?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Effects of multi-strain (Lactofeed) and mono-strain (Pediguard) probiotics on broiler breeder performance and expression of toll-like receptors (TLR) were evaluated. Three hundred broiler breeder hens (Ross 308) at the age of 51 weeks were randomly allocated into 1 of 5 dietary treatments with 6 replicates in each in a completely randomized design. The dietary treatments included (1) the basal diet (control), (2) control + 0.1 g/kg Lactofeed, (3) control + 0.1 g/kg Pediguard, (4) control + 0.1 g/kg Lactofeed + 0.1 g/kg Pediguard and (5) control + 0.5 g/kg oxytetracycline antibiotic. Compared to the control group, treatments had no effect on hen-day egg production and body weight of broiler breeders (P > .05). The egg yolk cholesterol concentration of broiler breeders fed probiotic-supplemented diet was decreased (P < .05). There were no differences in the immune response to PHA-P injection, serum glutathione peroxidase activity, malondialdehyde and cholesterol concentration and blood haematology of broiler breeder among different dietary treatments (P > .05) while TLR2 and TLR4 mRNA expression up-regulated (P < .05). It can be concluded Lactofeed and Pediguard did not improve broiler breeder performance and T-cell-mediated immune response and are not advisable for breeder nutrition.
Introduction
Because of public forbiddance of the application of sub-therapeutic levels of antibiotics, development and application of non-antibiotic alternatives like probiotics as performance enhancers are increased although there is little information about their effects especially on meat type broiler breeders. There are many species of probiotics around the world and their efficacy depends on their single- or multi-strain nature and their manufacturing process. Probiotics are defined as live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance and it depends greatly on the kind and population of probiotics bacterial content. Although probiotics manner of action remains to be clarified, they are thought to function by maintaining the presence of beneficial microorganisms, and competitive exclusion of pathogenic bacteria adherence in the intestine of broilers (Callaway et al. Citation2008). In this regards, incorporation of Lactobacillus fermentum and Saccharomyces cerevisiae improved the intestinal microflora balance in the rectum of broiler chickens and enhanced intestinal immunity in chickens (Lei et al. Citation2009).
Close relationship between the gastrointestinal tract (GIT) microflora and development and/or maintenance of a functional intestinal immune system is fully understood. For example, germ-free mammals have a higher susceptibility to intestinal infections (O’Hara and Shanahan Citation2006) and are unable to mount an effective antibody response until re-establishment of their gut microflora. Intestinal immunity was increased in chickens fed diets supplemented with Lactobacillus-based probiotic culture. Probiotics might augment toll-like receptor (TLR) signalling, regulate local mucosal cell-mediated immune responses, enhance dendritic cell-induced T-cell hypo-responsiveness and promote epithelial barrier integrity in avian species (Gao et al. Citation2008). TLRs recognize specific microbial components and induce the production of T-helper (Th1) cytokines through a process dependent on the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway (Murch Citation2001).
On the other hand, probiotics influence the host immune system in multiple and diverse ways, including increased antibody production, up-regulation of cell-mediated immunity, improvement of T-cell homing to mesenteric lymph nodes, and augmented TLR signalling (Corthésy et al. Citation2007). Specific antibody titres following immunization with New-Castle disease (ND) virus were enhanced in probiotic fed chicks illustrating the adjuvant role of probiotics with a practical relevance (Apata Citation2008).
However, little information is available about the effects of probiotic strain in broiler breeder performance, blood haematology and expression of TLRs. Recently, a new single- and multi-species probiotic product has been developed as a competitive exclusion product for poultry in Iran (single- and multi-strain probiotics, Lactofeed and Pediguard respectively). There is not any information about the effect of these probiotics on broiler breeder performance. Therefore, the present experiment has investigated the functionality of mono- and multi-strain probiotics on meat type broiler breeder performance and intestinal immunity and oxidative enzymes activity.
Material and methods
Animal welfare
All procedures including animal welfare, husbandry and experimental procedures were evaluated and approved by the Institutional Animal Care and Ethics Committee of the Iranian Council of Animal Care (Care ICoA Citation1995).
