 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study was conducted to investigate the effects of Macleaya cordata extract (MCE) on growth performance, immune responses, antioxidant capacity, intestinal morphology and microbiota in weaned piglets. A total of 36 weaned piglets [Duroc× (Large White × Landrace)] with an average body weight of 6.55 ± 0.32 kg at weaning were used in a 21-day experiment. Pigs were divided into three treatments (n = 12). Control (basal diet); MCE (basal diet plus MCE) and ABO (basal diet plus 20 mg/kg falvomycin & 100 mg/kg aureomycin). Compared with the control group, piglets in the MCE and ABO groups had higher average daily gain, lower feed efficiency and diarrhea rates (P < 0.05). Serum IgG level in MCE -fed piglets was higher (P < 0.05) than that of control -fed piglets. A higher (P < 0.05) serum the activity of total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) was observed in MCE-fed piglets than those in the control and ABO groups. The addition of MCE increased the amount of Lactobacillus spp. (P < 0.05), while decreased the amount of Salmonella spp. (P < 0.05) and tended to reduce the E. coli population (P < 0.10). Dietary MCE increased villus height and the villus height to crypt depth ratio in the duodenum, jejunum and ileum, and decreased the crypt depth in the jejunum of piglets (P < 0.05). In conclusion, dietary MCE supplementation would exert beneficial effects on growth performance, immune status, antioxidant capacity and the intestinal health and may be used as a potential alternative to antibiotics in weaned piglets.
1. Introduction
Weaning is probably the most stressful time in a pig’s life. From the physiological point of view, they are physiologically not fully competent to deal with the multiple social, nutritional, environmental and immunological changes associated with weaning (Pluske et al. Citation1997). Weaning is associated with changes to the architecture of the small intestine, disturbed intestinal microbiota and diminished immune responses (Spreeuwenberg et al. Citation2001; Boudry et al. Citation2004; Pieper et al. Citation2008), as well as accompanied by a decreased nutrient intake and digestibility, weight loss and growth performance depression (Kim et al. Citation2012). Antibiotics have been widely used to improve the health and growth performance in weaned piglets (Frydendahl Citation2002; Pluske Citation2013). However, the misuse of antibiotics has led to bacterial resistance in livestock animal and humans, as well as antibiotics-residue in animal products, which may affect for human health (Schwarz et al. Citation2001; Kolpin et al. Citation2002). Therefore, alternative feed additives should be investigated in livestock.
Macleaya cordata extract (MCE) is a rich source of sanguinarine. Many studies reported that sanguinarine is an antimicrobial (Newton et al. Citation2002; Kosina et al. Citation2010), anti-oxidant (Chaturvedi et al. Citation1997), anti-inflammatory (Tanaka et al. Citation1993; Yiu and Wei Citation1993; Niu et al. Citation2012), and immuno-modulatory products (Chaturvedi et al. Citation1997). Dietary supplementation with MCE has beneficial effects for pigs (Jeroch et al. Citation2009; Kantas et al. Citation2015), broiler chickens (Vieira et al. Citation2008), and fish (Zhang et al. Citation2013). Supplementation of MCE into the broilers’ diet at 20 or 50 ppm improved growth performance, increased relative jejunal or ileal length, and altered gut microbiota in broiler chickens (Lee et al. Citation2015). A recent study showed that supplementation of post-weaning piglet diets with 120 mg MCE per kg diet improved growth performance and nutrient digestibility (Goodarzi et al. Citation2018). Although MCE appears to increase the production performance of multiple species including swine, little data are available on its effects on weanling stage pigs. We hypothesized that MCE affects gastrointestinal tract integrity, immune response, and microbial populations in weanling pigs. Thus, we envision that growth performance and gastrointestinal tract health and function of MCE supplemented weanlings will be similar to antibiotic-treated animals. Therefore, the objective of this study was to investigate the effect of dietary MCE on growth performance, immune status, antioxidant capacity, intestinal microbiota and morphology in weaned piglets.
2. Materials and methods
All procedures were approved by the Committee of Animal Care at the Institute of Subtropical Agriculture, Chinese Academy of Sciences. Sangrovit® is a standardized pre-mixture of MCE, combined with a carrier of dried and ground plant material from the Papaveraceae family, standardized to provide at least 12.5 g/kg mixture of quaternary-benzo(c)phenanthridine alkaloids (e.g. sanguinarine and chelerythrine) and protopine alkaloids (e.g. protopine and allocryptopine), among which 1.5% w/w sanguinarine was used as a main marker, and is manufactured from extracts of M. cordata (Micolta Bioresource Co. Ltd., Changsha 410128, China).
2.1. Experimental design, animals, diets, and husbandry
A total of 36 crossed healthy weaned piglets [Duroc × (Large White × Landrace)] with an average body weight (BW) of 6.55 ± 0.32 kg (21 d of age) were used in this experiment. The piglet were randomly classified in to 3 groups including basal diet (Control), basal diet + 50 mg/kg MCE (MCE group), and basal diet + 20 mg/kg flavomycin +100 mg/kg aureomycin (ABO group). The feeding protocol was carried out from 22 days until 42 days of age. A corn-soybean meal basal diet containing no antibiotics was present in according to the nutritional requirements (NRC Citation2012). Individual animals were housed in stainless steel metabolic cages (150 cm long × 105 cm wide × 120 cm high) in a temperature-controlled nursery house (25°C to 27°C). Relative humidity was controlled at 60–70%. Each animal was housed in a stainless steel metabolic cage (150 cm long × 105 cm wide × 120 cm high). All the pigs had ad libitum access to the diets and clean drinking water.
