ABSTRACT
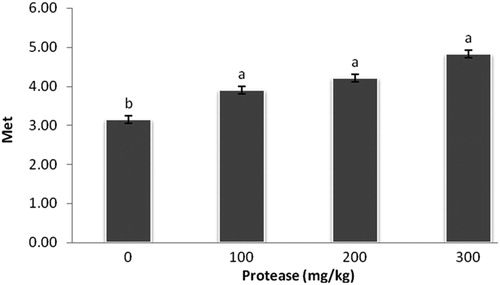
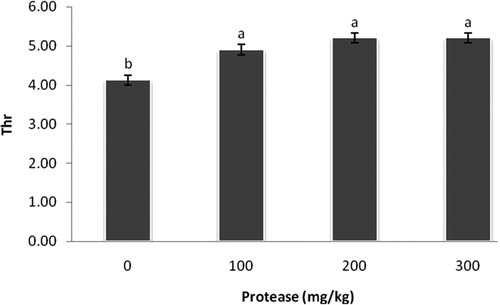
An experimental study was conducted to examine the effects of adding serine-protease from Bacillus licheniformis on performance and physiological parameters of broiler chickens under Egyptian condition. A total of 600 one-day-old chicks were randomly divided into four experimental treatments. The treatments consisted of the control diet with 0, 100, 200 and 300 mg/kg serine-protease. Protease supplementation increased (P < 0.05) body weights (BW). Feed conversion ratio (FCR) was improved (P < 0.05) due to 200 and 300 mg/kg protease supplementation. The dry matter and crude protein digestibilities were enhanced (P < 0.05) by both 200 and 300 mg/kg protease supplementation. Plasma albumin and high-density lipoprotein (HDL) concentrations were increased (P < 0.05), while plasma total cholesterol (CHO) and low-density lipoprotein (LDL) concentrations were decreased (P < 0.05) at 100, 200, and 300 mg/kg of protease. Liver malondialdehyde (MDA) levels were declined (P < 0.05) due to 200 and 300 mg /kg protease supplementation. Supplementing 100, 200, and 300 mg/kg of protease increased (P < 0.05) lysine, methionine, and threonine levels in breast muscle. In conclusion, exogenous serine-protease could be used as a feed additive in broiler nutrition and supplementing 200∼300 mg/kg was sufficient to improve growth performance, probably because of its mechanism to enhance protein digestibility.
1. Introduction
Protein is one of the most nutrients and the most expensive component of animal diets. The physiological function of proteases is necessary for all living organisms, and proteolytic enzymes can be classified based on their origin: microbial (bacterial, fungal, and viral). The endogenous intestinal proteases are sufficient to improve protein utilization (Le Heurou-Luron et al. Citation1993; Niban and Mahgna Citation1993; Baghban-Kanani et al. Citation2018). However, digestibility of crude protein (CP) and amino acid is not completed in all animals (Wang and Parsons Citation1998; Lemme et al. Citation2004). In addition, it has been reported that activities of digestive enzymes (lipase, amylase, and trypsin) increase with age (Noy and Sklan Citation1979; Nitsan et al. Citation1991; Jin et al. Citation1998). Therefore, supplemental exogenous enzymes could optimize the digestibility of CP and consequently growth performance (body weight gain and feed conversion ratio) of broiler chickens (Jiang et al. Citation2008; Angel et al. Citation2011; Kalmendal and Tauson Citation2012; Yegani and Korver Citation2013; Saleh et al. Citation2019a).
