?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
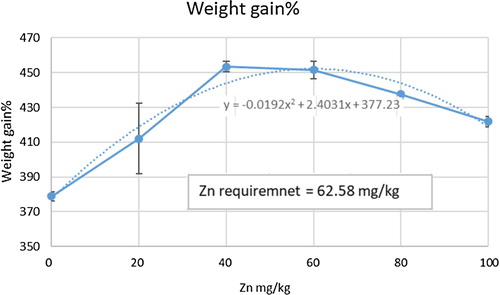
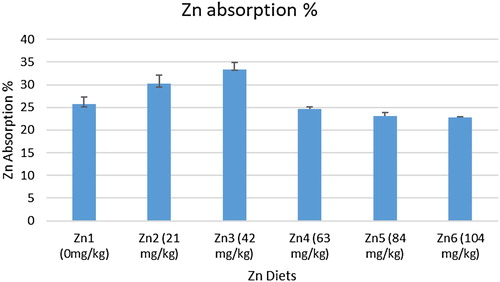
The present study was designed to estimate the zinc (Zn) requirement of Labeo rohita juveniles fed practical diet. Treatments used for the study were consisted of six experimental diets supplemented with graded levels of Zn (0, 21, 42, 63, 84 and 104 mg/kg diet) from Zn gluconate. For each experimental diet, two replicates were allocated, and 18 fish were stocked in each replicate. The feeding trial was lasted for 90 days. Results showed that final weight, absolute weight gain, weight gain% and specific growth rate increased with increasing dietary Zn levels up to 42 mg/kg and started to decrease with further increase in dietary Zn level. Quadratic regression analysis of weight gain% data indicated that L. rohita juveniles required 62.58 mg/kg Zn for normal growth. Maximum Zn absorption was observed in fish fed diet supplemented with 42 mg/kg Zn compared with other dietary treatments. Alkaline Phosphatase (ALP) activity in kidney and spleen of L. rohita juveniles increased with the increase in dietary Zn levels up to 42 and 63 mg/kg, respectively. Conclusively, supplementation of graded levels of dietary Zn-gluconate improved the growth performance and increased the Zn bioavailability and ALP activity up to a certain limit in L. rohita juveniles.
KEYWORDS:
Introduction
Mineral elements are vital nutrients as they play an important role in maintaining osmoregulation and formation of bones and scales. Trace minerals are mainly absorbed from water which make it difficult to determine their requirements for fish (National Research Council Citation1983).
Zinc (Zn) is an important inorganic trace element as it influences various physiological functions including growth performance, immune system and defense mechanism against free ions and radicals (Watanabe et al. Citation1997). As a cofactor, Zn participates in several enzyme systems to regulate protein and lipid metabolism (National Research Council Citation2011). It plays a key role in the formation of nucleoproteins as well as in prostaglandin metabolism (Watanabe et al. Citation1997). Korenek et al. (Citation2007) observed as increase in the activities of chymotrypsin and trypsin in the droppings of quails by the supplementation of Zn. Alexei et al. (Citation2002) reported that Zn stabilizes the activities of amylase and lipase in bacteria.
Growing aquatic species do not obtain the enough Zn because freshwater (Spry et al. Citation1988) and seawater (Willis and Sunda Citation1984) are deficient in normal Zn levels. Hence, Zn is considered an essential trace mineral in fish feeds (Lall Citation1989; National Research Council Citation1993; Wei et al. Citation1999). Zn appears to be less bioavailable to the fish fed high plant meal based diets as such diets contain low level of Zn and high levels of phytate, an anti-nutritional factor. Presence of phytate adversely affected the bioavailability of Zn in chinook salmon (Richardson et al. Citation1985). Similar results were also observed in channel catfish (Gatlin and Wilson Citation1984; Gatlin and Phiflip Citation1989). The requirement of dietary zinc for the juvenile grouper ranged between 28.9 and 33.7 mg/kg diet (Houng-Yung et al. Citation2014). Whereas, optimal level of total dietary Zn in practical diet (supplemented and contributed by ingredients) of rainbow trout was found to be 80 mg kg (Welker et al. Citation2016).