Table 1. Ingredients and chemical composition of experimental diets (as-fed basis).
2.2. Growth performance and diarrhea incidence
The individual BW and feed consumption were measured at the beginning and the end of the trial. Those data were used for calculating the average daily gain (ADG), average daily feed intake (ADFI), and the ratio of feed to gain (F/G). The clinical signs of diarrhea were visually assessed every day by observers blinded to treatments, and a scoring system was applied to indicate the presence and severity of diarrhea as follows: 1 = hard feces; 2 = slightly soft feces; 3 = soft, partially formed feces; 4 = loose, semiliquid feces; and 5 = watery, mucous-like feces. When the average score was over 3, pigs were identified as having diarrhea. Diarrhea rate was calculated according to the formula (Sun et al. Citation2008):
2.3. Sample collection and preparation
At the end of experiment (21 days of experiment), 5 mL blood samples were collected via anterior vena cava puncture from all of pigs and centrifuged at 3000 × g and 4°C for 15 min to separate out the serum. The serum samples were then stored at −20°C for immunoglobulin (Ig) and antioxidative index analysis. Then all of the animals were humanely killed by lethal intraperitoneal injections of sodium pentobarbital, as described by Yao et al. (Citation2011). Segments of the mid-duodenum, mid-jejunum and mid-ileum in each animal were collected for morphological examination. The luminal digesta of the cecum was collected and stored at −80°C until the gut microbial composition analysis.
2.4. Bacterial quantification by real-time PCR
Total bacterial DNA was extracted from the contents of each intestinal sample (0.2 g) according to a previously described protocol (Kraler et al. Citation2016), using a QIAamp DNA Stool Mini Kit (Qiagen, Germany). Those extracts were stored at −80°C. They were then quantified on a Nanodrop 2000 Spectrophotometer (Thermo Scientific, Courtaboeuf, France) before the results were adjusted to a concentration of 10 ng/μL.
Methods based on 16S rRNA were used to assess the abundances of Bifidobacterium spp., Escherichia coli, Lactobacillus spp., and Salmonella spp. as previously described (Chen et al. Citation2018). All PCR primers are listed in . Duplicate sample analyses were performed in mixtures (final volume, 10 μL) that contained 1 μL of diluted DNA sample and 0.2 μM of each primer, using a 1× of SYBR® Premix Ex Taq™ II Kit (TaKaRa Bio Inc., Shiga, Japan). The amplification programme included 95°C for 30 s; followed by 40 cycles of 95°C for 5 s and 60°C for 30 s; and then a final melting curve for SYBR Green tests. The melting curve analysis and size-determination of amplificates on agarose gels verified that the target fragments had been amplified. Standard curves were generated as described by Qi et al. (Citation2011). Results were expressed in log10 copies of 16S rRNA genes per gram of intestinal material in each sample and bacterial group (Metzler-Zebeli et al. Citation2015).
Table 2. Sequences of primers and probes used for group-specific quantitative PCRs.
2.5. Serum parameters assay
Serum antioxidant-related indices, including total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), catalase (CAT) and the concentration of malondialdehyde (MDA), were determined with commercially available reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Moreover, the levels of immunoglobulin (IgM, IgG and IgA) in serum was detected by an enzyme-linked immunosorbent assay (ELISA) kit (Bogoo Biotechnology Co. Ltd., Shanghai, China). All measurements were done at least in triplicate and according to the manufacturer’s instructions.
2.6. Morphological measurements
Histological samples were rapidly fixed in neutral-buffered formalin. Sections of the small intestine were excised, dehydrated, and embedded in paraffin wax before four transverse sections were cut, placed on glass slides, and stained with haematoxylin and eosin. The villus height and crypt depth of 10 well-oriented villi per segment were measured using a Nikon ECLIPSE 80i light microscope equipped with a computer-assisted morpho-metric system (Nikon Corporation, Tokyo, Japan) (Chen et al. Citation2016).
2.7. Statistical analysis
All data were analyzed by analysis of variance (ANOVA) as a randomized complete block design using the General Linear Model (GLM) procedure of the IBM SPSS v 20.0 (IBP SPSS Institute Inc., 2011). Individual pig served as the experimental unit for the analysis of all data. Significant differences between means were determined using Tukey’s multiple comparison test. The results were shown as mean and standard error of the mean (SEM). A value of P ≤ 0.05 was considered statistically significant, whereas 0.05 < P < 0.10 was considered as trend.
3. Results
3.1. Growth performance and diarrhea ratio
As shown in , piglets fed with MCE increased the ADG by 16.38% (P < 0.05) and ADFI by 6.35% (P < 0.05) compared with the piglets fed the control diet, decreased the F/G by 8.89% (P < 0.05). Supplementation with ABO also significantly increased ADG and ADFI (P < 0.05) in comparison to the control group. However, there was no significant effects between MCE and ABO groups.
Table 3. Effects of dietary MCE supplementation on growth performance of weaned piglets.
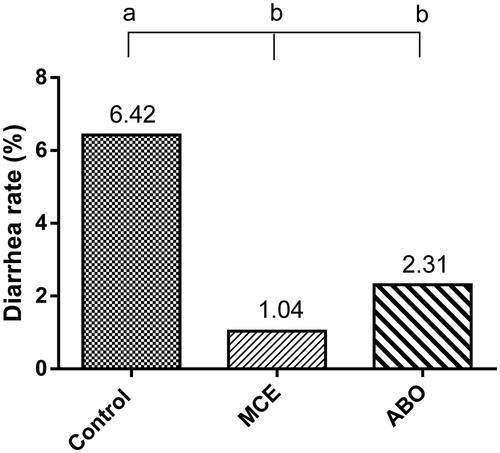
As shown in , supplemental MCE and ABO decreased the diarrhea ratio by 83.8% (P < 0.05) and 64.0% (P < 0.05), respectively, as compared with control group. There was no significant difference between MCE and ABO groups.