Experimental design and birds
This experiment was conducted in the experimental farm of the Department of Animal Science, University of Tehran, Iran. In a completely randomized design, 300 Ross 308 broiler breeder hens and 20 roosters (for artificial insemination) allocated to 5 dietary treatments with 6 replicates (10 birds in each). The trial was conducted in an open-sided breeder house under natural environmental conditions from March to July of 2017. The temperature in the layer house was set in the range of 18°C to 24°C. The trial lasted 10 weeks. Breeder hens were housed in the pens with 3 m2 floor spaces (1.5 × 2 m), while roosters were housed in a separate pen with 4 m2 floor space. A regime of 15.5 h light was provided and all hens and roosters were kept under uniform management conditions throughout the experimental period and each pen was illuminated with one 90-watt incandescent light bulb. Daily allocated feed was adjusted weekly to maintain body weight gain (BWG) as recommended by Ross 308 parent stock management manual. Total hen-day egg production and body weight gain of the hens were recorded at the end of each week from 51 to 61 weeks of age. The corn-soybean meal based basal diet (mash form) was formulated to meet or exceed the Ross 308 Parent Stock Nutrition Specifications (Aviagen Citation2016). The dietary treatments included (1) the basal diet as a control group, (2) the basal diet supplemented with 0.1 g/kg Lactofeed probiotic, (3) the basal diet supplemented with 0.1 g/kg Pedigaurd probiotic, (4) basal diet supplemented with 0.1 g/kg Lactofeed + 0.1 g/kg Pediguard and (5) basal diet supplemented with 0.5 g/kg oxytetracycline antibiotic. The probiotics supplemented to diets according to manufacturer’s recommendations. Lactofeed probiotic is a multi-strain probiotic comprising 4 bacteria strains including Lactobacillus acidophilus 2.5 × 107 cfu/g, Lactobacillus casei 2.5 × 107 cfu/kg, Bifidobacterium thermophilum 2.5 × 107 cfu/g, Enterococcus feacium 2.5 × 107 cfu/g and Pediguard probiotic consisted of Pediococcus acidilactici 1 × 1010 cfu/g. Administration level of probiotics was recommended by the manufacturer. The composition of basal diet and nutrient composition of feed ingredients were shown in . Daily feed allocated to broiler breeder ranged from 158 g/day/bird during the experiment. In order to check mixing condition and probiotic activity and growth in the feed, the populations of bacteria in the feed samples of each diet were measured as described by Lei et al. (Citation2009).
Table 1. Ingredients and calculated nutrient content of basal diet.
Response criteria
Ejaculates from 15 males were pooled and diluted to 2 × 109 viable spermatozoa per millilitre with poultry semen extender. All hens were artificially inseminated once in the afternoon between 3 and 4 pm, on two consecutive days (days 0 and 1) with 0.5 ml extended semen (1 × 108 spermatozoa) at the 50 weeks of age. Artificial insemination was carried out within 30 min after semen collection. Inseminations were standardized to prevent undesirable effect of sperm quality factors, number of spermatozoa, time of insemination and age, percentage and duration of fertility (Beaumont et al. Citation1992). Hen-day egg production (HDEP) was calculated at the end of the each week and at the end of the experiment as described below:
Finally, broiler breeder body weight, egg weight and egg mass, gram feed per each egg production percentage as FCR, fertility and hatchability were recorded.
Sample collections
At 60 weeks of age, 2 birds from each replicate were selected according to the average BW within the pen after a 12-h fasting, and weighted individually. Then birds were killed by cervical dislocation in a germ-free isolation chamber sterilized by ultra-violate radiation. The foregut about 12 cm from the end of the duodenum to the middle section of the jejunum of birds was removed and flushed with a cold phosphate buffer containing 154 mM NaCl, 3 mM KCl, 12 mM Na2HPO4, and 2 mM KH2PO4 (pH 7.4, 0°C) as described by (Sato et al. Citation2009). The foregut (about 1 g) was frozen in liquid nitrogen and stored at −80°C until analysis.
