ABSTRACT
The aim of the current study was to evaluate the protective effects of Minthostachys verticillata essential oil (Mv-EO) against aflatoxin B1-induced toxicity in Vero cells in vitro and in male rats. The cytotoxicity of AFB1 (0-30 µg/mL) and Mv-EO (0-500 µg/mL) was determined on Vero cells using the Neutral Red assays. The tested Mv-EO did not cause genotoxicity or cytotoxicity in vivo and was able to attenuate AFB1-caused cytotoxicity and genotoxicity. Animals were divided into four groups consisting of 5 (five) rats in each group: T1 basal feed (BF – negative control), T2 BF + Mv-EO [0.04%], T3 BF + AFB1 [100 µg/kg], T4 BF + food with AFB1 [100 µg/kg] + Mv-EO [0.04%]. Tissue samples were collected at the end of treatment period for genotoxic study and histological examination. Treatment with Mv-EO alone and even combined with AFB1 showed a significant improvement in the histomorphometry of intestinal villi, without alteration of productive parameters. Also, the micronucleus test demonstrated that Mv-EO reduced AFB1-induced DNA damage on bone marrow cells of male Wistar rats. This study demonstrated that Mv-EO could be used as protective against AFB1-induced cytotoxicity and genotoxicity, and as phytogenic feed additive.
1. Introduction
Aflatoxins (AFs) are toxic secondary metabolites produced by Aspergillus species which grow in a wide variety of animal feeds and foods (Kurtzman et al. Citation1987). Contamination of several feedstuffs for livestock and foods for human consumption with these toxins is a continuing worldwide problem as their effect on livestock productivity can lead to significant economic losses and public health issues (Gallo et al. Citation2015). Aflatoxin B1 (AFB1) is the most prevalent and the most carcinogenic, teratogenic, genotoxic and immunotoxic mycotoxin (IARC Citation2002; Strosnider et al. Citation2006). Exposure to and the adverse health effects of AFB1 can be limited by chemopreventive and chemoprotective strategies to reduce the AFB1-induced toxicity. This is a promissory strategy that combined with others could prevent /protect the toxic effects caused by mycotoxins. Numerous investigations have shown that AFB1-induced toxicity can be protected with different natural compounds (Stahl and Sies Citation2005; Yilmaz et al. Citation2018). Further, oils and extracts of plants have been previously reported to suppress the formation of DNA adducts of AFB1 (Hashim et al. Citation1994). Additionally, essential oils as well as numerous biological products have been assessed as phytogenic feed additives by improving animal productivity, properties of feed and food quality (Steiner Citation2009).
Minthostachys verticillata (Griseb.) Epling, also known as peperina is a species of the Lamiaceae family. It is a well-known South America aromatic plant rich in essential oils widely used in folk medicine for the treatment of a variety of diseases including gastroenteric disorders, carminative, antispasmodic and antirheumatic (Núñez and Cantero Citation2000). Several biological activities of M. verticillata essential oil (Mv-EO) have been reported; however, little is known about the interaction of Mv-EO with AFs (Primo et al. Citation2001; González Pereyra et al. Citation2005; Cariddi et al. Citation2007; Bluma et al. Citation2008; González and Marioli Citation2010). Previous acute and subchronic studies demonstrated that Mv-EO was neither cytotoxic in vitro nor cyto-genotoxic in vivo both at low nor high concentrations (Escobar et al. Citation2012, Citation2015). Its multiple properties including antiviral, antibacterial, antifungal and immunoenhancing activities, could be exploited in livestock industry, making Mv-EO an ideal candidate for be used as phytogenic feed additive.
Thus, the aim of the current study was to investigate the possible protective effects of Mv-EO against toxics effects caused by AFB1 on Vero cells. Furthermore, 45-day oral protective Mv-EO effects on AFB1-induced toxicity in male rats were evaluated. The bone marrow micronuclei assay was used to evaluate genotoxicity of dietary Mv-EO administered to Wistar rats. In addition, its ability to reduce genotoxicity caused by dietary AFB1 was studied. Also, productive parameters and internal organs were macroscopically and microscopically examined in order to assess potential impairment in Mv-EO-treated rats and controls.
