day 4 PI. The ulcers were covered by a thin whitish layer of necrotic intestinal tissue in pullets and greenish (bile stained) materials in broilers ((A and B)). The caecal tonsils were swollen, ulcerated, and haemorrhagic and often contained cheesy necrotic material on days 3–5 PI in both types of chickens but very severe in broilers ((A and B)). The lesion score was significantly higher (p < 0.05) in broilers than pullets. Some of the spleen samples were enlarged on day 3 PI with most of them mottled by dark or white spots on the serosal surface up to day 4 PI. But by day 5 PI, atrophy was observed in most samples in both infected broilers and pullets. Atrophy of the thymus was noticeable in some samples on days 3–5 PI in the infected broilers and pullets. The bursa showed clear atrophy from days 3 to 5 PI in both infected broilers and pullets ((A and B)).
Figure 1. (A) Proventriculus of VNDV infected broilers showing severe mucosal haemorrhages on day 4 PI. (B) Proventriculus of VNDV infected pullets showing mild mucosal haemorrhages on day 4 PI.
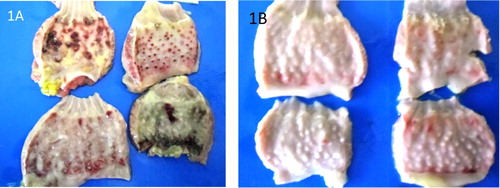
Figure 2. (A) Intestines of VNDV infected broilers showing severe haemorrhagic ulcers in broilers on day 4 PI. (B) Intestines of VNDV infected pullets showing less severe ulcers on day 4 PI.
Histopathology
There were no lesions in the UB and UI groups but infected groups showed severe lesions. Sections of bursa of infected broilers and pullets showed severe lymphocytic necrosis and depletion ((A and B)). The thymus of infected broilers and pullets showed severe lymphocytic necrosis and depletion. ((A and B)). Severe lymphocytic necrosis and depletion with deposition of fibrin were observed in the spleen of infected broilers and pullets. ((A and B)). But in the three organs the IP had more surviving lymphocytes than the IB ().
Figure 5. (A) Severe necrosis and depletion of lymphocytes in the bursa of VNDV infected broilers on day 4 PI. H&E × 400. (B) Less severe necrosis and depletion of the lymphocytes in the bursa of VNDV infected pullets on day 4 PI H&E × 400.
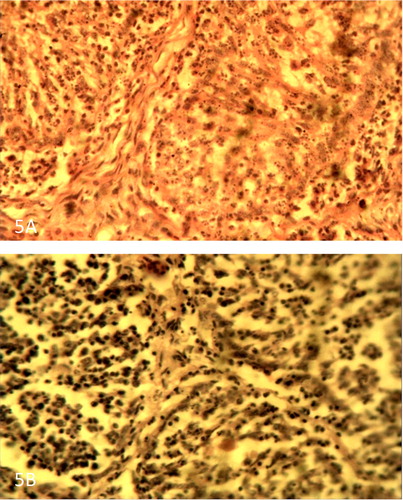
Figure 6. (A) Thymus of VNDV infected broiler showing severe necrosis and depletion of lymphocytes on day 4 PI. H&E × 400. (B) Thymus of VNDV infected pullet showing less severe necrosis and depletion of lymphocytes on day 4 PI. H&E × 400.
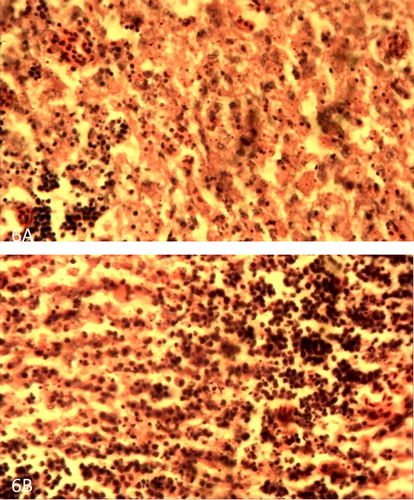
Figure 7. (A) Spleen of VNDV infected broiler showing severe lymphocytic depletion and fibrin deposition on day 4 PI. H&E × 400. (B) Spleen of VNDV infected pullet showing less severe lymphocytic depletion and fibrin deposition on day 4 PI. H&E × 400.
