ABSTRACT
This study investigated if increased doses of La Sota vaccine can increase antibody response to Newcastle disease without any serious adverse effect on the bursa. One hundred broilers aged four weeks were randomly assigned into four groups of 25 each: ZD, each drenched with phosphate-buffered saline, SS, DD and TD broilers were each drenched with single, double and triple dose of La Sota vaccine, respectively. The broilers were examined for signs and lesions. At weekly intervals, serum samples were collected post-vaccination (PV) and assayed for haemagglutination inhibition antibody. Groups DD and TD antibody titres were significantly (p < 0.05) higher than that of the SD on day 21 PV. But there was no significant (p > 0.05) difference between titres of DD and TD groups. Groups DD and TD geometrical mean titres were more than two times higher than that of SD group on day 21 PV. The bursa of all the vaccinated groups appeared reduced in size, with mild depletion of lymphocytes on day 10 PV. Generally, the integrity of the bursa was intact. This suggests that doubling the dose of La Sota vaccine may be considered in improving the performance of the vaccine in the control of velogenic Newcastle disease.
1. Introduction
Newcastle disease (ND) is one of the major diseases of poultry worldwide (Alexander et al. Citation2012). It affects a wide range of poultry, cage and wild birds (Cattoli et al. Citation2011). Chickens are the most susceptible and it is only in this specie that ND produces haemorrhagic and ulcerative lesions in the gastrointestinal tract (Igwe et al. Citation2018a). Turkeys are less susceptible (Okoroafor et al. Citation2018). Ducks, quails, and geese are resistant (Wakamatsu et al. Citation2006; Anis et al. Citation2013; Igwe et al. Citation2014) but constitute sources of infection to other poultry birds. ND is caused by the pathogenic strains of Newcastle disease virus (NDV) which is an avian paramyxovirus belonging to the genus Avulavirus, in the family Paramyxoviridae and order Mononegavirales (Lamb and Parks Citation2013). There are three pathotypes of the virus. The lentogenic pathotype includes the mild and nonpathogenic strains of the virus which are used in production of vaccines such as La Sota and Hitchner-B1 (H-B1). The mesogenic pathotype includes the moderately pathogenic which are also used in producing vaccines such as Komarov and Mukteswar, depending on the disease situation and national requirements. They cause mainly the respiratory form of ND. The velogenic pathotype causes the most devastating form of ND with mortalities of 70% to 100% within 7 days in susceptible chickens (Igwe et al. Citation2018a). The isolates are found worldwide in poultry and wild birds. They are enzootic in Africa, Middle East, Asia and some countries of Central and South America (Echeonwu et al. Citation1993; Liu et al. Citation2003; Perozo et al. Citation2008; Solomon et al. Citation2012) and they constitute a major impediment to poultry production in these areas. But it is exotic in North America and Europe. It is a World Animal Health Organisation (Office International des Epizooties (OIE)) reportable disease (OIE Citation2017). Outbreaks of the disease cause huge economic losses due to high mortalities, loss of egg production, high cost of stamping out policy used in controlling the disease in countries where it is exotic and the trade embargo placed on animal products by international community (OIE Citation2012). Control of ND is by vaccination using inactivated and live vaccines, and biosecurity. The La Sota vaccine has been effective in the control of ND caused by the mesogenic viruses in Europe and America. But there have been several reports that this vaccine is not effective in the control of ND caused by velogenic NDV (vNDV). Therefore, the aim of this study was to investigate the effects of increased doses of La Sota vaccine on the antibody response in broiler chickens.
2. Materials and Methods
2.1. Broiler chickens
One hundred Cobb broiler chicks were purchased at one-day-old from a reputable local commercial hatchery and randomly assigned into four groups of 25 broilers each. They were kept in isolation in the departmental facility. Brooding was on deep litter. Feed and water were supplied ad libitum and the broilers were not vaccinated against any disease. General care of the birds was provided in accordance with the Institutional Animal Care and Use Committee, as outlined in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (https://www.aaalac.org/about/Ag_Guide_3rd_ed.pdf).