Zn deficiency caused reduced growth rate, increased mortality, low body weight, skeletal deformities, cataracts and fin and skin erosion in fish (Tacon Citation1992). Nucleic acid and protein metabolism (Lall Citation2002) disorders were also observed in salmonids due to reduced activity of Zn-requiring enzymes (Ramseyer et al. Citation1999; Kucukbay et al. Citation2006; Rider et al. Citation2010). Therefore, adequate amount of Zn is required to avoid such deficiency symptoms. Zn sulphate (ZnSO4 .7H2O), an inorganic source, is usually used as Zn source to determine the requirements in fishes.
Recently, organically chelated trace elements are being supplemented in aquafeeds instead of inorganic minerals. Organic minerals are structurally stable and have low molecular weight, so, they compete with mineral inhibitors and increases the uptake of mineral in fish intestine (Ashmead Citation1992, Citation1993). Therefore, it may hypothesize that organic minerals are more bioavailable to the fish compared to inorganic salts (Zn-sulphate and Zn-oxide) (Ashmead Citation1992). Utilization of organic minerals in feed is like natural process of micro-mineral supplementation as natural feed ingredients consist of mineralized proteins and amino acids (Tucker and Taylor-Pickard Citation2005).
Indian major carps (Labeo rohita, Catla catla, and Cirrhinus mrigala) shares significant contribution in freshwater production of Indian subcontinent. The demand of L. rohita is dominated in the market due to its unique flavour, rapid growth and disease resistance capacity (Jhingran and Pullin Citation1988). However, dietary Zn requirement has not been determined until now for L. rohita juveniles. Therefore, the purpose of the present study is to evalute the Zn requirement from Zn-gluconate, an organic source, in practical diet for L. rohita juveniles.
Materials and methods
Feed ingredients and experimental diets
The present research work was carried out to study the Zn requirement from Zn gluconate, an organic source, in practical diet of L. rohita juveniles. By supplementing Zn at the levels of 0, 21, 42, 63, 84 and 104 mg/kg diet, six isolipidic, isocaloric and isonitrogenous experimental diets were formulated from the basal diet (). Feed ingredients were purchased from a commercial feed mill and experimental diets were formulated using these ingredients which were analyzed chemically (AOAC Citation1995). Cereal grinding machine (FFC-45, JIMO, China) was used to grind and sieve (0.05 mm) the feed ingredients. By using an electric mixer, chromic oxide, fish oil, feed ingredients, Zn free mineral mixture and vitamin premix were blended thoroughly. Composition of Zn free mineral mixture is presented in . The dough was prepared by adding 150 ml of distilled water/kg of diet and pelleted with hand pelletizer having a diameter of 3 mm. The moist pellets were dried at room temperature till the moisture was reduced up to 10% using an electric fan. Pellets were broken in small pieces, screened to desired sizes and refrigerated at −20°C in self-sealing plastic bags until fed. The proximate composition of experimental diets is given in .
Table 1. Composition of basal practical diet.
Table 2. Composition of Zn free mineral mixture.
Table 3. Proximate composition of experimental diets.
Fish husbandry
L. rohita juveniles were obtained from Government Fish Seed Hatchery. At arrival, juveniles were dipped in 5 g/L NaCl solution to reduce the incidence of ectoparasites and fungal infection. Fish were acclimatized in cemented tanks (1000 L water capacity) for two weeks. Juveniles were fed with basal diet to apparent satiation level. Eighteen juveniles with an average initial weight of 3.15 ± 0.01 g were assigned to each V-shaped tank (70 L capacity) for the feeding trial. The L. rohita juveniles were hand-fed once per day to the apparent satiation level for 3 months. After feeding session of three hours, the valves were opened to clean the tanks thoroughly and uneaten diet was drained out from the tanks by manual siphoning and filtered fresh water was supplied to each tank. The oxygen level was maintained in the rearing tanks through the capillary system.
Growth performance
Growth in term of absolute weight gain, weight gain%, feed conversion ratio (FCR) and specific growth rate (SGR) was calculated by standards formulae.