2. Materials and methods
2.1. M. verticillata essential oil and aflatoxin B1 production
2.1.1. Plant material and essential oil production
Leaves and thin stems from M. verticillata were used to obtain Mv-EO. Peperina was purchased from a local herb store and the voucher specimen was deposited in the herbarium of Universidad Nacional de Río Cuarto. Mv-EO was extracted by the hydrodistillation procedure for 2 h in Clevenger’s apparatus and dehydrated using anhydrous sodium sulphate. The chemical composition of peperina oil used in the present work was previously determined using GC–MS (Escobar et al. Citation2015) and the main components were found to be pulegone and menthone, representing 64.65% and 23.92% of the total oils, respectively. Thereafter, the purified Mv-EO was kept in 4°C in dark for further experiments. The chemical characterization of Mv-EO was performed by gas chromatography mass spectrometry (Shimadzu GC-R1A gas chromatograph fitted with a DB5 capillary column) according to Escobar et al. (Citation2015) where individual compounds (22) were identified based on their retention times against standard pure drugs injected in the same conditions.
2.1.2. Aspergillus parasiticus culture material
Aspergillus parasiticus cultures were prepared to obtain aflatoxin concentrations enough to contaminate feed for the experiment. Seven-day culture plugs of reference strain A. parasiticus NRRL 2999 were inoculated in 250 ml Erlenmeyer flasks containing 25 g autoclaved rice and 10 ml distilled water. Cultures were incubated in the dark, at 30°C for 15 d, manually stirring the flasks vigorously, for 1 min, once a day during the first 5 d to enhance the dissemination of conidia in the rice. After incubation, the cultures were autoclaved. The content of all flasks was placed in a metallic tray, covered with paper, let dry at 60°C in a forced air oven and ground with a laboratory mill. Aflatoxin B1 content of the resulting powder was quantified by high performance liquid chromatography (HPLC) according to Trucksess et al. (Citation1994).
2.2. In vitro cytotoxic assay
2.2.1. Cell culture and cytotoxic assay
Vero cells (ATCC CCL-76) were obtained from Asociación Banco Argentino de Células (ABAC). Cells were propagated in minimal essential medium (MEM; Gibco, USA) supplemented with 8% foetal calf serum (FCS; Natocor, Argentina), gentamycin 50 µg/mL and 2 mM glutamine (Sigma-Aldrich, Italy). Vero cells viability was measured by neutral red uptake assay (NRU). Cells were seeded in 96-well culture plates at 104 cells/well and, after monolayer formation, were exposed to increasing concentrations of AFB1 (0–30 µg/mL) and Mv-EO (0–500 µg/mL) during 48 h. AFB1 and Mv-EO were dissolved in 0.1% dimethyl sulfoxide (DMSO). Cells treated with 0.1% DMSO were used as controls. The medium was replaced with 150 µL of a 50 mg/mL solution of neutral red in MEM. After incubation at 37°C for 3 h, medium containing dye was removed and wells were washed twice with warmed PBS (150 µL/well). The dye within viable cells was released by extraction with a mixture of acetic acid, ethanol and water (1:50:49). After the cultures were shaking for 10 min, absorbance values were read at 540 nm on a microplate reader (Thermo Labsystems Multiskan MS). For comparisons, relative cell viability was expressed as percentage of NRU control group (%).
2.3. Subchronic toxicity assay
2.3.1. Experimental animals
Twenty male rats of Wistar strain, weighing about 150–200 g each (8-week-old) were obtained from the Bioterio Central of the Universidad Nacional de Río Cuarto. Animals were maintained in a temperature and humidity controlled room, with a 12-h light/dark cycles. The working protocol and the used techniques comply with the regulations of the Subcommittee on Animal Bioethics under the Ethics Committee of Scientific Research of the National University of Rio Cuarto, as established in Resolution 253/10 of the Superior Council.
2.3.2. Diet formulation
The diet was formulated with commercial pelleted rat chow (GEPSA FEEDS, Grupo Pilar S.A., Argentina). The composition of the feed was >24% protein, <7% fibre, 1–1.2% calcium, > 6% ether extract, 0.5–0.9% phosphorus, <8% total minerals and <13% moisture. The AFB1-contaminated diet was prepared weekly during the experiment in the same way as the control diet. Finely ground commercial basal feed (with no detectable levels of AFB1) was added with A. parasiticus culture powder to produce final concentration of 100 µg/kg AFB1 and dissolved in water. The mixture was homogenized manually for 20 min in a big plastic container; 30 g pieces were cut and stored at −20°C until use. Aflatoxin B1 concentration of the experimental diet was confirmed by HPLC as was described above.