2.2. La Sota vaccine and vaccination
The vaccine was manufactured and obtained from the National Veterinary Research Institute, Vom, Plateau State, Nigeria. It had a medium embryo infective dose (EID50) of 106.2 per ml. At the age of four weeks, the randomly assigned four groups were named ZD (Zero dose) group, SD (Single dose) group, DD (Double dose) group and TD (Triple dose) group. Each broiler in the ZD group received 0.5 ml of the phosphate-buffered saline (PBS) used in dissolving the vaccine orally as placebo. The SD broilers each was drenched with single dose of the vaccine in 0.5 ml. Each of the broilers in groups DD and TD received double and triple doses of the vaccine in 0.5 ml by drenching, respectively.
2.3. Clinical and Pathology Examinations
The chickens were observed twice daily for clinical signs, and on days 3, 6, 10 post-vaccination (PV), three chickens in each group were sacrificed and examined for gross lesions. Samples of the bursa of Fabricius (which will later be called bursa for the sake of clarity) were fixed in 10% formal saline for minimum of 24 hours, processed, embedded in paraffin wax, sectioned and stained with haematoxylin and eosin (H&E) using the method of Suvarna et al. (Citation2018). The slides were studied under the light microscope.
2.4. Serology
One ml of blood was collected from each of 10 chickens randomly selected in each group on days 0, 7, 14 and 21 PV. Sera were harvested and assayed for haemagglutination inhibition (HI) antibody titers using the method of OIE (Citation2012). The antigen used was a suspension of La Sota vaccine in PBS. The geometrical mean titres (GMT) were calculated using the Tube Method of Villegas (Citation2008).
2.5. Statistical analysis
The HI data were analysed using the one-way analysis of variance (ANOVA). Variant means were separated post hoc using the least significant difference method (Okafor Citation1992). All tests were performed with 5% level of significance (p < 0.05).
3. Results
3.1. Clinical signs and lesions
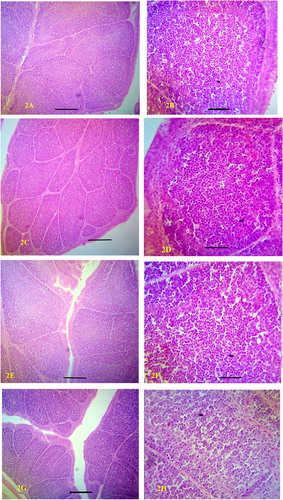
No clinical sign was observed in all the groups. Grossly the size of the bursa was normal on days 3 and 6 PV ((A,B)). But there was reduction in size of the bursa in all the vaccinated groups on day 10 PV ((C)). Histopathological sections of the organ showed mild inflammatory cellular infiltration of the bursa on day 3 PV in all the vaccinated groups. Mild depletion of lymphocytes which did not alter the normal architecture of the organ was observed on day10 PV in the SD, DD and TD groups ((A–H)).
Figure 1. (A). Bursa of broilers on day 3 PV showing no difference in sizes. (B). Bursa of broilers on day 6 PV showing no difference in sizes. (C). Bursa of broilers on day 10 PV. The bursa of the ZD group were bigger in size than the others. ZD = Zero dose SD = Single dose DD = Double dose TD = Triple dose.

Figure 2. (A). ZD bursa showing normal architecture on day 3 PV. H&E Bar = 200 micrometers. (B). ZD bursa showing normal lymphocytic population on day 3 PV. H&E Bar = 50 micrometers. (C). TD bursa showing normal architecture on day 3 PV. H&E Bar = 200 micrometers. (D). TD bursa showing normal lymphocytic population on day 3 PV. H&E Bar = 50 micrometers. (E). ZD bursa showing normal architecture on day 10 PV. H&E Bar = 200 micrometers. (F). ZD bursa showing normal population of lymphocytes on day 10 PV. H&E Bar = 50 microm. (G). TD bursa showing normal architecture on day 10 PV. H&E Bar = 200 microm. (H). TD bursa showing mild depletion of lymphocytes on day10 PV. H&E. Bar = 50 microm. ZD = Zero dose TD = Triple dose.