Cutaneous basophil hypersensitivity test and blood haematology
The lymphoproliferative response to phytohemagglutinin (PHA-P: L1668 Sigma Aldrich), as an indicator of a T-cell-induced delayed-type hypersensitivity reaction, was assessed as described previously (Corrier and DeLoach Citation1990). The toe web swelling reaction to PHA-P was measured in two broilers from each pen (marked with a black color) at 32 days of age. One-tenth millilitre of a PHA-P solution (1 mg/mL in phosphate buffer saline: PBS) was injected subcutaneously into two sites on the left toe web. As a sham control, 0.1 mL of PBS was injected into two sites on the right toe web. The thickness of each injection site was measured using a pressure-sensitive micrometer (model DC-516) before injection and at 4, 24 and 48 h after injection. The toe web swelling reaction to PHA-P was calculated using the following swelling index:
Blood samples were collected from the wing vein of two birds (those subjected for injection of phytohemagglutinin) from each pen and placed into EDTA anti-coagulant treated bottles at 59 weeks of age, then heterophil (H) and lymphocyte (L) percentage were determined and H:L ratio was calculated.
Serum malondialdehyde (MDA) and glutathione peroxidase activity
At the end of the study, blood samples were collected from 12 birds (2 per replicate) randomly chosen from each treatment. Blood samples were pooled and then centrifuged at 3000 g for 10 min and sera were collected. Serum malondialdehyde (MDA) and serum glutathione peroxidase (GPx3) activity were determined. Lipid peroxidation was assessed as thiobarbituric acid-reactive substance (TBARS) concentrations in samples by the method of (Uchiyama and Mihara Citation1978) and values are reported as the concentration of MDA. The activity of GPx3 was determined by using the indirect spectrophotometric procedure coupled with glutathione reductase (Lawrence and Burk Citation1976). Oxidation of NADPH was recorded for 1 min at 340 nm and the activity of GPx3 was calculated from the absorption difference. One unit was defined as 1 mmol NADPH oxidized per minute under the conditions described. A blank without the addition of plasma or cytosolic supernatant was carried out for each sample.
Quantitation of mRNA using real-time PCR
The total RNA was extracted from frozen foregut samples of 6 birds in each treatment with the TRIzol reagent (Invitrogen, USA) following the manufacturer’s instructions. The RNA quality (intact rRNA 28S/18S) was evaluated by agarose gel electrophoresis. One microgram of the total RNA was then reverse transcribed to cDNA using superscript III two-step reverse transcript kit (Invitrogen, USA). Chicken TLR-2, TLR-4 and β-actin genes were amplified using TaKaRa SYBR Green PCR Master Mix (TaKaRa, Japan). Primers’ sequences were shown in . The quantitative real-time PCR analysis was performed on a Bio-Rad Real-Time PCR detection system (Bio-radicycler version 3.0a, Bio-Rad, USA). Amplification was conducted with denaturation for 15 min at 95°C, followed by 40 cycles of denaturation for 5 s at 95°C, and annealing/elongation for 30 s at 60°C, and a final melting curve analysis. All target genes were normalized to the endogenous reference gene β-actin based on the previous analysis (Bai et al. Citation2008). Relative gene expression data were analysed using 2−ΔΔC(T) method (Livak and Schmittgen Citation2001).
Table 2. Oligonucleotide sequences of sense and antisense primers for real-time PCR products determined.
Statistical analysis
Sample size calculations of eight animals per group were determined based on previous works (Khan et al. Citation2007; Panda et al. Citation2008; Bai et al. Citation2013) with a statistical power of 0.8. All measured criteria on the effect of dietary probiotics on broiler breeder performance and reproductive characteristics were analysed by one way ANOVA using GLM procedure of SAS 9.4 (Sas Institute Citation2001) with diet as the main effects. Duncan’s multiple range tests were used to compare means (P < .05). A repeated measurements analysis was used to compare breeder groups for their performance and cell-mediated immunity over time (age), as well as their response patterns over age. Differences between treatments were examined using Tukey comparisons.
Results
Probiotics activity and their growth in the diet were detected. The population of Bacillus, Bifidobacterium and Enterococcus species in Lactofeed diet were 4.5 × 107 cfu/kg, 3.8 × 104 cfu/kg, 4 × 104 cfu/kg in Pediococcus species Pedigaurd diet was 1.4 × 106 cfu/kg. Since the amount of allocated mash fee to breeder hens was 158 g/day, therefore received bacteria for each breeder hen was very low. However, the use amount of each probiotic was recommended by the factory.
The results of this study did not show any significant increase in body weight, hen-day egg production percentage, egg weight and egg mass, fertility and hatchability percentages of broiler breeder hens fed diet containing Pediguard and Lactofeed probiotics as compared to control group (P > .05; ).