Previous experiments conducted on serine protease and threonine and found there benefit on growth performance (Cowieson and Ravindran Citation2008 Ayaşan et al. Citation2009; Angel et al. Citation2011; Fru-Nji et al. Citation2011; Vieira et al. Citation2013; Ayaşan and Okan Citation2014a, Citation2014b; Ding et al. Citation2016; Lin-Law et al. Citation2019; Ndazigaruye et al. Citation2019) and. In addition, it was reported that supplemental protease reduced nitrogen excretion (Aletor et al. Citation2000; Bregendahl et al. Citation2002; Canogullari et al. Citation2009) and decreased feed costs, and consequently broiler industry's production. However, other experiments have concluded inconsistent results (Simbaya et al. Citation1996; Marsman et al. Citation1997; Naveed et al. Citation1998). Additionally, protease enzymes have several benefits including decreasing undigested proteins in the diet, increasing amino acid availability, reducing protein needs in the diet, maintaining weight gain and feed efficiency, reducing proteolytic fermentation, and decreasing biogenic amines and bacterial toxins (Saleh et al. Citation2013; Bedford and Walk Citation2014). Therefore, protease enzymes are of interest to many poultry companies and nutrition supplementation companies for use as an important supplement digestive enzyme in broiler diets. There are few studies investigated the effects of serine protease on physiological change and amino acids composition in breast muscle of broiler chickens. Recently study by Giannenas et al. (Citation2017) reported that using Ronozyme®ProAct (serine protease) on performances of a broiler production system in Greece and they found that growth performance and meat quality were improved.
It could be hypothesized that supplementation of exogenous protease to broiler diets might be involved in improving the utilization of proteins in young chickens and consequently enhanced the growth performance of broilers. Therefore, the objective of the current study was to evaluate the effects of Ronozyme®ProAct serine protease from Bacillus licheniformis on blood biochemical permeates, lipid peroxidation, amino acids composition in breast muscle, nutrients utilization, and growth performance in broilers.
2. Material and methods
The study was approved by the Ethics Committee of Local Experimental Animals Care Committee and conducted in accordance with the guidelines of Kaferelsheikh University, Egypt (Number 4/2016 EC).
2.1 Design and diets
Ross 308 broiler chicks (n = 600), one-day-old were divided into four treatments, each included three replicates of 50 chicks (floor pens; ten birds/m2). Chicks were raised in a windowed experimental farm. The experiment was started during February and brooding heat was provided and the temperature inside the barn was maintained at around 32–34°C from day 1 to day 5 post-hatch, and gradually decreased to 24°C at 21d.
The control diet was based on corn, soybean meal, and corn gluten meal (without serine protease, ). Treatments two to four consisted of the control diet supplemented with graded levels of the exogenous serine-protease (Ronozyme® ProAct, DSM; activity 75,000 PROT kg−1 feed) at 100mg, 200mg, and 300mg/kg, respectively. The enzyme activity in all supplemental enzymes diets was about 98%. Ronozyme® ProAct is a preparation of serine protease produced by a genetically modified strain of Bacillus licheniformis. It is produced by fermentation of a sporulation-deficient Bacillus licheniformis strain which expresses a synthetic gene encoding a serine protease (EC 3.4.21.-). Broilers were fed ad-libitum starter, grower and finisher mash diets (day 1–32 of age) and water was available freely.
Table 1. Composition and calculated levels of the basal diets.
2.2 Growth performance
Mortality was registered daily through experiment period, while pen body weight (BW) and feed consumption were registered every week. Feed conversion ratio (FCR) was considered by dividing feed intake to the weight gain.
2.4 Blood and liver sampling
At day 32 of age, broiler chickens (n = 48) based on average BW were selected (12 birds/ treatment). They were weighed individually, slaughtered, and dissected to collect visceral organs. Weights of hot carcass, breast muscle, liver and abdominal fat were recorded. Blood samples were collected into heparinized test tubes and centrifuged (3000×g) for 20 min at 5oC to separate plasma and it was aspirated by pipette and stored in Eppendorf tubes at −20oC pending analysis. The livers from 12 broiler chickens were immediately collected after slaughter and were snap-frozen with liquid nitrogen, and stored at −80oC until analysis.
2.5 Nutrients digestibility
Forty-eight broiler chickens based on average BW were transferred into digestibility cages at day 33 to conduct a digestibility trial until day 36 of age (12 birds/treatment; 4/replicate). Excreta were collected, weighted, and dried by the drying oven at 60oC for 24 h from each replicate. The whole dried samples were then homogenized after drying. The proximate analysis in diets and excreta was conducted according to AOAC (Citation2003) for determination of dry matter, crude protein, and ether extract as described by Abdel-Moneim et al. (Citation2019) the calculation was as follows: Nitrogen digestibility (%) = (total nitrogen intake − total nitrogen excreted)/total nitrogen intake × 100.