3.2 Serology
The HI antibody titres were significantly (p < 0.5) higher in the DD and TD groups than the SD on day 21 PV (). The GMT figures are not subjected to statistical analysis (). Throughout the experiment, titres of the control group chickens, ZD, remained negative.
Figure 3. Newcastle disease haemagglutination inhibition antibody titres in the broilers. A = Zero dose (ZD) group B = Single dose (SD) group. C = Double dose (DD) group D = Triple dose (TD) group.
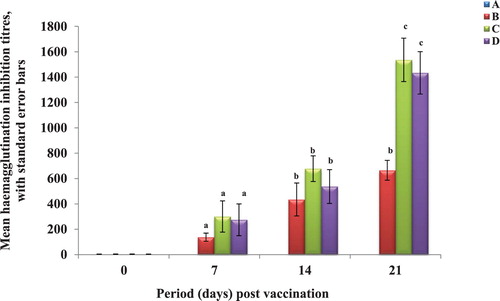
Table 1. The haemagglutination inhibition Newcastle antibody responses in geometrical mean titres (GMT).
4. Discussion
It has been reported that La Sota vaccine prevented the development of clinical signs but not the replication and shedding of the vNDV in the faeces, eggs and mouth of the vaccinated chickens and turkeys (Miller et al. Citation2007, Citation2013; Sá E Silva et al. Citation2016; Okoroafor et al. Citation2018; Wajid et al. Citation2018). This would result to environmental contamination and spread of the virus. Vaccines which were antigenically and genetically related to the challenge virus reduced viral shedding (Wajid et al. Citation2018). La Sota vaccine did not prevent a significant drop in egg production when laying chickens were infected with vNDV (Bwala et al. Citation2012; Igwe et al. Citation2018b) This causes huge economic losses to egg producers especially in countries where the virus is enzootic. Drastic fall in or complete cessation of egg production may be the only clinical sign of vNDV infection in vaccinated layers. La Sota vaccination did not prevent atrophy and depletion of lymphocytes in the lymphoid organs of chickens and turkeys infected with vNDV and this can lead to immunosuppression (Ezema et al. Citation2009, Citation2016; Okoroafor et al. Citation2018). Because economic losses from vNDV infection play an important role for poultry production, in this study, we investigated, if increased doses of La Sota vaccine can increase antibody response to Newcastle disease without any serious adverse effect on the bursa.
All NDV strains belong to the same serotype. Over the years the virus as an RNA virus has been evolving. Based on genetic characteristics NDV has been grouped into Class I and Class II viruses. Class I viruses are found mainly in wild birds and are of low virulence. Class II viruses have at least 18 genotypes with 7 genotypes already identified in Nigeria (Welch et al. Citation2019). Virulent strains of Class II viruses are frequently isolated in chickens and pet birds (Absalón et al. Citation2019). In order to improve protection by La Sota vaccination, there is need to find out if these genetic differences have any significant effect on the neutralizing antibody-antigen binding sites. Rohollahzadeh et al. (Citation2018) reported that administration of thermostable I-2 and B1 Newcastle vaccines could protect broilers against acute NDV infection and there were significant increments of CD4+ and CD8+ expression in the interstitial and tracheal tissues together with ascending interferon levels in the sera of vaccinated group when compared with the unvaccinated. Probiotic cultures and enzymes mixture did not significantly affect the titres of antibodies against avian influenza and Newcastle disease virus at day 42 post-infection (Seidavi et al. Citation2017). Poorghasemi et al. (Citation2015) observed that dietary fat source had no impact on (p > 0.05) humoural immune response to sheep red blood cell injections, NDV, infectious bursal disease virus and infectious bronchitis virus vaccinations in broilers. La Sota vaccine administered orally, did not cause clinical signs in all the vaccinated groups of broilers in this experimental study. Our results are in agreement with previous reports, that La Sota, a commonly used NDV live vaccine and also a lentogenic strain from genotype II, does not cause morbidity and mortality in chickens (Cornax et al. Citation2012; Rohollahzadeh et al. Citation2018).