Figure 1. Diarrhea rate of weaned piglets fed different dietary treatments (%) (n = 12). Treatments consisted of (1) Control; basal diet, (2) MCE; basal diet + 50 mg/kg MCE and (3) ABO; basal diet + 20 mg/kg flavomycin + 100 mg/kg aureomycin Different letters mean differ significantly (P < 0.05 or P < 0.01).
3.2. Serum immunity and antioxidant capacity
As shown in , the amount of IgG in MCE group was higher than control group (P < 0.05). However, there was no difference in serum IgA and IgM levels among the groups (P > 0.05).
Table 4. Effects of dietary MCE supplementation on immune response of weaned piglets.
The effects of experimental diets on the activities of antioxidant enzymes in the serum of piglets are presented in . A higher (P < 0.05) serum T-AOC, GSH-Px and SOD was observed in MCE-supplemented piglets than those in the control and ABO groups, while piglets fed with MCE decreased the serum MDA content (P < 0.05), and tended to increase the activities of serum CAT (P < 0.10) compared with the control and ABO groups. Antibiotics supplementation had no significant effect (P > 0.05) on the serum antioxidant indices compared to the control group.
Table 5. Effects of dietary MCE supplementation on serum antioxidant activity of weaned piglets.
3.3. Intestinal microflora
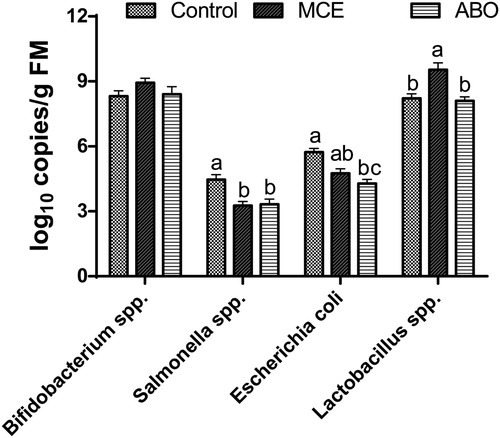
After a 21-d feeding, piglets fed the MCE diet had higher (P < 0.05)cecal Lactobacillus spp. population than piglets fed the control and ABO diet, and had lower (P < 0.05) Salmonella spp. than piglets fed the control diet(). Piglets fed the ABO diet had lower (P < 0.05) cecal E. coli population than piglets fed the control diet. Piglets fed the MCE diet had tended to decrease (P < 0.10) the E. coli than piglets fed the control diet. No treatment effect was found for Bifidobacterium spp. (P > 0.05).
Figure 2. Occurrence of bacterial groups in cecal samples of weaned piglets fed dietary treatments, determined by qPCR (log10) 16S rRNA gene copy number/g fresh matter. Treatments consisted of (1) Control; basal diet, (2) MCE; basal diet + 50 mg/kg MCE and (3) ABO; basal diet + 20 mg/kg flavomycin + 100 mg/kg aureomycin. Bars represent mean ± SD of 12 pigs per group. The letters a, b, c indicate the difference between treatment groups (P < 0.05).
3.4. Small intestinal morphology
Piglets fed the diet supplemented with MCE had greater (P < 0.05) villus height and the villus height to crypt depth ratio (VC) at the duodenum, jejunum and ileum than those fed the control diet (). The crypt depth in the jejunum of piglets fed the diets containing MCE was lower (P < 0.05) than those in the control piglets. However, there was no significant difference (P > 0.05) in the crypt depth of duodenum or ileum among the treatments. Furthermore, no difference in small intestinal morphology was observed between MCE treatment and ABO treatment (P > 0.05).
Table 6. Effects of dietary MCE supplementation on intestinal morphology of weaned piglets.
4. Discussion
Natural compounds extracted from M. cordata, such as the quaternary benzo[c]phenanthridine alkaloids (QBAs) sanguinarine has been used as feed additive in both swine and poultry (Vieira et al. Citation2008; Pellikaan et al. Citation2010). Gradually, they have evoked attention as a substitute to antibiotic growth promoters (Kim et al. Citation2012). Kosina et al. (Citation2004) conducted an in vivo safety assessment of sanguiritrin (a mixture of sanguinarine and a structurally similar alkaloid, chelerythrine) (5 mg/kg body weight) in pigs following its oral administration for 90 days. Similar to the findings with sanguiritrin, administration of MCE for 90 days at doses higher than the daily recommended dosage were well tolerated in rats (Zdarilova et al., Citation2008). They demonstrated no signs of toxicity and impairment in the health status of the animals, and the remaining alkaloid was unabsorbed and excreted in the feces (Psotova et al. Citation2006), Furthermore, M. cordata was showed up in the European Food Safety Authority (EFSA) database (Ni et al. Citation2016). Previous study demonstrated that dietary supplementation with MCE improved growth performance, gut health, immune systemand digestive function and reduced the diarrhea in nonruminant mammals (Gudev et al. Citation2004; Jankowski et al. Citation2009; Zhang and Coultas Citation2013; Liu et al. Citation2016). In the present study, piglets fed with MCE increased the ADG and ADFI and decreased F/G. In agreement with previous studies, improvement of growth performance (increase of BW, ADG and feed intake, reduction of feed conversion rate) in weaning pigs was found (Kantas et al. Citation2015). Similarly, supplementation of post-weaning piglet diets with 120 mg MCE per kg diet also caused improvement in body weight gain and feed conversion rate (Goodarzi et al. Citation2018). Moreover, the growing broilers fed MCE at 50 and 25 ppm (1–21 d and 22–42 d, respectively) had improved BW and feed conversion rate at 21 d of age (Vieira et al. Citation2008). It has been reported that sanguinarine can regulate the serotonin synthesis by employing tryptophan and finally lead to improvement in feed intake (Ni et al. Citation2016). Improvements in protein retention by reducing intestinal decarboxylation of aromatic amino acids through the inhibition of L-amino acid decarboxylase (Drsata et al. Citation1996). Furthermore, dietary MCE increased relative jejunal and ileal lengths in broiler chickens (Lee et al. Citation2015), changed the intestinal morphology, increased the nutrient digestibility, thus promoted effectively nutrition absorption, which may be associated with promote the growth performance of piglets in this study.