2.3.3. Study design
Animals were divided into four groups consisting of 5 rats in each group: T1 basal feed (BF – negative control), T2 BF + Mv-EO [0.04%], T3 BF + AFB1 [100 µg/kg], T4 BF + food with AFB1 [100 µg/kg] + Mv-EO [0.04%]. The Mv-EO was dissolved in water at 0.04% (phospholipids emulsifying agents) to be administered in drinking. Water and feed were provided ad libitum throughout the experimental period (45 d). At the end of the study, the rats were decapitated without being anesthetized. After dissection, the liver, the kidneys, and a section of intestine were removed, weighed and used for histopathological examinations.
2.3.4. Micronuclei assay
The assay was carried out following standard protocols as recommended by Schmid (Citation1975). After decapitation, the femurs were immediately excised from the body. Using a syringe, the bone marrow was then flushed into a glass tube containing 3 mL foetal calf serum (FCS). The collected cells were centrifuged at 1000 rpm for 5 min and the supernatant was carefully removed from the pellet. The cells were re-suspended in the remaining fluid, slides were prepared and air-dried. Then, the slides were stained with May-Grunwald–Giemsa. To establish the genotoxicity, 1000 polychromatic (PCE) and corresponding normochromatic erythrocytes (NCE) were scored for the presence of micronuclei (MN) from each animal. To detect possible cytotoxic effects, the effect on the proportion of 1000 PCE with respect to the number of normochromatic erythrocytes (NCE) per rat (PCE/NCE index) was observed. The slides were scored blindly using a light microscope at a 1000× magnification. The average number of micronucleated erythrocytes (MNE) in individual rats was used as the experimental unit, with variability based on differences among animals within the same group.
2.3.5. Morphological and histopathological analyses
The macroscopic external features (weight, size and colour) of the organs collected during necropsy (liver, kidney and intestine) were registered. These organs were fixed in 10% neutral buffered formalin (pH 7.4) for paraffin routine processing. These samples were cut at 4 μm thickness and subject to hematoxylin/eosin (H&E) staining for microscopic histological examination under 400× magnification. Photomicrographs were taken with a Zeiss Axiostar plus microscope using an Electronic Eyepiece camera with MIAS (Micro Image Analisis Software 2008, v 2.2) software and a Canon Power Shot G5 camera (Canon Inc., Japan). The villus height was measured from the villus tip to the bottom, without including the intestinal crypt.
2.3.6. Body weight, feed intake, feed conversion efficiency and general health status
Productive parameters such as body weight gain, feed intake and feed conversion efficiency were determined. The feed conversion efficiency (FCE) was determined as the ratio of feed intake (g)/gained weight (g). Mean daily intake of Mv-EO was determined taken into account the amount of Mv-EO present into the water, the mean body weight of rats and daily amount of water drink by rats (mg Mv-EO/kg bw/day). Liver index was calculated as follows: 100× [organ weight (g)/total body weight (g)]. Toxicity signs, body weight and food consumption were monitored daily.
2.4. Statistical analysis
All data were expressed as mean and standard deviation (SD). Body weight, liver weight and micronucleus assays were tested by conducting a one-way analysis of variance (ANOVA). Dunn’s Multiple Comparison Test was used to determine statistical significance (P < .05) among the control and treatment groups using the GraphPad Prism software, version 6.0.1 (San Diego, USA, 2012).
3. Results
3.1. In vitro cytotoxic assay
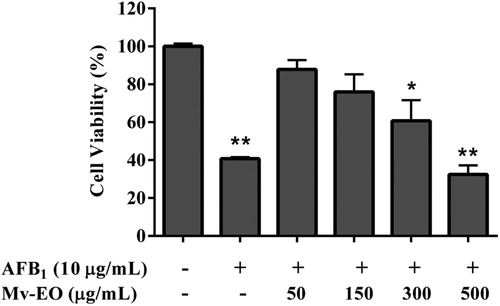
To investigate the protection of Mv-EO against AFB1-induced cytotoxicity, the NRU assay was performed. Vero cells were exposed to increasing concentrations of AFB1 (0–30 µg/mL) and Mv-EO (0–500 µg/mL), alone and in combination for 48 h. The cells treated with Mv-EO did not show cytotoxic effect. However, the treatments with AFB1 caused cytotoxicity in a dose-dependent manner (). The 50% inhibitory concentration (IC50) of AFB1 was 8.41 µg/mL. When cells were treated with AFB1 and Mv-EO, the essential oil decreased AFB1-induced cytotoxicity in an inverse doses dependent-manner. Highest concentrations of Mv-EO did not inhibit AFB1-induced cytotoxicity. As shown in , the cell viability was 40.72% at 10 µg/mL AFB1 exposure, and significantly increased to 87.82%, and 75.94%, when 50 and 150 µg/mL Mv-EO were added, respectively.