The current ND vaccination programme in Nigeria and some African countries is H-B1 at one-day-old, La Sota at three weeks of age, Komarov at six weeks of age and point of lay followed by La Sota re-vaccinations. Apart from the fact that this programme has not successfully controlled outbreaks of vNDV infections and huge losses in egg production, the Komarov vaccine is now phased out in developed countries where poultry production has metamorphosed into large industries where millions of chickens of the same age are assembled in one farm. The Komarov vaccine is administered in the breast muscle and requires individual handling of the chickens which is not possible in large poultry operations. For large poultry operations in countries where vNDV is enzootic, the solution may lie in having another look at the dose and vaccination programme of the La Sota vaccine. This project has shown that increasing the dose of the La Sota vaccine will increase the level of antibody response in broilers significantly. But there was no significant difference between the responses of DD and TD groups. This means that increasing the dose beyond double is of no benefit. This agrees with the observations of Cornax et al. (Citation2012) who reported that protection due to vaccination increased with vaccine titres until a threshold titre is reached beyond which little or no further benefit is elucidated. But they did not study the effect of the increased vaccine doses on the bursa. The GMT data showed that by day 21 PV, the GMTs of DD and TD groups were more than two times higher than that of the SD group.
Early experiments revealed that the bursa (controls humoural immunity) in the chicken grows rapidly after hatching, reaches maximum size between 4 and 12 weeks of age (Taylor and McCorkle Citation2009; Abdul-Aziz et al. Citation2016). The bursa contains lymphoid follicles whose medulla and cortex is separated by a layer of undifferentiated epithelial cells (intrafollicular epithelium) which give rise to bursa-derived (B) lymphocytes, the precursor cells of the antibody-synthesizing plasma cells (Cooper Citation2015; Abdul-Aziz et al. Citation2016). The study of the bursa was to ensure that the various doses of the vaccine had no serious adverse effect on the bursa as this may affect subsequent immune responses in the vaccinated chickens. On day 10 PV, grossly, the bursa of all the vaccinated groups appeared reduced in size with mild depletion of lymphocytes histologically which can be regenerated by intrafollicular epithelium in few weeks. The mild depletion of lymphocytes could indicate that La Sota vaccine when inoculated during the plateau phase of bursa growth triggered systemic immune response which is important in the development of ND immunity. These results could be explained as a common histological change that is generated by immune responses. Cooper Citation2015 reported that the immune responses include B cells, which secrete immunoglobulins of different classes and T cells, which play a role in cell-mediated immunity. represented by the circulating pool of lymphocytes, and do not synthesize antibodies but instead release various mediators upon interaction with the antigen. The antibody response to most antigens requires cooperation between T and B cells and macrophages as well. Histologically the bursa maintained its architecture in the present study and it can be said that all the doses used produced no adverse effects on the organ. This was not unexpected because La Sota vaccine virus is a lentogenic pathotype.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Amarachukwu O. Igwe http://orcid.org/0000-0003-0726-2962
Didacus C. Eze http://orcid.org/0000-0003-2773-2415
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Abdul-Aziz T , Fletcher OJ , Barnes HJ , Shivaprasad HL , Swayne DE , Riddell C. 2016. Avian histopathology. 4th ed. Jacksonville (FL ): American Association of Avian Pathologists.