Sample collection
Fish were starved fo4 24 hours and sacrificed using anaesthesia (MS-222) at the end of the feeding trial. Ten fish were selected to obtain muscles, kidney, intestine, bones, scales and spleen samples. Each pooled sample was referigerated at −20°C untill used for the determination of alkaline phosphatase activity (ALP), thiobarbituric acid reactive substances (TBARS), proximate composition and Zn content analysis.
Proximate analysis
The standard methods of AOAC (Citation1995) were followed to determine the proximate composition of experimental diets and muscle samples. Moisture was determined by drying the samples at 105°C for 12 h. Crude protein was measured by using Kjeldahl apparatus and petroleum aether extraction method was used to determine crude fat. Crude ash contents were obtained by igniting the samples at 600°C for 12 h in muffle furnace.
Zn content analysis
For this purpose, nitric acid and perchloric acid were used in 3:1 ratio (Wet digestion) to digest the samples of (bones, scales and intestine) digested samples were diluted to the appropriate level. Atomic absorption spectrophotometer was used to determine the Zn contents in samples (AOAC Citation1995).
Zn absorption
After three hours of feeding all uneaten diet was collected for determination of FCR and tanks were cleaned with filtered fresh water. Again, after two hours of tank cleaning, the faecal material was collected with care to avoid thin breakage of thin faecal strings through faecal collection tube of V-shaped tanks equipped with two valves. Collected faecal material was dried at 60°C, minced and referigerated for the analysis of Zn contents. Absorption of Zn was estimated by the following formula:
TBARS assay
For the estimation of peroxidation level in kidney and spleen, TBARS contents were measured by following Gatta et al. (Citation2000).
ALP enzyme assay
ALP activity in kidney and spleen of fish was determined by using Vitro Scient (ISO 13485) kit method. The ALP of the sample catalyses the magnesium-activated base hydrolysis of p-nitro-phenyl phosphate producing nitrophenolate and absorbanence was measured at 405 nm. The activity of enzyme was directly proportional to the rate of hydrolysis (Abbott Laboratories Citation1989).
Statistical analysis
The data were tested for normality and homogeneity of variances and finally, one-way analysis of variance was used for statistical analysis of data (Steel et al. Citation1996). The differences among different supplementation levels of Zn were compared by Tukey`s Honestly significant difference test (Snedecor and Conhran Citation1991). Quadratic regression was applied for the determination of optimum requirement of Zn. All of the statistical analysis was performed using CoStat computer package (Version 6.303, PMB 320, Monterey, CA, 93940 USA).
Results
Effect of graded levels of Zn on growth performance of L. rohita juveniles is given in . Final weight, absolute weight gain, weight gain% and SGR were increased with increase in dietary Zn levels up to 42 mg/kg and decreased with further increase in dietary Zn level, whereas, feed conversion ratio was reduced at this level (42 mg/kg) in L. rohita juveniles. Survival rate remained unaffected with the increase in Zn gluconate levels.
Table 4. Effect of graded levels of Zn on growth performance of L. rohita juveniles.
The data of weight gain% was analysed by the quadratic regression analysis to determine the requirement of Zn in diet for L. rohita juveniles. The result indicated that L. rohita required 62.58 mg/kg Zn for optimum growth ().
Effect of graded levels of Zn on muscle proximate composition of L. rohita juveniles is summarized in . Non-significant variations were observed among all the dietary treatments for dry matter, crude protein, crude fat and crude ash contents in muscles of L. rohita juveniles.
Table 5. Effect of graded levels of Zn on muscle proximate composition of L. rohita juveniles.
Effect of graded levels of Zn on Zn contents (µg/g) in bones, scales, intestine and faeces of L. rohita juveniles is given in . A linear increase was observed in the Zn concentration in intestine and faeces with the increase in dietary Zn levels from 0 to 104 mg/kg diet. Bones and scale Zn contents increased up to 84 and 42 mg/kg dietary Zn level, respectively.
Table 6. Effect of graded levels of Zn on Zn contents (µg/g) in bones, scales, intestine and faeces of L. rohita juveniles.