In general, diarrhea after weaning of weaned piglet is associated with rapid multiplication of E. coli and other pathogens in the intestine (Nagy et al. Citation1992; Wittig et al. Citation1995). Antibiotics have been widely used in the treatment of diarrhea. In the current study, we found that the antibiotics have an effect on the diarrhea. It has been reported that the supplementation of medicinal plant extracts decreases diarrhea incidence in pigs (Xu et al. Citation2018), mainly because the extract has antimicrobial properties and it aids in the regulation of organic functions, intestinal pH, and peristalsis (Kommera et al. Citation2006; Kong et al. Citation2007). The antimicrobial properties of sanguinarine have been clearly confirmed (Newton et al. Citation2002). We observed that the supplementation of MCE reduced the diarrhea. In agreement with the previous study found that isoquinoline alkaloids has decreased the prevalence of diarrhea in growing piglets (Liu et al. Citation2016). Furthermore, isoquinoline alkaloids in the extract were found to suppress or destroy these microorganisms, as well as modulating vital functions, such as peristalsis and the pH of intestines (Li et al. Citation2011). The results of the current study also revealed that dietary addition of MCE reduced diarrhea as effectively as the combination of antibiotics used. The results indicated that MCE may be used instead of antibiotics in preventing the weaned pigs from diarrhea.
Macleaya cordata extract is known to have anti-inflammatory effects and immunomodulatory properties (Lenfeld et al. Citation1981; Yiu and Wei Citation1993). It has been reported that MCE stimulates phagocyte activity and promotes host protective responses (Gudev et al. Citation2004). The immunoglobulin (IgG, IgA and IgM) protect the extravascular compartment against pathogenic bacteria and microorganisms (Kong et al. Citation2007; Lauridsen Citation2010). Furthermore, IgG also has antibacterial and antitoxin effects (Li et al. Citation2009). In the current study, piglets fed with MCE-containing diets increased the levels of serum IgG. Liu et al. (Citation2016) reported that supplementing pig diet with MCE increased the concentration of IgG which is in consistent with the present findings. In addition, dietary supplementation with MCE increased the levels of serum IgG and IgM in broilers (Yakhkeshi et al. Citation2011). The improvement on anti-inflammatory results in better immunomodulatory properties (Gessner et al. Citation2017). Chaturvedi et al. (Citation1997) have demonstrated that sanguinarine is a potent inhibitor of NF-κB activation via inhibiting the phosphorylation and degradation of IκBα, an inhibitory subunit of NF-κB. As the activity of the transcription factor NF-κB influences the immune system. These findings suggest that dietary MCE can improve humoral immunity of pigs.
Weaning has been demonstrated to cause oxidative stress reflected by oxidative imbalance in piglets (Zhu et al. Citation2012; Yin et al. Citation2014). Previous studies have shown that such oxidative stress problems can be alleviated by supplementation with herbal plant (e.g. alfalfa saponin and piper sarmentosum) (Shi et al. Citation2014; Wang et al. Citation2016). Sanguinarine may have exerted its anti-oxidative function by impairing the activity of the nicotinamide adenine dinucleotide phosphate (NADPH) enzyme, which is supported by a study by Qin et al. (Citation2006), which demonstrated that sanguinarine is an enzyme inhibitor rather than an reactive oxygen species (ROS) scavenger. Liu et al. (Citation2015) confirmed that sanguinarine can inhibit NADPH oxidase 2 (NOX2) NADPH oxidase activity and ROS generation of H9c2 cardiac cells induced by Angiotensin II. Antioxidant capacities of MCE were shown in this study by measuring some antioxidant parameters in serum, such as T-AOC, SOD, GSH-Px, CAT and MDA. The SOD, GSH-Px and CAT are the main parameters used to assess oxidative status in the enzymatic system. The SOD is a group of metalloenzymes that protect cells from superoxide radicals by degrading the superoxide radicals into hydrogen peroxide (Hao et al. Citation2015), and the CAT decompose hydrogen peroxide into water (Desagher et al. Citation1996). The GSH-Px can catalyze the reduction reaction of lipid peroxides caused by reduced glutathione, which plays a role in the cell membrane protection (Johnson et al. Citation2003). The T-AOC level indicates total antioxidative capacity and reflects the nonenzymatic antioxidant defense system (Tao et al. Citation2014). The MDA is one of the most frequently used indicators of lipid peroxidation. Increased MDA is the consequence of cellular membrane damage initially caused by increased formation of radicals (Niedernhofer et al. Citation2003). Supplementation of laying hens diet with sanguinarine decreased serum MDA concentration (Bavarsadi et al. Citation2016). Our results indicated that MCE supplementation can contribute to the improvement of antioxidant capacity for counteracting the oxidative stress caused by weaning.