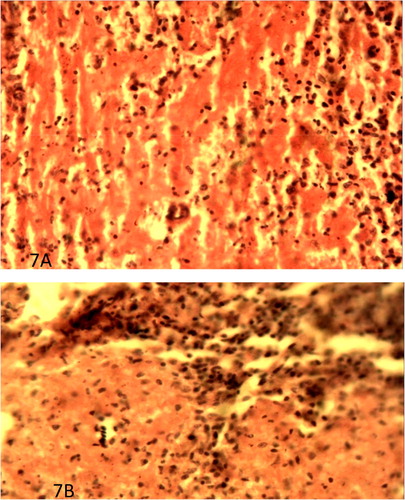
Table 3. Comparison of gross lesion scores of pullets and broilers challenged with vvNDV on day 4PI.
Virus isolation
Virus was isolated from all the pooled organs of the infected chickens on day 3 PI (). In the positive cases, the harvested allantoic fluids showed haemagglutination activity with washed chicken red blood cells. The haemagglutination was neutralized by known specific NDV antiserum. The organs of the uninfected chickens were negative for the virus.
Table 4. Virus isolation in pooled organs on day 3 PI.
Discussion
The lesions in the gastrointestinal tract only were scored in this experiment because they are found only in chickens in VNDV infections and are suspected to be responsible for the very high mortalities found in chickens compared with other avian species (Okoroafor et al. Citation2018) One proventricular sample in the infected broilers showed haemorrhages at the tip of the proventricular glands which is highly suggestive of ND and should be used to differentiate ND from infectious bursal disease (IBD) where there are also proventricular haemorrhages (Okoye Citation1984). This differentiation may not apply to avian influenza because the clinical signs and lesions of the VND and avian influenza are very close. Differentiation between the two is mainly by isolation and identification of the organism. The proventricular samples in both infected broilers and pullets showed haemorrhages as bands at proventricular-esophageal and at times at proventricular-gizzard junctions. This means that these lesions are neither suggestive of IBD nor ND. The haemorrhages in the proventriculus of the broilers had higher score and by visual inspection of the figures were more severe than those of the pullets. The same is true for the intestinal ulcers and caecal tonsils of the infected broilers and pullets. These haemorrhagic and ulcerative lesions of VND in the gastrointestinal tracts are due to the necrosis of aggregations of lymphocytes in the gastrointestinal tract. The lesions in the bursa, spleen and thymus were not scored. But the histopathological sections of these organs showed more surviving lymphocytes in the pullets than broilers. The above observations support the earlier report of Omeke et al. (Citation2018). Using a low dose of this virus for challenge, it was reported that the total mortalities in broilers and pullets were 30% and 0% respectively and resistance could be attributed to the higher NDV antibody titre found in the pullets. Serology was not done in this experiment because all the chickens died within 5 days PI. They recommended that low dose infection appeared more adequate for studies of comparative susceptibilities of chickens and other animal diseases that were very pathogenic at normal dose. King (Citation1996) reported that SPF White Leghorn layers were more susceptible to velogenic neurotropic NDV infection than White Rock broilers. Shi et al. (Citation2011) observed that when a virulent NDV was used to infect five different types of ducks, the mortality ranged from 6% to 66%. All the above observations are not in agreement with the statement by Alexander and Senne (Citation2008) that breed and genetic composition appeared to have no effect on the susceptibility to ND.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Didacus Chukwuemeka Eze http://orcid.org/0000-0003-2773-2415
Yusuf Abba http://orcid.org/0000-0001-7888-9004
Shodeinde Vincent Olu Shoyinka http://orcid.org/0000-0002-2058-7318
Emmanuel Chukwudi Okwor http://orcid.org/0000-0001-7168-7207
Wilfred Sunday Ezema http://orcid.org/0000-0002-4942-7721
John Ikechukwu Ihedioha http://orcid.org/0000-0002-0780-7531
John Osita Arinze Okoye http://orcid.org/0000-0003-2137-3626
References
- Alexander DJ , Senne DA. 2008. Newcastle disease, other avian parasmyxoviruses and pneumovirus infections. In: Saif YM (Editor-in Chief), Fadley AM , Glisson JR , McDougald LR , Nolan LK , Swayne DE (Associate Editors). Diseases of poultry. 12th ed. Ames (IA, USA ): Blackwell Publishing; p. 75–100.