Table 3. Effect of dietary treatments on breeder reproduction and performance from 51 to 61 weeks of age.†
The effect of probiotics on broiler breeder egg yolk cholesterol concentration and serum biochemical parameters and blood haematology were compared in and . There were no statistically significant differences between dietary treatments on broiler breeder serum cholesterol and MDA concentration and GPx3 activity (P > .05) while egg yolk cholesterol concentration affected by feed additives (P < .05). Inclusion of Lactofeed and Pediguard alone or in combination decreased egg yolk cholesterol (∼13%) compared to control and antibiotic group but there were no significant differences between that of probiotic fed broiler breeders (P > .05).
Table 4. Effect of dietary treatments on broiler breeder egg yolk cholesterol and serum biochemical parameters.
Table 5. Effect of dietary treatments on broiler breeder white blood cell count.
In addition, the current study found that there were no significant effects of probiotics on breeder white blood cells population (P > .05).
The result of cutaneous basophile hypersensitivity is presented in . There was no difference between treatments on toe web thickness index after PHA-P injection (P > .05) while this index affected by time of measurement (P < .05).
Table 6. Effect of dietary treatments on toe web thickness against PHA-P injection.
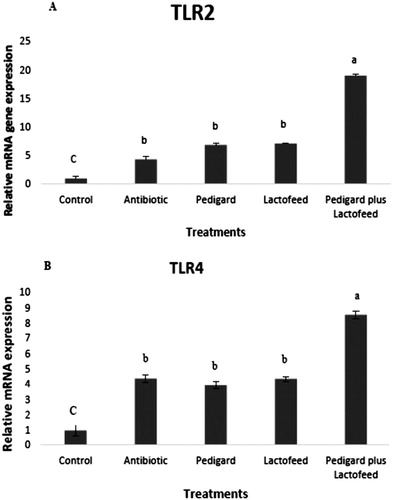
Changes in the expression of TLRs in the foregut are shown in . Dietary inclusion of each of probiotics alone increased (P < .05) the expression level of TLR2 and TLR4 compared to the control group while when broiler breeders fed diet containing both of the probiotics, the expression level of TLR2 and TLR4 were higher than the others (P < .05). However, there were no differences in TLR2 and TLR4 expression between antibiotic, Pediguard and Lactofeed probiotic groups (P > .05).
Discussion
Initial objective of this project was to determine the effect of probiotics (Pediguard and Lactofeed) on broiler breeder performance. The most obvious finding was that none of the detected and calculated parameters of this study did not affected by probiotics. Egg production (total laid eggs during 59 weeks of age) of broiler breeders in control, Pediguard, Lactofeed, both of probiotics and antibiotic fed groups were 408.83, 356, 372.5, 413.8 and 382, respectively, and it was not statistically different. Results of the current study suggest that the new probiotic did not increase hen-day egg production and hatchability. Our results were similar to others who reported that commercial probiotic (multi-strain) supplementation has no effect on laying hen egg production and supplementation of 100 mg Lactobacillus sporogenes per kg had no effect on white leghorn layer breeders performance (Panda et al. Citation2008).
In contrary, the findings of the current study do not support the previous researches which concluded that egg production increased by probiotic supplementation in white leghorn layer breeders (Panda et al. Citation2008). In addition, novel probiotics increased broiler chicken body weight (Olnood et al. Citation2015) and laying hen performance (Tang et al. Citation2015). As far as the final product is concerned, the probiotic dose levels should be based on the ones found to be efficacious in animal studies and the colony-forming units per gram of product is an important parameter. Although the information about the minimum impressive concentrations is still insufficient, it is mostly confirmed that probiotic products should have a minimum concentration of 106 cfu/mL (or gram) and generally, it has been suggested that in animals efficacy could be demonstrated for most probiotics with a daily intake of 108–109 microorganisms (Toma and Pokrotnieks Citation2006). It is generally suggested that a probiotic should have several billion microorganisms to increase the possibility of sufficient gut colonization. For example, typical doses of lactobacilli used in studies ranged from 1 to 20 billion colony-forming units per day (Williams Citation2010) while in our experiment the administration level of probiotics (factory recommendation) is lower than that of other researchers and the minimum level of common effective dose of probiotics. Indeed, in a previous experiment using the 5-bacterial strain probiotic product, probiotic efficacy in improving broiler growth and FCR was demonstrated with a probiotic addition at 109 cfu/kg of diet, resulting in an average daily intake of 2 × 108 microorganisms per broiler. However, no consistent conclusions could be drawn regarding the effect of increasing probiotic administration level on growth performance (Mountzouris et al. Citation2010).