Generally, microflora will maintain a dynamic equilibrium with each other in order to maintain normal physiological function in the host (Wang et al. Citation2009). It has been shown that sanguinarine was a potent inhibitor of E.coli, Aeromonas hydrophila, and Salmonella aureus infection (Miao et al. Citation2011). Dietary supplementation with MCE increased the amount of lactic-acid bacteria in the ileal and cecal contents in broiler chickens (Juskiewicz et al. Citation2011). Administering sanguinarine in drinking water reduced the Salmonella enteritidis count in the cecum of broilers (Pickler et al. Citation2013). In addition, the administration of sanguinarine suppressed ileal counts of E.coli and Salmonella spp. in laying hens (Bavarsadi et al. Citation2016). In the present study, we found that piglets fed diets supplemented with MCE increased the amount of the beneficial microorganisms Lactobacillus spp., and decreased those of the harmful microorganism E. coli and Salmonella spp. Other studies on medicinal plant additives have shown a reduction of Streptococcus spp. and Clostridium Cluster XIVa in pigs fed polyphenol-rich plant products (Fiesel et al. Citation2014). This suggested that MCE has the potential to resist harmful intestinal microflora and enhanced beneficial microflora, thus maintaining intestinal microfloral homeostasis (Zhao et al. Citation2018). Therefore, supplementation with MCE may improve the intestinal microflora and decreased diarrhea rates, leading to enhance growth performance and health status.
It has been reported that dietary sanguinarine is not metabolized into potentially harmful benz[c]acridine and passes along the small intestine with almost no absorption (Psotova et al. Citation2006). In this study, an increase in villus height and the ratio of villus height to crypt depth in the duodenum, jejunum and ileum was observed in piglets fed 50 mg/kg MCE after weaning compared with control diet. Similar results were also reported by Bavarsadi et al. (Citation2016), supplementation of laying hens diets with sanguinarine (3.75 and 7.5 mg/kg) increased the ratio of villus height to crypt depth and decreased crypt depth in the jejunum. Moreover, MCE supplementation at 60 mg/kg post-weaning piglets diets increased villus length (Goodarzi et al. Citation2018). Furthermore, broiler chickens fed diets supplemented with 20 mg/kg MCE had increased relative jejunal and ileal lengths (Lee et al. Citation2015). In addition, supplementation with isoquinoline alkaloids also reduced the occurrence of lesions in the duodenum, jejunum, and ileum of broiler chickens that were challenged with Eimeria spp. (Xue et al. Citation2017). Neither sinus dystrophy nor inflammation of the mucosal epithelium was observed when pigs were given a diet supplemented with either 2 ppm (0.0002%) pure sanguinarine or 100 ppm (0.01%) of a sanguinarine preparation (Kosina et al. Citation2004). Commercial products that contain QBAs (primarily sanguinarine) exhibit the antimicrobial properties that are inherent to those QBAs (Lenfeld et al. Citation1981). Sanguinarine improved the time of recovery of the gut walls from infections (e.g. clostridia and E. coli) by 60% within hours of the first treatment (Mellor Citation2001), indicating that sanguinarine inhibited the action of harmful bacteria in the intestinal wall and reduced the production of toxic compounds and damage to intestinal epithelial cells, thereby protecting the intestinal mucosa (Yakhkeshi et al. Citation2011). Therefore, supplemented with MCE had higher villus height and lower crypt depth at the small intestinal mucosa could result in the low diarrhea and good growth performance.
5. Conclusions
In conclusion, the present data demonstrated that supplementation of weaning piglet diets with MCE improved growth performance, reduced rate of diarrhea among supplemented weanlings. These MCE effects were associated with increased serum IgG and changes in intestinal morphology and function including increased villus height, greater intestinal antioxidant activity, and a higher proportion of beneficial bacteria. Together findings support that MCE supplementation may be a good alternative to adding antibiotics to weanling piglet diet.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Jiashun Chen http://orcid.org/0000-0002-4474-6803
Additional information
Funding
References
- Bavarsadi M , Mahdavi AH , Ansari-Mahyari S , Jahanian E. 2016. Effects of different levels of sanguinarine on antioxidant indices, immunological responses, ileal microbial counts and jejunal morphology of laying hens fed diets with different levels of crude protein. J Anim Physiol Anim Nutr. 101:936–948. doi: 10.1111/jpn.12528
- Boudry G , Péron V , HuërouLuron IL , Lallès JP , Sève B. 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 134:2256–2262. doi: 10.1093/jn/134.9.2256
- Chaturvedi MM , Kumar A , Darnay BG , Chainy GB , Agarwal S , Aggarwal BB. 1997. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-kappa B activation, Ikappa B alpha phosphorylation, and degradation. J Biol Chem. 272:30129–30134. doi: 10.1074/jbc.272.48.30129
- Chen YP , Cheng YF , Li XH , Zhang H , Yang WL , Wen C , Zhou YM. 2016. Dietary palygorskite supplementation improves immunity, oxidative status, intestinal integrity, and barrier function of broilers at early age. Anim Feed Sci Technol. 219:200–209. doi: 10.1016/j.anifeedsci.2016.06.013
- Chen JS , Kang BJ , Zhao YR , Yao K , Fu CX. 2018. Effects of natural dietary supplementation with Macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets. J Anim Physiol Anim Nutr. 102:1666–1674. doi: 10.1111/jpn.12976
- Desagher S , Glowinski J , Premont J. 1996. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996
- Drsata J , Ulrichová J , Walterová D. 1996. Sanguinarine and chelerythrine as inhibitors of aromatic amino acid decarboxylase. J Enzyme Inhib. 10:231–237. doi: 10.3109/14756369609036530
- Fiesel A , Gessner DK , Most E , Eder K. 2014. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet Res. 10:196. doi: 10.1186/s12917-014-0196-5
- Fleury MA , Jouy E , Eono F , Cariolet R , Couet W , Gobin P , Le Goff O , Blanquet-Diot S , Alric M , Kempf I. 2016. Impact of two different colistin dosing strategies on healthy piglet fecal microbiota. Res Vet Sci. 107:152–160. doi: 10.1016/j.rvsc.2016.06.003
- Frydendahl K. 2002. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet Microbiol. 85:169–182. doi: 10.1016/S0378-1135(01)00504-1
- Gessner DK , Ringseis R , Eder K. 2017. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr. 101:605–628. doi: 10.1111/jpn.12579
- Goodarzi BF , Manner K , Zentek J. 2018. The impacts of Macleaya cordata extract and naringin inclusion in post-weaning piglet diets on performance, nutrient digestibility and intestinal histomorphology. Arch Anim Nutr. 72:178–189. doi: 10.1080/1745039X.2018.1459342
- Gudev D , Popova RS , Moneva P. 2004. Effect of supplemental Sangrovit on some biochemical indices and leukocytes phagocytic activity in growing pigs. Arch Zootec. 7:123–134.