- Absalón AE , Cortés-Espinosa DV , Lucio E , Miller PJ , Afonso CL. 2019. Epidemiology, control, and prevention of Newcastle disease in endemic regions: Latin America. Trop Anim Health Prod. 51:1033–1048. doi: 10.1007/s11250-019-01843-z
- Alexander DJ , Aldous EW , Fuller CM. 2012. The long view: a selective review of 40 years of Newcastle disease research. Avian Pathol. 41:329–335. doi: 10.1080/03079457.2012.697991
- Anis Z , Morita T , Azuma K , Ito H , Ito T , Shimada A. 2013. Histopathological alterations in immune organs of chickens and ducks after experimental infection with virulent 9a5b Newcastle disease virus. J Comp Pathol. 149:82–93. doi: 10.1016/j.jcpa.2012.09.011
- Bwala DG , Clift S , Duncan NM , Bisschop SPR , Oludayo FF. 2012. Determination of the distribution of lentogenic vaccine and virulent Newcastle disease virus antigen in the oviduct of SPF and commercial hen using immunohistochemistry. Res Vet Sci. 93:520–528. doi: 10.1016/j.rvsc.2011.06.023
- Cattoli G , Susta L , Terregino C , Brown C. 2011. Newcastle disease: a review of field recognition and current methods of laboratory detection. J Vet Diagn Invest. 23:637–656. doi: 10.1177/1040638711407887
- Cooper MD. 2015. The early history of B cells. Nat Rev Immunol. 15:191–197. doi: 10.1038/nri3801
- Cornax I , Miller PJ , Afonso CL. 2012. Characterization of live LaSota vaccine strain–induced protection in chickens upon early challenge with a virulent Newcastle disease virus of heterologous genotype. Avian Dis. 56:464–470. doi: 10.1637/10043-122011-Reg.1
- Echeonwu GON , Ireogbu CI , Emeruwa AC. 1993. Recovery of velogenic Newcastle disease virus from dead and healthy free roaming birds in Nigeria. Avian Pathol. 22:383–387. doi: 10.1080/03079459308418928
- Ezema WS , Eze DC , Shoyinka SVO , Okoye JOA. 2016. Atrophy of lymphoid organs and suppression of antibody response caused by velogenic Newcastle disease virus infection in chickens. Trop Anim Health Prod. 48:1703–1709. doi: 10.1007/s11250-016-1147-x
- Ezema WS , Okoye JOA , Nwanta JA. 2009. La Sota vaccination may not protect against the lesions of velogenic Newcastle disease in chickens. Trop Anim Health Prod. 41:477–484. doi: 10.1007/s11250-008-9210-x
- Igwe AO , Afonso CL , Ezema WS , Brown CC , Okoye JOA. 2018b. Pathology and distribution of velogenic viscerotropic Newcastle disease virus in the reproductive system of vaccinated and unvaccinated laying hens (Gallus gallus domesticus) by immunohistochemical labelling. J Comp Pathol. 159:36–48. doi: 10.1016/j.jcpa.2017.12.009
- Igwe AO , Ezema WS , Eze DC , Okoye JOA. 2014. Experimental velogenic Newcastle disease can be very severe and viscerotropic in chickens but moderate and neurotropic in guinea fowl. Int J Poult Sci. 13:582–590. doi: 10.3923/ijps.2014.582.590
- Igwe AO , Shittu I , Okoye JOA. 2018a. Response of cyclophosphamide-treated broiler chickens to challenge with velogenic Newcastle disease virus. J Appl Anim Res. 46:938–946. doi: 10.1080/09712119.2018.1434078
- Lamb R , Parks G. 2013. Paramyxoviridae. In: DM Knipe , PM Howley , editors. Fields virology. Philadelphia (PA ): Lippincott Williams & Wilkins; p. 957–995.
- Liu XF , Wan HQ , Ni XX , Wu YT , Liu WB. 2003. Pathotypical and genotypical characterization of strains of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1985–2001. Arch Virol. 148:1387–1403.
- Miller PJ , Afonso CL , El Attrache J , Dorsey KM , Courtney SC , et al . 2013. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Dev Comp Immunol. 41:505–513. doi: 10.1016/j.dci.2013.06.007
- Miller PJ , King DJ , Afonso CL , Suarez DL. 2007. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine. 25:7238–7246. doi: 10.1016/j.vaccine.2007.07.017
- [OIE] Office International des Epizooties . 2012. Manual of diagnostic tests and vaccines for terrestial animals. Newcastle disease. In: Marian Truszczynski, editor. OIE standard commission publication, office international epizooties, 2012 version, part 2, Section 2.3, chapter 2.3.14. p. 555–573.
- [OIE] Office International des Epizooties . 2017. http://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases2017/.