Effect of graded levels of Zn on TBARS and ALP contents in kidney and spleen of L. rohita juveniles is summarized in and , respectively. Kidney and spleen TBARS contents decreased with increasing Zn levels up to 63 mg/kg and thereafter an increase in TBARS contents was observed. ALP activity in kidney and spleen of L. rohita juveniles increased with the increase in dietary Zn levels up to 42 and 63 mg/kg, respectively. Moreover, the effect of graded levels of Zn on Zn absorption in L. rohita juveniles is given in . Maximum Zn absorption was observed in fish fed diet supplemented with 42 mg/kg Zn compared with other dietary treatments.
Table 7. Effect of graded levels of Zn on TBARS (mg/g protein) contents in kidney and spleen of L. rohita juveniles.
Table 8. Effect of graded levels of Zn on ALP activity (U/g protein) in kidney and spleen of L. rohita juveniles.
Discussion
Among trace minerals, Zn is an important nutrient as it is involved in various metabolic pathways such as protein synthesis, growth, immunity and energy metabolism in fish like other animals (Hayashi et al. Citation2001; Carpene et al. Citation2003; Lin et al. Citation2013; Houng-Yung et al. Citation2014). It performs its functions as a cofactor in more than 200 metalloenzymes including DNA polymerase, carbonic anhydrase and carboxy peptidase (Salim et al. Citation2012). The importance of Zn as antioxidant has also been illustrated in many aquatic organisms (Feng et al. Citation2011; Trevisan et al. Citation2014; Huang et al. Citation2015). However, high Zn ingestion showed negative effects on feed utilization and growth in Jian carp, Cyprinus carpio var. Jian (Tan et al. Citation2011), yellow catfish, Pelteobagrus fulvidraco (Luo et al. Citation2011) and Nile tilapia, Oreochromis niloticus (Do Carmo e Sa et al. Citation2004). Therefore, it is necessary to supplement the optimum level of dietary Zn to avoid negative effects on fish health. Its optimum supplementation may reduce the feed cost and mineral leaching in aquatic water bodies (Buentello et al. Citation2009; Huang et al. Citation2015).
In the current study, final weight, absolute weight gain, weight gain% and SGR increased with increasing dietary Zn gluconate levels up to 42 mg/kg and decreased with further increase in dietary Zn level whereas FCR was reduced at this level (42 mg/kg) in L. rohita juveniles. Survival rate remained unaffected at all levels of Zn. Like our results, a significant increment was also observed in SGR of juvenile Jian carp fed Zn supplemented diet (Tan et al. Citation2011). Similar observations were also recorded by Gatlin and Wilson (Citation1983), Do Carmo e Sa et al. (Citation2004) and Ogino and Yang (Citation1979) in channel catfish, Nile tilapia and common carp, respectively. Moreover, weight gain of juvenile grouper was significantly increased by increasing the concentration of Zn in diet up to 36.7 mg/kg and then levelled off with further increase in dietary Zn level, which may owe to the pro-oxidative effect of Zn (Houng-Yung et al. Citation2014). Bhagawati et al. (Citation2016) reported improved weight gain in golden mahseer fry fed diet supplemented with 40 mg/kg Zn and decreased significantly with further increase in dietary Zn level. Similarly, findings of Liang et al. (Citation2012) and Tan et al. (Citation2011) are also in accordance with our results that higher dietary Zn levels increased the FCR in Ctenopharyngodon Idella and Cyprinus carpio to a certain limit. In contrast to our results, weight gain in Nile tilapia remained unaffected when fed 150 mg/kg dietary Zn as Zn oxide, Zn amino acid complex or Zn sulphate monohydrate (Do Carmo e Sa et al. Citation2005). Luo et al. (Citation2011) observed no significant effect of Zn supplementation on feed intake in yellow catfish.
In the current study, non-significant changes were observed in dry matter, crude protein, crude fat and ash contents of L. rohita juveniles fed on Zn-gluconate supplemented diets. Similar to our study, crude protein and moisture contents in the whole body remained unaffected among different dietary Zn treatments in turbot (Ma et al. Citation2014). Contrast to our results, enhanced body lipid content and decreased moisture and ash contents were observed when fish were fed higher Zn level (53 mg/kg) (Liang et al. Citation2012). Ma et al. (Citation2014) reported improved ash and crude lipid contents in turbot fed Zn supplemented diets. This may be due to increase in anti-oxidative activities (super oxide dismutase and glutathione peroxidase) that significantly lower the lipid peroxidation thereby increasing the lipid contents in turbot (Mallick and Mohn Citation2000).