- Hao R , Li Q , Zhao J , Li H , Wang W , Gao J. 2015. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest Sci. 178:237–242. doi: 10.1016/j.livsci.2015.06.004
- Jankowski J , ZduńCzyk Z , Juśkiewicz J , Kozłowski K , Lecewicz A , Jeroch H. 2009. Gastrointestinal tract and metabolic response of broilers to diets with the Macleaya cordata alkaloid extract. Arch Geflügelk. 73:95–101.
- Jeroch H , Kozlowski K , Jeroch J , Lipinski K , Zduńczyk Z , Jankowski J. 2009. Efficacy of the phytogenic (Papaveraceae) additive Sangrovit® in growing monogastric animals. Züchtungskunde. 81:279–293.
- Johnson RJ , Kang DH , Feig D , Kivlighn S , Kanellis J , Watanabe S , Tuttle KR , Rodriguez-Iturbe B , Herrera-Acosta J , Mazzali M. 2003. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5
- Juskiewicz J , Gruzauskas R , Zdunczyk Z , Semaskaite A , Jankowski J , Totilas Z , Jarule V , Sasyte V , Zdunczyk P , Raceviciute-stupeliene A , et al. 2011. Effects of dietary addition of Macleaya cordata alkaloid extract on growth performance, caecal indices and breast meat fatty acids profile in male broilers. J Anim Physiol Anim Nutr. 95:171–178. doi: 10.1111/j.1439-0396.2010.01037.x
- Kantas D , Papatsiros VG , Tassis PD , Athanasiou LV , Tzika ED. 2015. Effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim Sci J. 86:92–98. doi: 10.1111/asj.12240
- Kim JC , Hansen CF , Mullan BP , Pluske JR. 2012. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 173:3–16. doi: 10.1016/j.anifeedsci.2011.12.022
- Kolpin DW , Furlong ET , Meyer MT , Thurman EM , Zaugg SD , Barber LB , Buxton HT. 2002. Pharmaceuticals, Hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: a national reconnaissance. Environ Sci Technol. 36:1202–1211. doi: 10.1021/es011055j
- Kommera SK , Mateo RD , Neher FJ , Kim SW. 2006. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian-Aust J Anim Sci. 19:1784–1789. doi: 10.5713/ajas.2006.1784
- Kong XF , Wu GY , Liao YP , Hou ZP , Liu HJ , Yin FG , Li TJ , Huang RL , Zhang YM , Deng D. 2007. Dietary supplementation with Chinese herbal ultra-fine powder enhances cellular and humoral immunity in early-weaned piglets. Livest Sci. 108:94–98. doi: 10.1016/j.livsci.2007.01.002
- Kosina P , Gregorova J , Gruz J , Vacek J , Kolar M , Vogel M , Roos W , Naumann K , Simanek V , Ulrichova J. 2010. Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia. 81:1006–1012. doi: 10.1016/j.fitote.2010.06.020
- Kosina P , Walterová D , Ulrichová J , Lichnovský V , Stiborová M , Rýdlová H , Vičar J , Krečman VR , Brabec MJ , Šimánek VM. 2004. Sanguinarine and chelerythrine: assessment of safety on pigs in ninety days feeding experiment. Food Chem Toxicol. 42:85–91. doi: 10.1016/j.fct.2003.08.007
- Kraler M , Ghanbari M , Domig KJ , Schedle K , Kneifel W. 2016. The intestinal microbiota of piglets fed with wheat bran variants as characterised by 16S rRNA next-generation amplicon sequencing. Arch Anim Nutr. 70:173–189. doi: 10.1080/1745039X.2016.1160534
- Lauridsen C. 2010. Evaluation of the effect of increasing dietary vitamin E in combination with different fat sources on performance, humoral immune responses and antioxidant status of weaned pigs. Anim Feed Sci Technol. 158:85–94. doi: 10.1016/j.anifeedsci.2010.03.015
- Lee KW , Kim JS , Oh ST. 2015. Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J Poult Sci. 52:15–22. doi: 10.2141/jpsa.0140073
- Lenfeld J , Kroutil M , Marsálek E , Slavík J , Preininger V , Simánek V. 1981. Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonium majus. Planta Med. 43:161–165. doi: 10.1055/s-2007-971493
- Li LL , Wu X , Peng HZ , Fan MZ , Hou ZP , Kong XF , Yin YL , Zhang B , Li TJ , Hou YQ , et al. 2009. The effect of dietary addition of a polysaccharide from Atractylodes macrophala Koidz on growth performance, immunoglobulin concentration and IL-1β expression in weaned piglets. J Agr Sci. 147:625–631. doi: 10.1017/S002185960999013X
- Li LL , Yin FG , Zhang B , Peng HZ , Li FN , Zhu NS , Hou DX , Yin YL , Luo JJ , Tang ZR. 2011. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livest Sci. 142:33–41. doi: 10.1016/j.livsci.2011.06.013
- Liu G , Guan G , Fang J , Martinez Y , Chen S , Bin P , Duraipandiyan V , Gong T , Tossou MC , Al-Dhabi NA , et al. 2016. Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets. Biomed Res Int. 2016:1069585.