Figure 1. Viability of Vero cells exposed to different concentrations of aflatoxin B1 (0–30 µg/mL) for 48 h determined by NRU assays. The results are presented as percentage (mean ± SD) of three independent experiments.
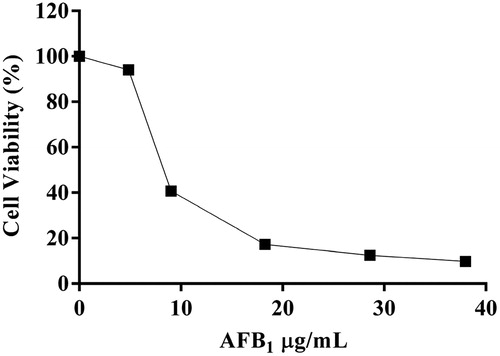
Figure 2. Viability of Vero cells exposed to aflatoxin B1 (10 µg/mL) and different concentrations of M. verticillata essential oil (0–500 µg/mL) for 48 h determined by NRU assays. The results are presented as percentage (mean ± SD) of three independent experiments. *P < .05 and **P < .01 indicates a significant difference compared with the control group (One-way ANOVA test).
3.2. Subchronic toxicity assay
The dosage of the Mv-EO at 0.04% in water was equivalent to a mean daily intake 35 mg/kg bw/day and the dosage of the AFB1 at 100 µg/kg feed was equivalent to a mean daily intake 1 mg/kg bw/day. The animals from all groups were healthy and no signs of toxicity or death were observed throughout the study for 45 consecutive days demonstrating that Mv-EO did not cause any genotoxicity by itself. In contrast, the AFs-contaminated diet caused genotoxicity in T3 (). However, the administration of Mv-EO was able to reduce the genotoxicity in group 4 compared with groups 1 and 2 (P ≤ .05).
Table 1. Micronucleated erythrocytes in the bone marrow cells of male Wistar rat fed aflatoxin B1-contaminated diet (100 mg/kg) and treated with M. verticillata essential oil (0.04%).
Gross pathological examination of the organs such as liver, kidney and intestine of all animals did not show differences no detectable abnormalities when compared to control group, indicating that the Mv-EO did not induce any organ damage. Macroscopic analyses of the different organs of Mv-EO-treated rats did not show significant changes in colour and texture when compared to the control group. Representative microscopic findings in liver, lungs, kidneys and intestine of Mv-EO-treated rats and controls are shown in .
Figure 3. Histological examination of internal organs of male Wistar rats fed aflatoxin B1-contaminated diet (100 mg/kg) and treated with M. verticillata essential oil (0.04%) for 45 d. Representative sections of liver, kidney (×400) and intestine (×200).
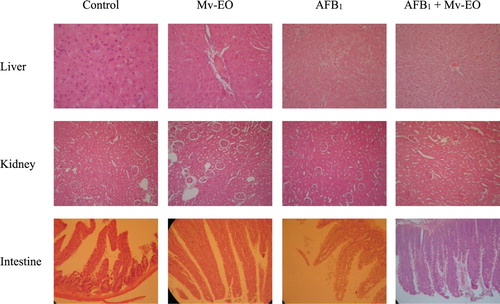
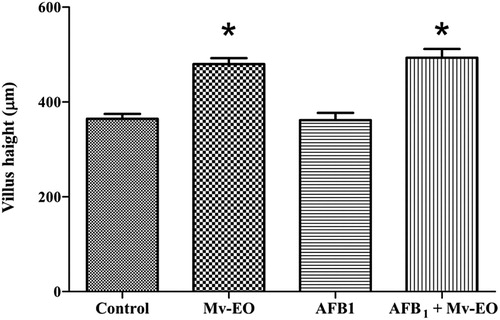
The histological examinations revealed there were no differences between Mv-EO-treated rats and controls. Moreover, rats orally treated with Mv-EO increased (P < .05) intestinal villus height (). The average intestinal villus height of control group was 364.3 ± 10.39 while the average intestinal villus height of rats treated with Mv-EO was significantly greater: 479.7 ± 12.52 Mv-EO alone and 493.1 ± 18.44 co-treatment with Mv-EO + AFB1.