Trace minerals as essential nutrients are vital to maintain the structural and functional activities in fish as well as in terrestrial animals. Bone mineral contents are always considered a specific indicator for estimating the mineral status of an organism. In the present study, bone and intestine Zn contents were significantly increased with increase in dietary Zn level. The increase in Zn contents may be due to its chelation with gluconate that easily transport the Zn across intestinal mucosa by preventing the formation of insoluble complexes in the digestive tract (Ashmead Citation1992). Chelated ligands release in the body at the specific site where they are needed otherwise these ligands remain intact in fish body (Ashmead Citation1992). Similar results were observed in channel catfish (Gatlin and Wilson Citation1984) and Nile tilapia (Do Carmo e Sa et al. Citation2004). In contrast, Zn supplementation at the level of 30 mg/kg diet had no significant effect on bone Zn content in Nile tilapia (Eid and Ghonim Citation1994). Gatlin and Wilson (Citation1983) observed the similar responses in channel catfish.
Thiobarbituric acid reactive substances (TBARS) contents are used to quantify the malondialdehyde (MDA) contents and serve as a useful indicator of lipid peroxidation and antioxidant status of fish. Zn may contribute to improve the antioxidant status and reduce the lipid peroxidation level in fish (Anderson et al. Citation2001). In the present study, TBARS contents in spleen and kidney were significantly decreased with increase in dietary Zn level whereas their level started to increase at higher levels (84 and 104 mg/kg Zn level). The optimum concentration of dietary Zn might be required to decrease MDA level in fish (Onderci et al. Citation2003; Kucukbay et al. Citation2006). Like current study, elevated levels of dietary Zn significantly increased the MDA levels in juvenile yellow catfish (Luo et al. Citation2011).
Alkaline phosphatase (ALP) activity is also considered a sensitive indicator of Zn status in animal (Swinkels et al. Citation1996). In the present study, supplementation of dietary Zn showed dose dependent effect on ALP activity in kidney and spleen. Increased ALP activity may attribute to the increased Zn availability to the fish as Zn acts as cofactor for this enzyme. Dietary Zn concentration strongly influenced the activity of ALP in juvenile abalone (Tan and Mai Citation2001). The study conducted by Do Carmo e Sa et al. (Citation2004) on Nile tilapia showed a significant increase in ALP activity in response to dietary Zn supplementation up to 50 mg/kg Zn level. Similar effects were recorded in various tissues of rats, rainbow trout and channel catfish by Huber and Gershoff (Citation1973), Apines et al. (Citation2001) and Gatlin and Wilson (Citation1983).
Absorption of Zn occurs mainly in upper small intestine and usually follows the first order kinetics (Wood et al. Citation2011). In the current study, Zn absorption increased up to 42 mg/kg dietary Zn level and then decreased with further increase in dietary Zn level. Similar to our study, amino acid chelated Zn (an organic source) showed higher absorption of Zn in rainbow trout at 40 mg/kg Zn level (Apines et al. Citation2001).
Conclusively, supplementation of graded levels of dietary Zn-gluconate improved the growth performance and Zn bioavailability up to a certain level while higher showed negative influence on the performance of L. rohita juveniles.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbott Laboratories . 1989. Vision system operator’s manual. Abbott Park, IL : Abbott Laboratories. p. 7.25–7.27.
- Alexei S , Claire V , Sull K , Gregory ZJ. 2002. Pyrococcus furiosus α-amylase is stabilized by calcium and Zn. Biochemistry. 41:6193–6201. doi: 10.1021/bi012106s
- Anderson RA , Roussel AM , Zouari N , Mahjoub S , Matheau JM , Kerkeni A. 2001. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 20:212–218. doi: 10.1080/07315724.2001.10719034
- AOAC . 1995. Official methods of analysis. Association of official analytical chemist. 15th ed. Washington, DC : AOAC International. p. 1094.