- Liu Y , Jiao R , Ma ZG , Liu W , Wu QQ , Yang Z , Li FF , Yuan Y , Bian ZY , Tang QZ. 2015. Sanguinarine inhibits angiotensin II-induced apoptosis in H9c2 cardiac cells via restoring reactive oxygen species-mediated decreases in the mitochondrial membrane potential. Mol Med Rep. 12:3400–3408. doi: 10.3892/mmr.2015.3841
- Mellor S. 2001. Natural appetisers from plants. Feed Mix. 9:29–31.
- Metzler-Zebeli BU , Schmitz-Esser S , Mann E , Grüll D , Molnar T , Zebeli Q. 2015. Adaptation of the cecal bacterial microbiome of growing pigs in response to resistant starch type 4. Appl Environ Microbiol. 81:8489–8499. doi: 10.1128/AEM.02756-15
- Miao F , Yang XJ , Zhou L , Hu HJ , Zheng F , Ding XD , Sun DM , Zhou CD , Sun W. 2011. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat Prod Res. 25:863–875. doi: 10.1080/14786419.2010.482055
- Nagy B , Arp LH , Moon HW , Casey TA. 1992. Colonization of the small intestine of weaned pigs by enterotoxigenic Escherichia coli that lack known colonization factors. Vet Pathol. 29:239–246. doi: 10.1177/030098589202900308
- Newton SM , Lau C , Gurcha SS , Besra GS , Wright CW. 2002. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria Canadensis . J Ethnopharmacol. 79:57–67. doi: 10.1016/S0378-8741(01)00350-6
- Ni H , Martínez Y , Guan G , Rodríguez R , Más D , Peng H , Navarro MV , Gang L. 2016. Analysis of the impact of isoquinoline alkaloids, derived from Macleaya cordata extract, on the development and innate immune response in swine and poultry. Biomed Res Int. 2016:1352146. doi: 10.1155/2016/1352146
- Niedernhofer LJ , Daniels JS , Rouzer CA , Greene RE , Marnett LJ. 2003. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 278:31426–31433. doi: 10.1074/jbc.M212549200
- Niu X , Fan T , Li W , Wei X , Huang H. 2012. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Eur J Pharmacol. 689:262–269. doi: 10.1016/j.ejphar.2012.05.039
- NRC . 2012. Nutrient requirements of swine, 11th rev. ed. Washigton (DC ): Natl. Acad. Press.
- Pellikaan WF , Andrés-Elias N , Durand A , Bongers LJGM , SvL-v S , Torrallardona D. 2010. Effect of carob bean gum, spray dried porcine plasma and sanguinarine on fermentation activity in the gut of weanling pigs. Livest Sci. 133:164–168. doi: 10.1016/j.livsci.2010.06.054
- Pickler L , Beirão BCB , Hayashi RM , Durau JF , Lourenço MC , Caron LF , Santin E. 2013. Effect of sanguinarine in drinking water on Salmonella control and the expression of immune cells in peripheral blood and intestinal mucosa of broilers. J Appl Poultry Res. 22:430–438. doi: 10.3382/japr.2012-00649
- Pieper R , Janczyk P , Zeyner A , Smidt H , Guiard V , Souffrant WB. 2008. Ecophysiology of the developing total bacterial and lactobacillus communities in the terminal small intestine of weaning piglets. Microb Ecol. 56:474–483. doi: 10.1007/s00248-008-9366-y
- Pluske JR. 2013. Feed-and feed additives-related aspects of gut health and development in weanling pigs. J Anim Sci Biotechnol. 4:83–89. doi: 10.1186/2049-1891-4-1
- Pluske JR , Hampson DJ , Williams IH. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2
- Psotova J , Vecera R , Zdarilova A , Anzenbacherova E , Kosina P , Svobodova A , Hrbac J , Jirovsky D , Stiborova M , Lichnovsky V. 2006. Safety assessment of sanguiritrin, alkaloid fraction of Macleaya cordata, in rats. Vet Med. 51:145–155. doi: 10.17221/5534-VETMED
- Qi H , Xiang Z , Han G , Yu B , Huang Z , Chen D. 2011. Effects of different dietary protein sources on cecal microflora in rats. Afr J Biotechnol. 10:3704–3708.