Figure 4. Effects of administration aflatoxin B1-contaminated diet (100 mg/kg) and M. verticillata essential oil (0.04%) on intestinal villus height of male Wistar rats through 45 d. All data are mean ± SEM of 5 rats/group. *P < .05 (Dunnett’s Multiple Comparison Test).
Also, shows the performance of Wistar rats fed AFB1-contaminated diet and Mv-EO. Results showed that the administration of Mv-EO did not cause any adverse effects on body weight gain, feed intake and feed conversion efficiency during the experiment. Animals fed AFB1-contaminated diet showed a slight but not significant bodyweight decrease. However, animals fed AFB1-contaminated diet and treated with Mv-EO showed a performance similar to control. Body weight gain, feed intake and FCE for all rats fed with Mv-EO tended to be improved after 45 days compared with control group. Regarding the general health status of animals, rats from all groups appeared healthy, inquisitive and active throughout the experiment. The Mv-EO did not cause mortality, illness or significant changes in the general behavior of animals. No significant difference in feed and water intake or fur quality between groups were observed either.
Table 2. Effect of M. verticillata essential oil on male Wistar rat performance with an AFB1-contaminated diet.
4. Discussion
In view of the economic losses and public health issues caused by aflatoxins-contaminated feed, the detoxification by effective and eco-friendly agents has been increased. The use of natural products in diet as a chemoprotection strategy is an alternative to the control of aflatoxicosis. On the other hand, the use of natural products as phytogenic feed additives has been gaining considerable attention in feed industry. In the current study, the ability of M. verticillata essential oil to protect Vero cells on in vitro assay and male Wistar rats in a subchronic AFB1 toxicity assay was evaluated.
Phytogenic feed additives are well known to exert in vitro antiviral, antimicrobial, antifungal and other bioactivities. In this sense, M. verticillata essential oil (Mv-EO) has been reported with antiviral activity against herpes and pseudorabies viruses. Studies on the antibacterial properties of the same Mv-EO have shown that it was active against Bacillus subtilis, Staphylococcus aureus, Streptococcus faecalis, Bacillus cereus var. mycoides, Proteus mirabilis, Escherichia coli, Salmonella typhi, as well as against the causative agent of American Foulbrood, Paenibacillus larvae (De Feo et al. Citation1998; Primo et al. Citation2001; González and Marioli Citation2010). Bluma et al. (Citation2008) demonstrated that Mv-EO has antifungal effect against Aspergillus section Flavi sporulation and inhibition of AFB1 production. Further, the immune modulation potential of Mv-EO was reported on in vitro and in vivo assays in our laboratory (González Pereyra et al., Citation2005; Cariddi et al. Citation2007, Citation2011). As regards safety about Mv-EO, previous reports demonstrated that Mv-EO was not cytotoxic in vitro (up to 1000 µg/mL). Moreover, in acute doses (up to 500 mg/kg bw), Mv-EO did not produce signs of toxicity or death in mice. Furthermore, a 90 days subchronic toxicity and genotoxicity assay indicated that administration of Mv-EO did not promote toxic effects in rats up to on diet administration of 7 g/kg (Escobar et al. Citation2012, Citation2015).
In this study, Vero cells were exposed to AFB1 in combination with Mv-EO. Cell viability was reduced after exposure to AFB1 alone, and addition of Mv-EO reduced this damage in an inverse doses dependent-manner. This result indicated that the lower concentrations of Mv-EO (50–150 µg/mL) exerted protective effects on AFB1- cytotoxicity in vitro, whereas higher concentration did not. Some reports informed that Thymus vulgaris, Nigella sativa and Syzygium aromaticum essentials oils had a potential antioxidant activity and a protective effect against AFs toxicity (Abdel-Wahhab and Aly Citation2005; El-Nekeety et al. Citation2011).