However, there are a few reports focusing on the effects of probiotics on serum and egg yolk cholesterol concentration in broiler breeders. It is interesting to note that in this study egg yolk cholesterol decreased by inclusion of Lactofeed and Pediguard probiotics to broiler breeder diet while serum cholesterol did not affected and this finding was unexpected. Interestingly as seen in , decreased yolk cholesterol levels were related to probiotic inclusion to breeder diets since the lowest yolk cholesterol levels were belonged to the eggs of breeders that received both of probiotics (16.78 vs. 14.97 mg/g yolk). Similar to our results, total yolk cholesterol levels of 100 and 150 mg/kg probiotic-supplemented groups (172.6 and 168.1 mg/yolk, respectively) were lower than that of the control (216.8 mg/yolk) in laying hens and reduction of egg yolk cholesterol concentration could be attributable to reduced absorption and synthesis of cholesterol in the GIT (Mohan et al. Citation1995). In addition, findings of the current study somehow are in agreement with those obtained by the previous research which reported that probiotic supplementation can depress serum and egg yolk cholesterol concentrations (Panda et al. Citation2008; Tang et al. Citation2015) in laying hens. In most animals, cholesterol is eliminated by catabolism and excretion in the faeces, but hens eliminate considerable amounts of cholesterol into egg and egg cholesterol originates from serum cholesterol. It is also possible that some of the microorganisms present in the probiotic preparation could assimilate the cholesterol present in the GIT for their own cellular metabolism. Some probiotic bacteria precipitate the cholesterol with de-conjugated bile salts by the ability of probiotics to produce bile salt hydrolase enzyme (BSH) (EC 3.5.1.24) for bile salt deconjugation in the enterohepatic circulation, retardation of cholesterol synthesis via the inhibition of hydroxymethylglutaryl coenzyme A (HMG CoA) reductase and conversion of cholesterol by probiotics in the intestine into coprostanol, which is directly excreted with the faeces (Ooi and Liong Citation2010) thereby preventing them from acting as precursors in cholesterol synthesis. The hypocholesterolemic effect on the host due to probiotic feeding is also dependent on duration of probiotic feeding and 12-week feeding is required for serum cholesterol reduction (Abdulrahim et al. Citation1996) while in this experiment serum cholesterol were estimated after 11 weeks of continuous feeding of probiotic, and non-significant effect of probiotics on serum cholesterol were observed.
Glutathione peroxidase displays its activity mainly in the cellular cytoplasm and only about ten percent of its activity is displayed in mitochondria. In this manner, the safe removal of hydrogen peroxide is attained through the joint action of GPx and catalase. Malondialdehyde level endogenously reflects lipid peroxidation, which is the consequence of diminished anti-oxidant protection as reactive oxygen species (ROS) levels increase. In this experiment serum GPx3 activity and MDA concentration were not different between dietary treatments and this may be attributed to the probiotic inability to confer adequate anti-oxidant protection against lipid peroxidation during the productive phase of broiler breeders. However, the findings of the current study do not support the previous research that fed probiotics to broiler chickens and showed raise in the activity of superoxide dismutase and glutathione peroxidase and reduce serum MDA concentration (Xiumei et al. Citation2004) and Bacillus subtilis decreased serum MDA, nitric oxide and liver MDA concentration (Yu et al. Citation2010). Similarly, broilers were fed diet with medicinal plant (green tea) with the same probiotics (0.5% and 1.0%) levels reduced lipid oxidation (Sarker et al. Citation2009) and in this regard authors are unaware of any research on Lactofeed and Pediguard as anti-oxidant and their application to study oxidative stress during production period of broiler breeders. A possible explanation for the results of current study might be that used probiotics do not have efficient anti-oxidation effect or selenium is absent in their composition. Another possible explanation is that the dose of administration was lower than that of others used, so using higher dose of probiotics may be effective in achieving positive effect of probiotics on serum GPx3 activity and MDA concentration.