- Okafor LC. 1992. Biometry – basic principles and approaches. Onitsha. Nigeria: Geelink. 141–172.
- Okoroafor OA , Eze PC , Ezema WS , Nwosu C , Okorie-Kanu C , Animoke PC , Anene B , Okoye JOA . 2018. La Sota vaccination may not protect against virus shedding and lesions of velogenic Newcastle disease in commercial turkeys. Trop Anim Health Prod. 50:345–351. doi: 10.1007/s11250-017-1439-9
- Perozo F , Merino R , Afonso CL , Villegas P , Calderon N. 2008. Biological and phylogenetic characterization of virulent Newcastle disease virus circulating in Mexico. Avian Dis. 52:472–479. doi: 10.1637/8276-022908-Reg.1
- Poorghasemi M , Seidavi A , Qotbi AAA , Chambers JR , Laudadio V , Tufarelli V. 2015. Effect of dietary fat source on humoral immunity response of broiler chickens. Europ Poult Sci (Archiv Fur Geflugelkunde). 79:1–8.
- Rohollahzadeh H , Nili H , Asasi K , Mokhayeri S , Najjari AHA. 2018. Respiratory and GIT tract immune responses of broiler chickens following experimental infection with Newcastle disease’s virus. Comp Clin Path. 27:1241–1255. doi: 10.1007/s00580-018-2728-z
- Sá E Silva M , Susta L , Moresco K , Swayne DE. 2016. Vaccination of chickens decreased Newcastle disease virus contamination in eggs. Avian Pathol. 45:38–45. doi: 10.1080/03079457.2015.1112876
- Seidavi A , Dadashbeiki M , Alimohammadi-Saraei MH , van den Hoven R , Payan-Carreira R , Laudadio V , Tufarelli V. 2017. Effects of dietary inclusion level of a mixture of probiotic cultures and enzymes on broiler chickens immunity response. Environ Sci Pollut Res Int. 24:4637–4644. doi: 10.1007/s11356-016-8206-8
- Solomon P , Abolnik C , Joannis TM , Bisschop S. 2012. Virulent Newcastle disease virus in Nigeria: identification of a new clade of sub-lineage 5f from livebird markets. Virus Genes. 44:98–103. doi: 10.1007/s11262-011-0678-5
- Suvarna KS , Layton C , Bancroft JD. 2018. Bancroft’s theory and practice of histological techniques. 8th ed., Oxford : Elsevier.
- Taylor RL , McCorkle FM. 2009. A landmark contribution to poultry science–Immunological function of the bursa of Fabricius. Poult Sci. 88:816–823. doi: 10.3382/ps.2008-00528
- Villegas P. 2008. Titration of biological suspensions. In: DE Dufour Zavala , L Swayne , DE Glisson , JR Pearson , JE Reed , et al ., editors. A laboratory manual for the isolation identification and characterization of avian pathogens. 5th ed. Jackson Ville : American Association of Avian Pathologists; p. 217–221.
- Wajid A , Basharat A , Bibi T , Rehmani SF. 2018. Comparison of protection and viral shedding following vaccination with Newcastle disease virus strains of different genotypes used in vaccine formulation. Trop Anim Health Prod. 50:1645–1651. doi: 10.1007/s11250-018-1607-6
- Wakamatsu N , King DJ , Kapczynski DR , Seal BS , Brown CC. 2006. Experimental pathogenesis for chickens, turkeys, and pigeons of exotic Newcastle disease virus from an outbreak in California during 2002–2003. Vet Pathol. 43:925–933. doi: 10.1354/vp.43-6-925
- Welch CN , Shittu I , Abolnik C , Solomon P , Dimitrov KM , Taylor TL , Williams-Coplin D , Goraichuk IV , Meseko CA , Ibu JO , et al. 2019. Genomic comparison of Newcastle disease viruses isolated in Nigeria between 2002 and 2015 reveals circulation of highly diverse genotypes and spillover into wild birds. Arch Virol. 164:2031–2047. doi: 10.1007/s00705-019-04288-9