2.6 Biochemical analysis
Total protein, albumin, total cholesterol (CHO), triglycerides (TG), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, glucose, creatinine, glutamic oxalacetic transaminase (SGOT), and glutamate pyruvate transaminase (SGPT) were measured by using kits from (Diamond Diagnostics, Egypt).
2. 7 amino acids levels in breast muscle
Lysine, methionine, and threonine contents in breast muscle were analysed according to (Ceylan and Mİ Citation2011). Ten-grams of breast meat and 40 ml of 0.1 N HCl were homogenized for 45 s at 4°C then centrifuged 15,000 × g for 50 min at 4°C. The supernatants were filtered and were analyzed using (GC-4 CM-PFE, Shimadzu gas chromatograph, Tokyo, Japan) equipped with a flame ionization detector (FID). The amino acids determined were lysine, methionine, and threonine. The amino acids determined were lysine, methionine, and threonine. The values were expressed in grams of amino acid per 100 grams of breast meat.
2.8 Assaying malondialdehyde
Lipid peroxidation was evaluated by assaying the level of malondialdehyde (MDA) in the liver by using kits from Cell Biolabs Inc. (San Diego, CA, USA).
2.9 Statistical analysis
Data were analyzed using SAS software 9.2 (SAS Institute, Cary, North Carolina) and the GLM procedure was used in the analysis. Dunnett's t Test was used to compare each protease level to the nun-supplemented control. Direct regression was utilized to examine if there are any linear or quadratic responses of the dependent variables to the supplemental protease.
3. Results
Dietary protease supplementation at any level increased (P < 0.05) the final body weights of broiler chickens compared to the non-supplemented chickens (). FCR was significantly improved due to dietary 200 and 300 mg/kg protease supplementation. The addition of protease enzyme had no significant influence on the average daily feed intake.
Table 2. Effect of dietary serine protease supplementation on broiler performance at day 32 of age1,2,3.
Digestibility of dry matter was enhanced (P < 0.05) by the addition of both 200 and 300 mg/kg of protease compared to the control and a linear response was observed (). Similarly, a linear increase in crude protein digestibility was observed with increasing dietary protease levels. Ether extract digestibility was the highest when protease was supplemented at 100 mg/kg.
Table 3. Effect of dietary serine protease supplementation on nutrients digestibility1,2.
Plasma total protein was elevated (P < 0.05) in a quadratic fashion with an increase of 200 mg of supplemental protease then a further increased at 300 mg (). Similarly, plasma albumin levels were increased quadratically by protease supplementation at all levels. Plasma GOT was declined (P < 0.05) at 300 mg/kg of protease, while plasma GPT was declined at 200 and 300 mg protease /kg. Plasma creatinine concentrations were decreased linearly (P < 0.05) as protease supplementation increased from 100 to 300 mg/kg. Plasma cholesterol and LDL-cholesterol concentrations were decreased in a quadratic manner with increasing protease supplementation. However, plasma HDL- cholesterol concentration was increased linearly (P < 0.05) with increasing protease supplementation. In addition, plasma triglycerides and glucose concentrations were decreased at only 200 or 300 mg of protease and their response to the enzyme was linear.
Table 4. Effect of dietary serine protease supplementation on blood biochemical indices1,2.
Hot carcass and breast weights were increased (P < 0.05) when protease was included at any level compared to the control group (). Whereas, thigh muscle weight was increased only at 200 and 300 mg of protease. A significant reduction in abdominal fat was observed due to the addition of 300 mg protease. Regarding the internal organ weights, liver weight was increased by supplementation of 200 and 300 mg protease, while heart and gizzard were not significantly affected by protease supplementation.
Table 5. Effect of dietary serine protease supplementation on carcass, cuts, internal organ and abdominal fat (g/100g BW)1,2.
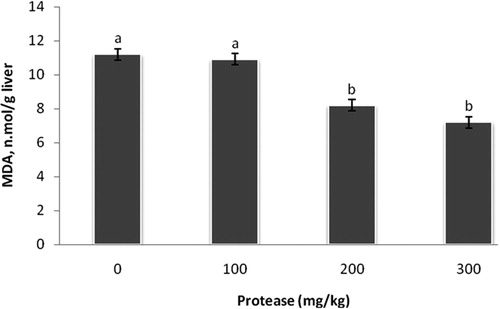
It is noteworthy to mention that, liver MDA concentrations were declined (P < 0.05) due to dietary supplementation of 200mg and 300 mg protease /kg ().