- Bwala DG , Clift S , Duncan NM , Bisschop SPR , Oludayo Fasina F. 2012. Determination of the distribution of lentogenic vaccine and virulent Newcastle disease virus angtien in the oviduct of SPF and commercial hen using immunohistochemistry. Res Vet Sci. 93:520–528. doi: 10.1016/j.rvsc.2011.06.023
- Cattoli G , Susta L , Terregino C , Brown C. 2011. Newcastle disease: a review of field recognition and current methods of laboratory detection. J Vet Diag Invest. 23:637–656. doi: 10.1177/1040638711407887
- Chatfield C. 1983. Statistics for technology: a course in applied statistics. 3rd ed. London (UK ): Chapman and Hull.
- Echeonwu GON , Ireogbu CI , Emeruwa AC. 1993. Recovery of velogenic Newcastle disease virus from dead and healthy free roaming birds in Nigeria. Avian Path. 22:383–387. doi: 10.1080/03079459308418928
- Eze CP , Okoye JOA , Ogbonna IO , Ezema WS , Eze DC , Okwor EC , Ibu JO , Salihu EA. 2014. Comparative study of the pathology and pathogenesis of a local velogenic newcastle disease virus infection in ducks and chickens. Int J Poult Sci. 13:52–61. doi: 10.3923/ijps.2014.52.61
- Ezema WS , Eze DC , Shoyinka SVO , Okoye JOA. 2016. Atrophy of the lymphoid organs and suppression of antibody response caused by velogenic Newcastle disease virus infection in chickens. Trop Anim Hlth Prod. 48:1703–1709. doi: 10.1007/s11250-016-1147-x
- Ezema WS , Okoye JOA , Nwanta JA. 2009. Lasota vaccination may not protect against the lesions of velogenic Newcastle disease in chickens. Trop Anim Hlth Prod. 41:477–484. doi: 10.1007/s11250-008-9210-x
- Igwe AO , Ezema WS , Eze DC , Okoye JOA. 2014. Experimental velogenic Newcastle disease can be very severe and viscerotropic in chickens but moderate and neurotropic in guinea fowl. Int J Poult Sci. 13:582–590. doi: 10.3923/ijps.2014.582.590
- Igwe AO , Ihedioha JI , Okoye JOA. 2018. Changes in serum calcium and phosphorus levels and their relationship with egg production in laying chickens infected with velogenic Newcastle disease virus. J Appl Anim Res. 46:523–528. doi.org/10.1080/09712119.2017.1352506.
- Kaleta EF , Baldauf C. 1988. Newcastle disease in free-living and pet birds. In: Alexander DJ , editor. Newcastle Disease. Boston : Kluwer Academic Publishers; p. 197–246.
- King DJ. 1996. Influence of chicken breed on pathogenicity evaluation of velogenic neurotropic Newcastle disease virus isolates from cormorants and turkeys. Avian Dis. 40:210–217. doi: 10.2307/1592391.
- Lamb RA , Collins PL , Kolakofsky D , Melero JA , Nagai Y , Oldstone MBA , Pringle CR , Rima BK. 2005. Family paramyxoviridae. In: Fauquet CM , Mayo MA , Maniloff J , Desselberger U , Ball LA , editors. Virus taxonomy, VIIIth report of the international committee on taxonomy of viruses. San Diego, USA : Elsevier Academic Press; p. 655–668.