In the present work, animals fed AFB1-contaminated diet showed a significant increase in micronucleated erythrocytes. These results were consistent with reports describing AFB1 as a potent carcinogen (Madrigal-Santillan et al. Citation2006). Our treatment with Mv-EO reduced the micronucleus levels compared to the control. The observed protective effect of Mv-EO could be due to the phenolic compounds present in the oil that suppress the formation of DNA adducts by aflatoxin B1 (Hashim et al. Citation1994). The AFB1 doses were chosen to simulate subchronic aflatoxicosis-causing doses found in naturally contaminated feeds (Theumer et al. Citation2008; Benford et al. Citation2010). The results of the present work indicated that the presence of Mv-EO did not cause organs damage and histopathological study showed all treatments were similar.
Several researchers have demonstrated the extract plant and essential oils can be used as phytogenic feed additives due to their beneficial effect on gut health, immunity response and growth performance (Li et al. Citation2012; Zhai et al. Citation2018). The intestinal villus height increase was informed to improve animal growth performance by enhancing nutrient absorption (Ruttanavut and Yamauchi Citation2010; Wu et al. Citation2013; Chowdhury et al. Citation2018). Consistent with this, the present results showed that there was significant (P < .05) increasement in the intestinal villus height in the animals treated with Mv-EO. This positive effect of Mv-EO in intestinal villus height, suggested that this extract could be used as a phytobiotic on farmyard animals. In further studies, Mv-EO should be added swine and poultry diets to stimulate production performance, and to improve health and welfare. The effects and modes of actions of Mv-EO on production performance, intestinal and general health, as well as to protect the toxic effects caused by mycotoxins, should be assessed.
5. Conclusion
In conclusion, AFB1 treatment was cytotoxic to Vero cells and genotoxic for male Wistar rat bone marrow cells. Treatment with Mv-EO could inhibit the cytotoxicity and genotoxicity induced by AFB1 in both in vitro and in vivo. Moreover, the treatment with Mv-EO showed a significant increase of intestinal villus height, without alteration of productive parameters. Consequently, M. verticillata essential oil has potential to counteract the effects of AFB1-induced cytotoxicity and genotoxicity, and as feed additive. Further studies for the use of Mv-EO as phytogenic feed additive should be assessed.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Franco Matías Escobar http://orcid.org/0000-0002-4292-9809
Additional information
Funding
References
- Abdel-Wahhab MA , Aly SE. 2005. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 25(3):218–223.
- Benford D , Leblanc JC , Woodrow SR. 2010. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic. Food Chem Toxicol. 48(Suppl 1):S34–S41.
- Bluma R , Amaiden MR , Daghero J , Etcheverry M. 2008. Control of Aspergillus section Flavi growth and aflatoxin accumulation by plant essential oils. J Appl Microbiol. 105(1):203–214.
- Cariddi L. , Escobar FM. , Moser M. , Panero A. , Alaniz F. , Zygadlo J. , Sabini LI. , Maldonado A. 2011. Monoterpenes isolated from Minthostachys verticillata (Griseb.) Epling essential oil modulates immediate-type hypersensitivity responses in vitro and in vivo. Planta Medica. 77(15):1687–1694.
- Cariddi LN , Panero A , Demo MS , Sabini LI , Maldonado AM , Grosso M , Zyglado J. 2007. Inhibition of immediate-type allergic reaction by Minthostachys verticillata (Griseb.) Epling essential oil. J Essent Oil Res. 19(2):190–196.
- Chowdhury S , Mandal GP , Patra AK , Kumar P , Samanta I , Pradhan S , Samanta AK. 2018. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim Feed Sci Technol. 236:39–47.
- De Feo V , Ricciardi A , Biscardi D. 1998. Chemical composition and antimicrobial screening of the essential oil of Minthostachys verticillata (Griseb.) Epl. (Lamiaceae). J Essent Oil Res. 10(1):61–65.
- El-Nekeety AA , Mohamed SR , Hathout AS , Hassan NS , Aly SE , Abdel-Wahhab MA. 2011. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon. 57(7-8):984–991.
- Escobar FM , Cariddi LN , Sabini MC , Reinoso E , Sutil SB , Torres CV , Zanon SM , Sabini LI. 2012. Lack of cytotoxic and genotoxic effects of Minthostachys verticillata essential oil: studies in vitro and in vivo . Food Chem Toxicol. 50(9):3062–3067.
- Escobar FM , Sabini MC , Cariddi LN , Sabini LI , Mañas F , Cristofolini A , Bagnis G , Gallucci MN , Cavaglieri LR. 2015. Safety assessment of essential oil from Minthostachys verticillata (Griseb.) Epling (peperina) 90-days oral subchronic toxicity study in rats. Regul Toxicol Pharmacol. 71(1):1–7.