The findings of the current study do not completely support the previous research who reported that administration of multi-species probiotic did not influence blood eosinophil and monocytes percentage while increased lymphocyte and decreased heterophil and H:L ratio (Khan et al. Citation2011). The increase in lymphocyte percentage in layers received either multi-strain probiotic paralleled with decrease of heterophil percentage and H:L ratio, is indicating reduction of possible stress effect by dietary treatments. Generally, heterophil number increase during mildly or moderately stressful conditions and consequently H:L ratio can be used to detect the presence of physiological stress for most stressors, so probiotic may exert its beneficial effects by modulating immune system of the host against potentially harmful antigen via activation of lymphocytes and antibody production (Maxwell and Robertson Citation1998).
A cutaneous basophil hypersensitivity test to PHA-P was performed to assess cellular immunity in the birds. Phytohemagglutinin is a plant lectin and has the ability to crosslink T-cell surface receptors and initiate proliferation of T-cells without need of antigen-presenting cells. After injection of PAH, the heterophils, basophils, macrophages, and almost every cell of the immune system migrate to the site of PHA injection (Vinkler et al. Citation2010). The PHA swelling test is widely employed as a measure of disease resistance and acquired immune capacity of an animal (Salaberria et al. Citation2013). Phytohemagglutinin is a CD8+ T-cell-mediated response. The importance of CD8+ T-cells in avian immunity is critical since they help in the protective immunity against Marek’s disease, infectious bronchitis and other viral diseases. Decrease in CD8+ T-cell response could render the birds susceptible to infectious diseases (Erf Citation2004). Overall, probiotics did not affect both toe web thickness index and white blood cells in our experiment and a possible explanation for non-effectiveness of probiotics on both toe web thickness index might be that dietary probiotics did not affect lymphocyte and heterophil of broiler breeder.
Important objective of this project was to identify the effect of Lactofeed and Pediguard on broiler breeder gut TLRs gene expression. Lactofeed and Pediguard had positive effect on mRNA expression of TLR2 and TLR4 while the most interesting finding was that administration of probiotics together hade more positive effect on mRNA expression of TLRs as compared to control group. These results suggested that the probiotics stimulated the T-cell immune system via TLR2 and TLR4 in the gut without decreasing the growth performance of broiler breeders in the current study. Results are in accord with recent studies indicating that incorporation of L. fermentum and S. cerevisiae probiotic increased the mRNA expression levels of broiler chicken TLR2 and TLR4 at 21 days (Bai et al. Citation2013) and zymosan derived from S. cerevisiae increased mRNA expression of TLR2 in broiler chickens (Sato et al. Citation2009). On the other hand, results do not support the previous research which reported that inclusion of yeast to broiler diets did not influenced mRNA expression of TLR2 and TLR4 in the bursa of fabricius (Yitbarek et al. Citation2013). The TLRs are a family of conserved trans-membrane proteins that recognize conserved molecular motifs derived from bacteria, viruses, fungi, and parasites and play a crucial role in activating T-cells in the intestinal immune system, especially via the MyD88 dependent TLR/IL-1R signalling pathway (Kunikata et al. Citation2002). The TLR2 is involved in recognition of lipotechoic acid of gram-positive bacteria and zymosan of yeasts and TLR4 is the principal receptor for lipopolysaccharide, which is a major component of the outer membrane of gram-negative bacteria (Kannaki et al. Citation2010). A down regulation of TLR4 expression in the intestine of broiler breeders fed the probiotic-supplemented diet up-regulated because dietary inclusion of the probiotics had no change on the population of gram-negative bacteria such as coliform in the small intestine of breeders (Lei et al. Citation2009) and the higher expression of TLR4 might relate to the polysaccharides presented on the surface of bacteria in the present study.