- Apines MJ , Satoh S , Kiron V , Watanabe T , Nasu N , Fujita S. 2001. Bioavailability of amino acids chelated and glass embedded zinc to rainbow trout, Oncorhynchus mykiss, fingerlings. Aquacult Nutr. 7:221–228. doi: 10.1046/j.1365-2095.2001.00178.x
- Ashmead HD. 1992. The roles of amino acid chelates in animal nutrition. Park Ridge, NJ : Noyes Publications; p. 479.
- Ashmead HD. 1993. Comparative intestinal absorption and subsequent metabolism of metal amino acid chelates and inorganic metal salts. The roles of amino acid chelates in animal nutrition. Park Ridge, New Jersey, USA : Noyes; p. 47–75.
- Bhagawati K , Chadha NK , Sarma D , Akhtar MS , Sawant PB , Borah S. 2016. Effect of dietary zinc on the growth and metabolic enzyme activities of golden mahseer (Tor putitora) fry. J Appl Nat Sci. 8:1692–1698. doi: 10.31018/jans.v8i3.1024
- Buentello JA , Goff JB , Gatlin DM. 2009. Dietary zinc requirement of hybrid striped bass, Morone chrysops × Morone saxatilis, and bioavailability of two chemically different zinc compounds. J World Aquacult Soc. 40:687–694. doi: 10.1111/j.1749-7345.2009.00288.x
- Carpene E , Andreani G , Monari M , Kindt M , Isani G. 2003. Biochemical changes during post-larval growth in white muscle of gilthead sea bream (Sparus aurata) fed zinc-fortified diets. Vet Res Commun. 27:215–218. doi: 10.1023/B:VERC.0000014143.28892.34
- Do Carmo e Sa MV , Pezzato LE , Barros MM , Padilha PM. 2005. Relative bioavailability of zinc in supplemental inorganic and organic sources for Nile tilapia Oreochromis niloticus fingerlings. Aquacult Nutr. 11:273–281. doi: 10.1111/j.1365-2095.2005.00352.x
- Do Carmo e Sa MV , Pezzato LE , Lima MMBF , Padilha PM. 2004. Optimum zinc supplementation level in Nile tilapia Oreochromis niloticus juveniles diets. Aquaculture. 238:385–401. doi: 10.1016/j.aquaculture.2004.06.011
- Eid AE. , Ghonim SI. 1994. Dietary zinc requirement of fingerling Oreochromis niloticus. Aquaculture. 119:259–264. doi: 10.1016/0044-8486(94)90180-5
- Feng L , Tan LN , Liu Y , Jiang J , Jiang WD , Hu K , Li SH , Zhou XQ. 2011. Influence of dietary zinc on lipid peroxidation, protein oxidation and antioxidant defence of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr. 17:875–882. doi: 10.1111/j.1365-2095.2011.00858.x
- Gatlin DM , Phiflip HF. 1989. Dietary calcium, phytate and Zn interactions in channel catfish. Aquaculture. 79:259–266. doi: 10.1016/0044-8486(89)90466-3
- Gatlin DM , Wilson RP. 1983. Dietary zinc requirement of fingerling channel catfish. J Nutr. 113:630–635. doi: 10.1093/jn/113.3.630
- Gatlin DM , Wilson RP. 1984. Zinc supplementation of practical channel catfish diets. Aquaculture. 41:31–36. doi: 10.1016/0044-8486(84)90387-9
- Gatta PP , Pirini M , Testi S , Vignola G , Monetti PG. 2000. The influence of different levels of dietary vitamin E on sea bass Dicentrarchus labrax flesh quality. Aquacult Nutr. 6:47–52. doi: 10.1046/j.1365-2095.2000.00127.x
- Hayashi K , Hara H , Asvarujanon P , Aoyama Y , Luangpituksa P. 2001. Ingestion of insoluble dietary fibre increased zinc and iron absorption and restored growth rate and zinc absorption suppressed by dietary phytate in rats. Br J Nutr. 86:443–451. doi: 10.1079/BJN2001417
- Houng-Yung C , Yu-Chun C , Li-Chi H , Meng-Hsien C. 2014. Dietary Zn requirements of juvenile grouper, Epinephelus malabaricus . Aquaculture. 432:360–364. doi: 10.1016/j.aquaculture.2014.05.020
- Huang F , Jiang M , Wena H , Wua F , Liua W , Tian J , Yang C. 2015. Dietary zinc requirement of adult Nile tilapia, Oreochromis niloticus, fed semi-purified diets, and effects on tissue mineral composition and antioxidant responses. Aquaculture. 439:53–59. doi: 10.1016/j.aquaculture.2015.01.018
- Huber AM , Gershoff SN. 1973. Effects of dietary zinc on zinc enzymes in the rat. J Nutr. 103:1175–1181. doi: 10.1093/jn/103.8.1175
- Jhingran VG , Pullin RSV. 1988. A hatchery manual for common, Chinese and Indian major carps under field condition. ICLARM Studies and Reviews 11. Manila : Asian Development Bank.