- Gallo A , Giuberti G , Frisvad JC. 2015. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins (Basel). 7(8):3057–3111.
- González MJ , Marioli JM. 2010. Antibacterial activity of water extracts and essential oils of various aromatic plants against Paenibacillus larvae, the causative agent of American Foulbrood. J Invertebr Pathol. 104(3):209–213.
- González Pereyra ML , Cariddi LN , Ybarra F , Isola MC , Demo MS , Sabini LI , Maldonado AM. 2005. Immunomodulating properties of Minthostachys verticillata on human lymphocytes and basophils. Rev Alergia México. 52(3):105–112.
- Hashim S , Aboobaker VS , Madhubala R , Bhattacharya RK , Rao AR. 1994. Modulatory effects of essential oils from spices on the formation of DNA adduct by aflatoxin B1 in vitro . Nutr Cancer. 21(2):169–175.
- International Agency for Research on Cancer. (IARC) . 2002. IARC monographs on the evaluation of carcinogenic risks to humans: some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. 82:1–590.
- Kurtzman CP , Horn BW , Hesseltine CW. 1987. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii . Antonie van Leeuwenhoek. 53(3):147–158.
- Li SY , Ru YJ , Liu M , Xu B , Péron A , Shi XG. 2012. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livestock Sci. 145(1-3):119–123.
- Madrigal-Santillan E , Madrigal-Bujaidar E , Márquez-Márquez R , Reyes A. 2006. Antigenotoxic effect of Saccharomyces cerevisiae on the damage produced in mice fed with aflatoxin B1 contaminated corn. Food Chem Toxicol. 44(12):2058–2063.
- Núñez CM , Cantero JJ. 2000. Las plantas medicinales del Sur de la provincia de Córdoba. Editorial De la Fundación Universidad Nacional de Río Cuarto, Argentina.
- Primo V , Rovera M , Zanon S , Oliva M , Demo M , Daghero J , Sabini L. 2001. Determination of the antibacterial and antiviral activity of the essential oil from Minthostachys verticillata (Griseb.) Epling. Rev Argent Microbiol. 33(2):113–117.
- Ruttanavut J , Yamauchi K. 2010. Growth performance and histological alterations of intestinal villi in broilers fed dietary mixed minerals. Asian J Anim Sci. 4(3):96–106.
- Schmid W. 1975. The micronucleus test. Mutation Res. 31(1):9–15.
- Stahl W , Sies H. 2005. Bioactivity and protective effects of natural carotenoids. Acta Biochim Biophys Sin. 1740(2):101–107.
- Steiner T. 2009. Phytogenics in animal nutrition natural concepts to optimize gut health and performance. Nottingham, UK : Nottingham University Press.
- Strosnider H , Azziz-Baumgartner E , Banziger M , Bhat RV , Breiman R , Brune M , DeCock K , Dilley A , Groopman J , Hell K , et al. 2006. Workgroup report: public health strategies for reducing aflatoxin exposure in developing countries. Environ Health Perspect. 114(12):1898–1903.
- Theumer MG , Lopez AG , Aoki MP , Canepa MC , Rubinstein HR. 2008. Subchronic mycotoxicoses in rats. histopathological changes and modulation of the sphinganine to sphingosine (Sa/So) ratio imbalance induced by Fusarium verticillioides culture material, due to the coexistence of aflatoxin B1 in the diet. Food Chem Toxicol. 46(3):967–977.
- Trucksess MW , Stack ME , Nesheim S , Albert RH , Romer TR. 1994. Multifunctional column coupled with 625 liquid chromatography for determination of aflatoxins B1, B2, G1, and G2 in corn, almonds, Brazil nuts, peanuts, and pistachio nuts: collaborative study. J AOAC Int. 77(6):1512–1521.
- Wu QJ , Zhou YM , Wu YN , Wang T. 2013. Intestinal development and function of broiler chickens on diets supplemented with clinoptilolite. Asian-Australas J Anim Sci. 26(7):987–994.
- Yilmaz S , Kaya E , Karaca A , Karatas O. 2018. Aflatoxin B1 induced renal and cardiac damage in rats: protective effect of lycopene. Res Vet Sci. 119:268–275.
- Zhai H , Liu H , Wang S , Wu J , Kluenter A-M. 2018. Potential of essential oils for poultry and pigs. Anim Nutr. 4(2):179–186.