Figure 1. Effect of serine protease on the levels of MDA in the liver. Values are means ± standard SEM. Means not sharing a common superscript letter with the control differ (P < 0.05) as indicated by Dunnett's t Test.
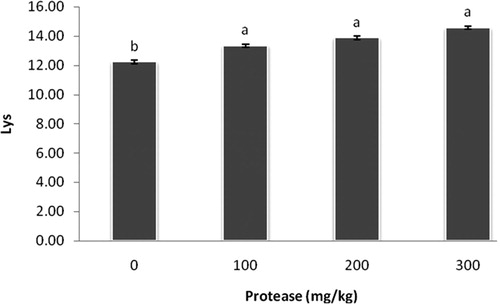
Interestingly, dietary supplementation of 100, 200, and 300 mg/kg protease increased (P < 0.05) lysine, methionine, and threonine levels in breast muscle compared with the control group (–).
Figure 2. Effect of serine protease on lysine content in the breast muscle. The values (means ± standard SEM) were expressed on a gram of amino acid per 100 grams of breast meat. Means not sharing a common superscript letter with the control differ (P < 0.05) as indicated by Dunnett's t Test.
4. Discussion
The protease used in the present study was a serine protease (EC 3.4.21.-) produced by the bacterial species Bacillus licheniformis and its activity is 75,000 protease/g and is stable in poultry feed manufacturing in Egypt. Supplemental protease in diets has been applied as a zootechnical feed additive to enhance digestibility and feed utilization. Results of the present study indicated that protease supplementation improved growth and FCR. These results are in coincidence with previous studies illustrated the positive effects of exogenous proteases added to poultry diets on growth performance (Cowieson and Ravindran Citation2008; Saleh et al. Citation2018). In the present study, the digestibility of dry matter and crude protein were significantly increased with protease supplementation. Therefore, the improvement of the growth performance in the present study might be clarified in the light of enhancements in digestibility of crude protein and dry matter. Previous studies found that protease supplementation improved true nitrogen digestibility (Ghazi et al. Citation2003; Bertichini et al. Citation2009; Sorbara Citation2009; Saleh et al. Citation2014, Citation2018). In addition, the improvement of the performance might be associated with blood biochemical parameters in the present study. In the present study, plasma total protein was elevated at 200 and 300 mg of supplemental protease, while albumin level in the plasma was elevated by supplement graded levels of protease 100, 200, and 300 mg/kg. It has been showed that the concentration of plasma albumin related to improving the immunity status in birds (Laborde et al. Citation1995; Ding et al. Citation2016; Saleh Citation2017; Ndazigaruye et al. Citation2019). Our results are in harmony with Allouche et al. (Citation2015) who found that plasma protein concentrations were enhanced by dietary protease supplementations. Plasma GOT was decreased by the addition of 300 mg protease/kg, while plasma GPT was declined at levels of 200 and 300 mg protease/kg. Elevation in plasma GOT activity indicates hepatic injury. More GOT and GPT are released into the bloodstream when the liver or heart cells are impaired (Saleh Citation2014; Choi et al. Citation2018). It has been reported that the alteration of GPT might be attributed to the synthesized fat level (Dongare et al. Citation2013; Saleh Citation2016). Results presented in showed that plasma total cholesterol concentration was decreased in connection with protease supplementation. In addition, the impaired liver induces lipid peroxidation, which causes hepatotoxicity (Wang et al. Citation2011). As graphically presented in , the levels of liver MDA were declined in 200 and 300 mg of protease/kg diet treatments. These findings might be attributed to the reduction of plasma triglycerides, total cholesterol and LDL- cholesterol (Wang et al. Citation2011; Saleh et al. Citation2018) as illustrated in .
Plasma creatinine concentration as an indicator of kidney functions was significantly decreased due to dietary protease supplementation. These results are in accordance with Dongare et al. (Citation2013) who reported that blood creatinine level was decreased by adding protease. The elevating creatinine level in the blood is correlated with the breakdown of creatine and creatine phosphate in the muscles. Current findings may point out overall better amino acid utilization.