- Korenek M , Korenekova B , Vasko L , Skalicka M , Nad P , Sutiak V , Neushi J. 2007. Effect of cadmium and Zn on the activity of chymotrypsin and trypsin in dropping of Japanese quails. Int J Environ Stud. 64:221–227. doi: 10.1080/00207230701257259
- Kucukbay Z , Yazlak H , Sahin N , Tuzcu M , Cakmak MN , Gurdogan F , Juturu V , Sahin K. 2006. Zinc picolinate supplementation decreases oxidative stress in rainbow trout (Oncorhynchus mykiss). Aquaculture. 257:465–469. doi: 10.1016/j.aquaculture.2006.03.005
- Lall SP. 1989. The minerals. In: Halver JE , editor. Fish nutrition. San Diego, CA : Academic Press; p. 219–257.
- Lall SP. 2002. The minerals. In: Halver JE , Hardy RW , editors. Fish Nutrition, 3rd edition. San Diego, CA : Academic Press; p. 259–308.
- Liang JJ , Yang HJ , Luo YJ , Tian LX , Liang GY. 2012. Dietary zinc requirement of juvenile grass carp (Ctenopharyngodon idella) based on growth and mineralization. Aquacult Nutr. 18:380–387. doi: 10.1111/j.1365-2095.2011.00935.x
- Lin S , Lin X , Yang Y , Li F , Luo L. 2013. Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp, Litopenaeus vannamei . Aquaculture. 406–407:79–84. doi: 10.1016/j.aquaculture.2013.04.026
- Luo Z , Tan X , Zheng J , Chen Q , Liu C. 2011. Quantitative dietary zinc requirement of juvenile yellow catfish, Pelteobagrus fulvidraco, and effects on hepatic intermediary metabolism and antioxidant responses. Aquaculture. 319:150–155. doi: 10.1016/j.aquaculture.2011.06.047
- Ma R , Hou H , Mai K , Bharadwaj AS , Ji F , Zhang W. 2014. Comparative study on the bioavailability of chelated or inorganic zinc in diets containing tricalcium phosphate and phytate to turbot (Scophthalmus maximus). Aquaculture. 420-421:187–192. doi: 10.1016/j.aquaculture.2013.11.003
- Mallick N , Mohn FH. 2000. Reactive oxygen species: response of algal cells. J Plant Physiol. 157:183–193. doi: 10.1016/S0176-1617(00)80189-3
- National Research Council . 1983. Nutrient requirements of warm water fishes and shellfishes. Washington, DC : National Academy Press. p. 102.
- National Research Council . 1993. Nutrient requirements of fish. Washington, DC : National Academy Press. p. 114.
- National Research Council . 2011. Nutrient requirements of fish and shrimp. Washington, DC : National Academy Press.