- Liu XF , Wan HQ , Ni XX , Wu YT , Liu WB. 2003. Pathotypical and genotypical characterisation of strains of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1885–2001. Arch Virol. 148:1387–1403.
- Miller PJ , Afonso CL , Attrache J , Dorsey KM , Courtney SC , Guo Z , Kapczynski DR. 2013. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Develop Comp Immunol. 41:505–513. doi: 10.1016/j.dci.2013.06.007
- Miller PJ , King DJ , Afonso CL , Suarez DL. 2007. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine. 25:7238–7246. doi: 10.1016/j.vaccine.2007.07.017
- Okoroafor OA , Eze PC , Ezema WS , Nwosu C , Okorie-Kanu C , Animoke PC , Anene B , Okoye JOA. 2018. La Sota vaccination may not protect against virus shedding and lesions of velogenic Newcastle disease in commercial turkeys. Trop Anim Hith Prod. 50:345–351. doi:10.1007//s 1125-017-1439-9 doi: 10.1007/s11250-017-1439-9
- Okoye JOA. 1984. Infectious bursal disease chickens. Vet Bulletin. 54:425–436.
- Okwor EC , Eze DC , Echeonwu GON , Ibu JO , Eze PC , Okoye JOA. 2016. Comparative studies on the effects of La Sota and Komarov vaccine antibodies on organ distribution, persistence and shedding of KUDU 113 virus in chickens. J Anim Plant Sci. 26:1226–1235.
- Omeke JN , Ezema WS , Eze DC , Okoye JOA. 2018. Low dose velogenic viscerotropic Newcastle disease virus infection caused thirty percent mortalities in Anak broilers but none in Lohmann Brown layer chickens. J Appl Anim Res. 46:1352–1357. doi:10.1080/09712119.2018.1505620.
- Sa e Silva MS , Susta L , Moresco K , Swayne DE. 2016. Vaccination of chickens decreased Newcastle disease virus contamination in eggs. Avian Pathol. 45:38–45. doi: 10.1080/03079457.2015.1112876
- Shi SH , Huang Y , Cui SJ , Cheng LF , Fu GH , Li X , Cheng Z , Peng CX , Lin F , Lin JS , Su JL. 2011. Genomic sequence of an avian paramyxovirus type 1 strain isolated from Muscovy duck (Cairinamoschata) in China. Arch Virol. 156:405–412. doi:10.1007/s00705-010-0866-y.
- Shittu I , Sharma P , Joannis TM , Volkenin JD , Odaibo GN , Olaleye DO , Williams-Coplin D , Solomon P , Abolnik C , Miller PJ , et al. 2016. Complete genome sequence of a genotype XVII Newcastle disease virus, isolated from an apparently healthy domestic duck in Nigeria. Genome Announce. 4:e01716–15. doi:10.1128/genomeA.01716-15.
- Solomon P , Aboinik C , Joannis TM , Bisschop S. 2012. Virulent Newcastle disease virus in Nigeria: identification of a new clade of sub-lineage 5f from live bird markets. Virus Genes. 44:98–103. doi: 10.1007/s11262-011-0678-5
- Spradbrow PS. 1988. Geographical distribution. Newcastle disease in free living and pet birds. In: DJ Alexander , editor. Newcastle disease. Boston : Kluwer Academic Publishers; p. 247–255.
- Suvarna SK , Layton C , Bancroft JD. 2008. Bancroft’s tdddheory and practice of histological techniques. 7th ed. England : Churchill Livingstone. Elsevier.
- World Organisation for Animal Health (OIE) . 2012. Manual of diagnostic tests and vaccines for terrestial animals. In: M Truszczynski , editor. Newcastle disease. OIE Standard commission Publication, Office International Epizooties, 2.