Increasing the supplemental protease levels were associated with decreasing the plasma levels of CHO, GLU, TG, and LDL-cholesterol and elevated HDL-cholesterol level in the current study. However, in another recent study (Ndazigaruye et al. Citation2019) it has been reported that serum total protein, albumin, TG, CHO, creatinine, HDL-cholesterol, GOT, and GPT levels did not change in broiler fed diets with protease. The disagreement between our data and their data may be due to some factors such as the nutrient composition of the diets (sufficient/insufficient protein), enzyme used (source of enzymes, doses), and raw protein sources used which could alter some conditions of the digestive tract such as the pH.
Hot carcass, cuts (breast and thigh muscles), and liver were increased with protease supplementation, while abdominal fat was decreased. It has been reported that enzymes improved amino acid utilization and decreased the quantity of nutrients excreted in faeces (Huo et al. Citation1993; Hajati et al. Citation2009; Tactacan et al. Citation2016). Proteases may cleave anti-nutrients such as trypsin inhibitors resulting in improve utilization and bio-availability of amino acids (Marsman et al. Citation1997; Hajati et al. Citation2009). In addition, it has been found that amino acids digestibility increased with protease inclusions by 5.4% for lysine, 7.8% for threonine, and 6.5% for methionine (Angel et al. Citation2011). Therefore, protease supplementation could increase the concentration of free amino acids in breast muscle. It has been reported that the levels of total amino acids in serum, liver, feather, and carcass can reflect the amino acids utilization (Kaczmarek et al. Citation2014; Azzam et al. Citation2015, Citation2017; Dong et al. Citation2016; Morales et al. Citation2017; Wecke et al. Citation2018; Middendorf et al. Citation2019). In addition, amino acids concentrations in carcass could be used to determine amino acid requirements accurately in broiler diets (Stilborn et al. Citation2010; Abd El-Moneim et al. Citation2019; Saleh et al. Citation2019b).
5. Conclusion
Based in the data presented above, it could be concluded that exogenous serine-protease (Ronozyme® ProAct) supplementation at levels 200∼300 mg/kg might be involved in enhancing protein utilization and resulted in positive effects on growth performance, nutrients utilization, lipid peroxidation and modified plasma lipids profile in broiler chickens.
Author contributions
A.A.S., M.M.D., M.M.A and K.A.A.; methodology, A.A.S., M.M.D and M.M.A; software, A.A.S. and M.M.A.; validation, A. A. S., M.M.A. and N.A.B.; formal analysis, A.A.S., M.M.A., T.A.E.; investigation, A.A.S.; resources, A.A.S. and M.M.A.; data curation, A.A.S., M.M.D. and T.A.E.; writing – original draft preparation, A.A.S., T.A.E. and M.M.A.; writing – review and editing, A.A.S., M.M.A. and T.A.E.; visualization, A.A.S.; supervision, A.A.S. and K.A.A and N.A.B.
Acknowledgement
The authors would like to extend their sincere appreciation to the staff members of the Poultry Production Department, Faculty of Agriculture, Kafrelsheikh University, Egypt; Also, The authors would like to thank Dr. Rashed Alhotan (King Saud University) for data analysis assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Ahmed A. Saleh http://orcid.org/0000-0002-2052-5248
References
- Abdel-Moneim AME, Selim DA, Basuony HA, Sabic EM, Saleh AA. 2019. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop Anim Health Prod. doi:10.1007/s11250-019-02055-1.
- Abd El-Moneim EA, El-Wardany I, Abu-Taleb AM, Wakwak MM, Saleh AA. 2019. Assessment of In Ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob Proteins. doi: 10.1007/s12602-019-09549-2
- Aletor VA, Hamid II, Nieß E, Pfeffer E. 2000. Low-protein amino acid-supplemented diets in broiler chickens: effects on performance, carcass char-acteristics, whole-body composition and efficiencies of nutrient utilisation. J Sci Food Agric. 80:547–554. doi:10.1002/(SICI)1097-0010(200004)80:5<547::AID-JSFA531>3.0.CO;2-C doi: 10.1002/(SICI)1097-0010(200004)80:5<547::AID-JSFA531>3.0.CO;2-C
- Allouche L, Madani T, Ait A, Hamouda Z, Boucherit MR. 2015. Effect of addition of exogenous enzymes in hypocaloric diet in broiler chicken on performance. Biochemical parameters and meat characteristics. Biotech Anim Husb. 31(4):P551–P565. doi:10.2298/BAH1504551A.