- Ogino C , Yang GY. 1979. Requirement of carp for dietary zinc. Bull Jpn Soc Sci Fish. 45:967–969. doi: 10.2331/suisan.45.967
- Onderci M , Sahin N , Sahin K , Kilic N. 2003. The antioxidant properties of chromium and zinc: in vivo effects on digestibility, lipid peroxidation, antioxidant vitamins and some minerals under a low ambient temperature. Biol Trace Elem Res. 92:139–150. doi: 10.1385/BTER:92:2:139
- Ramseyer L , Garlin D , Hill G , Link J. 1999. Effect of dietary Zn supplementation and phytase pre-treatment of soybean meal or corn gluten meal on growth, Zn status and Zn-related metabolism in rainbow trout (Ictalurus punctatus). Fish Physiol Biochem. 20:251–261. doi: 10.1023/A:1007719722459
- Richardson NL , Higgs DA , Beam RM , McBride JR. 1985. Influence of dietary calcium, phosphorus, Zn and sodium phytate level on cataract incidence, growth and histopathology in juvenile Chinook salmon (Oncorhynchur rshuwytscha). J Nutr. 115:553–567. doi: 10.1093/jn/115.5.553
- Rider SA , Davies SJ , Jha AN , Clough R , Sweetman JW. 2010. Bioavailability of co-supplemented organic and inorganic Zn and selenium sources in a white fishmeal-based rainbow trout (Oncorhynchus mykiss) diet. J Anim Physiol Anim Nutr. 94:99–110. doi: 10.1111/j.1439-0396.2008.00888.x
- Salim H , Lee H , Jo C , Lee S , Lee B. 2012. Effect of dietary zinc proteinate supplementation on growth performance, and skin and meat quality of male and female broiler chicks. Br Poult Sci. 53:116–124. doi: 10.1080/00071668.2012.658757
- Snedecor GW , Conhran WG. 1991. Statistical methods. Ames : Iowa State University Press.
- Spry DJ , Hodson PV , Wood CM. 1988. Relative contributions of dietary and waterborne Zn in the rainbow trout Salmo Gairdneri . Can J Fish Aquat Sci. 45:32–41. doi: 10.1139/f88-005
- Steel RGD , Torrie JH , Dickey DA. 1996. Principles and procedures of statistics. 3rd ed. New York : McGraw Hill International Book.
- Swinkels JW , Kornegay ET , Zhou W , Lindermann MD , Webb KE , Verstegen MV. 1996. Effectiveness of a zinc amino acid chelate and ZnSO4 in restoring serum and soft tissue zinc concentration when fed to zinc-depleted pigs. J Anim Sci. 74:2420–2430. doi: 10.2527/1996.74102420x
- Tacon AGJ. 1992. Dietary essential mineral deficiency, nutritional fish pathology. Morphological signs of nutrient deficiency and toxicity in farmed Fish. FAO Fish Technical Paper. No.330, Rome.
- Tan LN , Feng L , Liu Y , Jiang J , Jiang WD , Hu K , Li SH , Zhou XQ. 2011. Growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary zinc. Aquacult Nutr. 17:338–345. doi: 10.1111/j.1365-2095.2010.00793.x
- Tan B , Mai K. 2001. Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture. 192:67–84. doi: 10.1016/S0044-8486(00)00435-X
- Trevisan R , Flesch S , Mattos JJ , Milani MR , Bainy ACD , Dafre AL. 2014. Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna . Comp Biochem Phys C. 159:22–30.
- Tucker LA , Taylor-Pickard JA. 2005. Re-defining mineral Nutrition. Nottingham, UK : Nottingham University Press.
- Watanabe T , Kiron V , Satoh S. 1997. Trace minerals in fish nutrition. Aquaculture. 151:185–207. doi: 10.1016/S0044-8486(96)01503-7
- Wei W , Li A , Li D. 1999. Effect of dietary supplemented Zn on the growth and some biochemical parameters of juvenile flounder Paralichthys olivaceus . J Ocean Univ Qingdao. 18:60–66.
- Welker T. , Barrows F. , Overturf K. , Gaylord G. , Sealey W. 2016. Optimizing zinc supplementation levels of rainbow trout(Oncorhynchus mykiss) fed practical type fishmeal-and plant-based diets. Aquacult nutr. 22:91–108. doi: 10.1111/anu.12232
- Willis JN , Sunda WG. 1984. Relative contributions of food and water in the accumulation of Zn by two species of marine fish. Mar Biol. 80:273–279. doi: 10.1007/BF00392822
- Wood CM , Farrell AP , Brauner CJ. 2011. Homeostasis and toxicology of essential metals. Cambridge, UK : Academic Press. p. 520.