In conclusion, based on the results, Pediguard and Lactofeed had no significant effect on broiler breeder performance, cell-mediated immunity, serum GPx3 activity, MDA, cholesterol concentrations and blood haematology while egg yolk cholesterol concentration was decreased and TLR2 and TLR4 mRNA expression levels were up-regulated. These results indicated that Lactofeed and Pediguard did not improve broiler breeder performance and T-cell-mediated immune response but increased expression of gut TLRs, and therefore they are not advisable for breeder nutrition. In this experiment feed intake of broiler breeder was ranged from 165 to 172 g per day/bird and consumed probiotic in the experiment was 1.7 × 107/bird while a total of some 108–109 probiotic microorganisms should be consumed daily to probiotic effect could be transferred to the consumer. Probable reasons for our result are: (1) the concentration or CFU of probiotics that used in this study was 1 × 108 cfu/g while others used higher concentrations (1 × 1013 cfu/g) and the minimum dose of probiotics effectiveness is 1 × 108/g and (2) population or concentration of the bacteria (lactobacillus or bifidobacterium) in the small intestine of probiotic fed broilers was lower than that of the control group and it means that proliferation of bacteria in small intestine was not occurred.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ali Khatibjoo http://orcid.org/0000-0002-9214-8146
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Abdulrahim S , Haddadin M , Hashlamoun E , Robinson R. 1996. The influence of Lactobacillus acidophilus and bacitracin on layer performance of chickens and cholesterol content of plasma and egg yolk. Br Poult Sci. 37:341–346. doi: 10.1080/00071669608417865
- Apata D. 2008. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture of Lactobacillus bulgaricus . J Sci Food Agric. 88:1253–1258. doi: 10.1002/jsfa.3214
- Aviagen . 2016. Parent stock nutrition specifications.
- Bai S , Lu L , Luo X , Liu B. 2008. Kinetics of manganese absorption in ligated small intestinal segments of broilers. Poult Sci. 87:2596–2604. doi: 10.3382/ps.2008-00117
- Bai S , Wu A , Ding X , Lei Y , Bai J , Zhang K , Chio J. 2013. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult Sci. 92:663–670. doi: 10.3382/ps.2012-02813
- Beaumont C , Brillard J , Millet N , De Reviers M. 1992. Comparison of various characteristics of duration of fertility in hens. Br Poult Sci. 33:649–661. doi: 10.1080/00071669208417503
- Callaway T , Edrington T , Anderson R , Harvey R , Genovese K , Kennedy C , Venn D , Nisbet D. 2008. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev. 9:217–225. doi: 10.1017/S1466252308001540
- Care ICoA . 1995. Guide to the care and use of experimental animals. Isfahan University of Technology Isfahan, Iran.
- Corrier D , DeLoach J. 1990. Evaluation of cell-mediated, cutaneous basophil hypersensitivity in young chickens by an interdigital skin test. Poult Sci. 69:403. doi: 10.3382/ps.0690403
- Corthésy B , Gaskins HR , Mercenier A. 2007. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 137:781S–790S. doi: 10.1093/jn/137.3.781S
- Erf G. 2004. Cell-mediated immunity in poultry. Poult Sci. 83:580. doi: 10.1093/ps/83.4.580
- Gao J , Zhang H , Yu S , Wu S , Yoon I , Quigley J , Gao Y , Qi G. 2008. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult Sci. 87:1377–1384. doi: 10.3382/ps.2007-00418
- Kannaki T , Reddy M , Shanmugam M , Verma P , Sharma R. 2010. Chicken toll-like receptors and their role in immunity. Worlds Poult Sci J. 66:727–738. doi: 10.1017/S0043933910000693
- Khan SH , Atif M , Mukhtar N , Rehman A , Fareed G. 2011. Effects of supplementation of multi-enzyme and multi-species probiotic on production performance, egg quality, cholesterol level and immune system in laying hens. J Appl Anim Res. 39:386–398. doi: 10.1080/09712119.2011.621538
- Khan M , Raoult D , Richet H , Lepidi H , La Scola B. 2007. Growth-promoting effects of single-dose intragastrically administered probiotics in chickens. Br Poult Sci. 48:732–735. doi: 10.1080/00071660701716222
- Kunikata T , Tanaka A , Miyazawa T , Kato S , Takeuchi K. 2002. 16, 16-Dimethyl prostaglandin E2 inhibits indomethacin induced small intestinal lesions through EP3 and EP4 receptors. Dig Dis Sci. 47:894–904. doi: 10.1023/A:1014725024519
- Lawrence RA , Burk RF. 1976. Glutathione peroxidase activity in selenium-deficient rat liver. BBRC. 71:952–958.