- Angel CR, Saylor W, Vieria SL, Ward N. 2011. Effects of a monocomponent protease on performance and protein utilization in 7- to 22-day-old broiler chickens. Poult Sci. 90:2281–2286. doi: 10.3382/ps.2011-01482.
- Association of Official Analytical Chemists, AOAC. 2003. Methods of analysis, 15th ed. Washington, DC: AOAC.
- Ayaşan T, Okan F. 2014a. The effect of choice feeding based on threonine on performance characteristics and Carcas parameters of female broiler chicks. Ksu J Nat Sci. 17(2):1–9.
- Ayaşan T, Okan F. 2014b. The effect of choice feeding based on threonine on performance and carcass parameters of male broiler chicks. Turkish J Agri – Food Sci And Technol. 2(4):190–196. doi: 10.24925/turjaf.v2i4.190-196.117
- Ayaşan T, Okan F, Hizli H. 2009. Threonine requirement of broilers from 22 To 42 days. Int J Poult Sci. 8(9):862–865. doi: 10.3923/ijps.2009.862.865
- Azzam MMM, Dong XY, Dai L, Zou XT. 2015. Effect of excess dietary L-valine on laying hen performance, egg quality, serum free amino acids, immune function and antioxidant enzyme activity. Br Poult Sci. 56:72–78. doi: 10.1080/00071668.2014.989487.
- Azzam MMM, Dong XY, Zou XT. 2017. Effect of dietary threonine on laying performance and intestinal immunity of laying hens fed low-crude-protein diets during the peak production period. J Anim Physiol An N. 101:e55–e66. doi: 10.1111/jpn.12559.
- Baghban-Kanani P, Hosseintabar-Ghasemabad B, Azimi-Youvalari S, Seidavi A, Ayaşan T, Laudadio V, Tufarelli V. 2018. Effect of different levels of sunflower meal and multi-enzyme complex on performance, biochemical parameters and antioxidant status of laying hens. S Afr J Anim Sci. 48(2):390–399. doi: 10.4314/sajas.v48i2.20
- Bedford M, Walk C. 2014. Enzymes and their effect on amino acid nutrition. Marlborough: AB Vista Feed Ingredients.
- Bertichini AG, Carvalho JCC, Mesquita FR, Castro SF, Meneghetti C. 2009. Use of a protease to enhance the utilisation of soybean meal amino acids by broilers. Poult Sci. Abstract. 221: Raleigh NC.
- Bregendahl K, Sell JL, Zimmerman DR. 2002. Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult Sci. 81:1156–1167. doi:10.1093/ps/81.8.1156.
- Canogullari S, Baylan M, Ayasan T. 2009. Threonine requirement of laying Japanese Quails. J Anim Vet Adv. 8(8):1539–1541.
- Ceylan S, Mİ A. 2011. Free amino acids profile and quantities of ‘sırt,‘bohca’and ‘sekerpare’pastirma, dry cured meat products. J Sci Food Agr. 91(5):956–962. doi: 10.1002/jsfa.4273.
- Choi Y, Lee EC, Na Y, Rak S. 2018. Lee effects of dietary supplementation with fermented and non-fermented brown algae by-products on laying performance, egg quality, and blood profile in laying hens. Asian Austral J Anim. 31(10):1654–1659. doi: 10.5713/ajas.17.0921.
- Cowieson AJ, Ravindran V. 2008. Effect of exogenous enzymes in maize-based diets varying in nutrient density for young broilers: growth performance and digestibility of energy, minerals and amino acids. Br Poult Sci. 49:37–44. doi: 10.1080/00071660701812989.