- Qin F , Patel R , Yan C , Liu W. 2006. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free. Radical Bio Med. 40:236–246. doi: 10.1016/j.freeradbiomed.2005.08.010
- Schwarz S , Kehrenberg C , Walsh TR. 2001. Use of antimicrobial agents in veterinary medicine and food animal production. Int J Antimicrob Agents. 17:431–437. doi: 10.1016/S0924-8579(01)00297-7
- Shi YH , Wang J , Guo R , Wang CZ , Yan XB , Xu B , Zhang DQ. 2014. Effects of alfalfa saponin extract on growth performance and some antioxidant indices of weaned piglets. Livest Sci. 167:257–262. doi: 10.1016/j.livsci.2014.05.032
- Spreeuwenberg MA , Verdonk JM , Gaskins HR , Verstegen MW. 2001. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J Nutr. 131:1520–1527. doi: 10.1093/jn/131.5.1520
- Sun P , Li D , Li Z , Dong B , Wang F. 2008. Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. J Nutr Biochem. 19:627–633. doi: 10.1016/j.jnutbio.2007.08.007
- Tanaka T , Metori K , Mineo S , Hirotani M , Furuya T , Kobayashi S. 1993. Inhibitory effects of berberine-type alkaloids on elastase. Planta Med. 59:200–202. doi: 10.1055/s-2006-959651
- Tao X , Xu ZR , Wang YZ. 2014. Effects of dietary fluoride levels on growth, serum indexes and antioxidant systems in growing pigs. Turk J Vet Anim Sci. 30:65–70.
- Vieira SL , Berres J , Reis RN , Oyarzabal OA , Coneglian JLB , Freitas DM , Peña JEM , Torres CA. 2008. Studies with sanguinarine like alkaloids as feed additive in broiler diets. Brazi J Poult Sci. 10:67–71. doi: 10.1590/S1516-635X2008000100010
- Wang D , Ma W , She R , Sun Q , Liu Y , Hu Y , Liu L , Yang Y , Peng K. 2009. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult Sci. 88:967–974. doi: 10.3382/ps.2008-00533
- Wang M , Yang J , Gai Z , Huo S , Zhu J , Li J , Wang R , Xing S , Shi G , Shi F. 2017. Comparison between digital PCR and real-time PCR in detection of Salmonella typhimurium in milk. Int J Food Microbiol. 266:251–256. doi: 10.1016/j.ijfoodmicro.2017.12.011
- Wang DF , Zhou LL , Zhou HL , Hou GY , Zhou X , Li W. 2016. Effects of Piper sarmentosum extract on the growth performance, antioxidant capability and immune response in weaned piglets. J Anim Physiol Anim Nutr. 101:105–112. doi: 10.1111/jpn.12517
- Wittig W , Klie H , Gallien P , Lehmann S , Timm M , Tschäpe H. 1995. Prevalence of the fimbrial antigens F18 and K88 and of enterotoxins and verotoxins among Escherichia coli isolated from weaned pigs. Zentralbl Bakteriol. 283:95–104. doi: 10.1016/S0934-8840(11)80895-9
- Xu Y , Wang Z , Wang Y , Yan S , Shi B. 2018. Effects of chitosan as growth promoter on diarrhea, nutrient apparent digestibility, fecal microbiota and immune response in weaned piglets. J Appl Anim Res. 46:1437–1442. doi: 10.1080/09712119.2018.1531763
- Xue GD , Wu SB , Choct M , Pastor A , Steiner T , Swick RA. 2017. Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers. Poult Sci. 96:3581–3585. doi: 10.3382/ps/pex164
- Yakhkeshi S , Rahimi S , Naseri KG. 2011. The effects of comparison of herbal extracts, antibiotic, probiotic and organic acid on serum lipids, immune response, GIT microbial population, intestinal morphology and performance of broilers. J Med Plants Res. 10:80–95.
- Yao K , Guan S , Li TJ , Huang RL , Wu GY , Ruan Z , Yin YL. 2011. Dietary L-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Brit J Nutr. 105:703–709. doi: 10.1017/S000711451000365X
- Yin J , Wu MM , Xiao H , Ren WK , Duan JL , Yang G , Li TJ , Yin YL. 2014. Development of an antioxidant system after early weaning in piglets. J Anim Sci. 92:612–619. doi: 10.2527/jas.2013-6986
- Yiu CK , Wei SH. 1993. Clinical efficacy of dentifrices in the control of calculus, plaque, and gingivitis. Quintessence Int. 24:181–188.
- Zdarilova A , Vrublova E , Vostalova J , Klejdus B , Stejskal D , Proskova J , Kosina P , Vecera R , Hrbac J , Cernochova D , et al. 2008. Natural feed additive of Macleaya cordata: safety assessment in rats a 90-day feeding experiment. Food Chem Toxicol. 46:3721–3726. doi: 10.1016/j.fct.2008.09.054
- Zhang SM , Coultas KA. 2013. Identification of plumbagin and sanguinarine as effective chemotherapeutic agents for treatment of schistosomiasis. Int J Parasitol Drugs Drug Resist. 3:28–34. doi: 10.1016/j.ijpddr.2012.12.001
- Zhang C , Ling F , Chi C , Wang GX. 2013. Effects of praziquantel and sanguinarine on expression of immune genes and susceptibility to Aeromonas hydrophila in goldfish (Carassius auratus) infected with Dactylogyrus intermedius. Fish Shellfish Immunol. 35:1301–1308. doi: 10.1016/j.fsi.2013.08.001
- Zhao P , Zhang Z , Lan R , Li T , Kim IH. 2018. Comparison of efficacy of lactic acid bacteria complex and Enterococcus faecium DSM 7134 in weanling pigs. J Appl Anim Res. 46:888–892. doi: 10.1080/09712119.2017.1420655
- Zhu LH , Zhao KL , Chen XL , Xu JX. 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 90:2581–2589. doi: 10.2527/jas.2011-4444