- Lei Y , Zhang K-Y , Ding X-M , Bai S-P , Choi JS. 2009. Effect of probiotics on growth performance, development of small intestinal tract and microbial populations in broilers. J Agri Sci Technol. 3:24–31.
- Livak KJ , Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262
- Maxwell M , Robertson G. 1998. The avian heterophil leucocyte: a review. Worlds Poult Sci J. 54:155–178. doi: 10.1079/WPS19980012
- Mohan B , Kadirvel R , Bhaskaran M , Natarajan A. 1995. Effect of probiotic supplementation on serum/yolk cholesterol and on egg shell thickness in layers. Br Poult Sci. 36:799–803. doi: 10.1080/00071669508417824
- Mountzouris KC , Tsitrsikos P , Palamidi I , Arvaniti A , Mohnl M , Schatzmayr G , Fegeros K. 2010. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci. 89:58–67. doi: 10.3382/ps.2009-00308
- Murch SH. 2001. Toll of allergy reduced by probiotics. Lancet. 357:1057–1059. doi: 10.1016/S0140-6736(00)04305-1
- O’Hara AM , Shanahan F. 2006. The gut flora as a forgotten organ. EMBO Rep. 7:688–693. doi: 10.1038/sj.embor.7400731
- Olnood CG , Beski SS , Choct M , Iji PA. 2015. Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens. Animal Nutr. 1:184–191. doi: 10.1016/j.aninu.2015.07.003
- Ooi L-G , Liong M-T. 2010. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 11:2499–2522. doi: 10.3390/ijms11062499
- Panda AK , Rama Rao SS , Raju MV , Sharma SS. 2008. Effect of probiotic (Lactobacillus sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of White Leghorn layer breeders. J Sci Food Agric. 88:43–47. doi: 10.1002/jsfa.2921
- Salaberria C , Muriel J , de Luna M , Gil D , Puerta M. 2013. The PHA test as an indicator of phagocytic activity in a passerine bird. PLoS One. 8:e84108. doi: 10.1371/journal.pone.0084108
- Sarker M , Ko S , Kim G , Yang C. 2009. Effect of green tea and green tea probiotics on meat quality in broilers. Proceedings of the Sixth Int’l Poult Show and Seminar, World’s Poultry Science Association Bangladesh Branch.
- SAS Institute . 2001. SAS/WATTM user’s guide. Cary, NC : SAS Institute.
- Sato K , Takahashi K , Tohno M , Miura Y , Kamada T , Ikegami S , Kitazawa H. 2009. Immunomodulation in gut-associated lymphoid tissue of neonatal chicks by immunobiotic diets. Poult Sci. 88:2532–2538. doi: 10.3382/ps.2009-00291
- Tang SGH , Sieo CC , Kalavathy R , Saad WZ , Yong ST , Wong HK , Ho YW. 2015. Chemical compositions of egg yolks and egg quality of laying hens fed prebiotic, probiotic, and synbiotic diets. J Food Sci. 80:C1686–C1695. doi: 10.1111/1750-3841.12947
- Toma MM , Pokrotnieks J. 2006. Probiotics as functional food: microbiological and medical aspects. Acta Univ Latvien. 710:117–129.
- Uchiyama M , Mihara M. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. AnBio. 86:271–278.
- Vinkler M , Bainová H , Albrecht T. 2010. Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct Ecol. 24:1081–1086. doi: 10.1111/j.1365-2435.2010.01711.x
- Williams NT. 2010. Probiotics. Am J Health Syst Pharm. 67.
- Xiumei D , Chaofan Z , Ping W. 2004. The effect of compound probiotics on intestinal bacteria and anti-oxidation in broilers. China Poult. 14:003.
- Yitbarek A , Rodriguez-Lecompte J , Echeverry H , Munyaka P , Barjesteh N , Sharif S , Camelo-Jaimes G. 2013. Performance, histomorphology, and toll-like receptor, chemokine, and cytokine profile locally and systemically in broiler chickens fed diets supplemented with yeast-derived macromolecules. Poult Sci. 92:2299–2310. doi: 10.3382/ps.2013-03141
- Yu DY , Mao XF , Qin Y , Li WF. 2010. Effects of B. subtilis on growth performance, antioxidant and immunity of broilers. Chin J Anim Sci. 3:009.