- Ding XM, Li DD, Li ZR, Wang JP, Zeng QF. 2016. Effects of dietary crude protein levels and exogenous protease on performance, nutrient digestibility, trypsin activity and intestinal morphology in broilers. Livest Sci. 193:26–31. doi:10.1016/j.livsci.2016.09.002.
- Dong XY, Azzam MM, Zou XT. 2016. Effects of dietary L-isoleucine on laying performance and immunomodulation of laying hens. Poult Sci. 95:2297–2305. doi: 10.3382/ps/pew163.
- Dongare P, Dhande SR, Kadam VJ. 2013.;Standardization of carbon tetrachloride-induced hepatotoxicity in the rat. Am J Pharmtech Res. 3:438–445.
- Fru-Nji F, Kluenter AM, Fischer M, Pontoppidan KA. 2011. Feed serine protease improves broiler performance and increases protein and energy digestibility. J Poult Sci. 48(4):239–246. doi:10.2141/jpsa.011035.
- Ghazi S, Rooke JA, Galbraith H. 2003. Improvement of the nutritive value of soybean meal by protease and α-galactosidase treatment in broiler cockerels and broiler chicks. Br Poult Sci. 44:410–418. doi:10.1080/00071660310001598283.
- Giannenas I, Bonos E, Anestis V, Filioussis G, Papanastasiou DK. 2017. Effects of protease addition and replacement of soybean meal by corn gluten meal on the growth of broilers and on the Environmental performances of a broiler production system in Greece. PLoS ONE. 12(1):e0169511. doi:10.1371/journal.pone.0169511.
- Hajati H, Rezaei M, Sayyahzadeh H. 2009. The effects of enzyme supplementation on performance, carcass characteristics and some blood parameters of broilers fed on corn-soybean meal-wheat diets. Int J Poult Sci. 8(12):1199–1205. doi: 10.3923/ijps.2009.1199.1205.
- Huo GC, Fowler VR, Inborr J, Bedford MR. 1993. The use of enzymes to denature antinutritive factors in soybean. Proceedings of the 2nd Workshop on ‘Antinutritional Factors (ANFs) in Legume Seed’, Wageningen; December 1–3, The Netherlands, p. 60.
- Jiang Z, Zhou Y, Lu F, Han Z, Wang T. 2008. Effects of different levels of supplementary alpha-amylase on digestive enzyme activities and pancreatic amylase mRNA expression of young broilers. Asian Austral J Anim. 21:97–102. doi:10.5713/ajas.2008.70110.
- Jin SH, Corless A, Sell JL. 1998. Digestive system development in post-hatch poultry. World’s Poult Sci J. 54:335–345. doi:10.1079/WPS19980023.
- Kaczmarek SA, Rogiewicz A, Mogielnicka M, Rutkowski A, Jones RO. 2014. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult Sci. 93:1745–1753. doi: 10.3382/ps.2013-03739.
- Kalmendal R, Tauson R. 2012. Effects of a xylanase and protease, individually or in combination, and an ionophore coccidiostat on performance, nutrient utilization, and intestinal morphology in broiler chickens fed a wheat-soybean meal-based diet. Poult Sci. 91:1387–1393. doi: 10.3382/ps.2011-02064.
- Laborde CJ, Chapa AM, Burleigh DW, Salgado DJ, Fernandez JM. 1995. Effects of processing and storage on the measurement of nitrogenous compounds in ovine blood. Small Ruminant Res. 17:159–166. doi:10.1016/0921-4488(95)00665-8.
- Le Heurou-Luron I, Lhoste E, Wickerplanquarl C, Dakka N, Toullec R . 1993. Molecular aspects of enzyme synthesis in the exocrine pancreas with emphasis on development and nutritional regulation. Prod Nutr Soc. 52:301–313. doi:10.1079/pns19930066.
- Lemme A, Ravindran V, Bryden WL. 2004. Ileal digestibility of amino acids in feed ingredients for broilers. Worlds Poult Sci J. 60:423–437. doi:10.1079/WPS200426.
- Lin-Law F, Idrus Z, Soleimani Farjam A, Juan Boo L. 2019. Effects of protease supplementation of low protein and/or energy diets on growth performance and blood parameters in broiler chickens under heat stress condition. Ital J Anim Sci. 2(18(1)):679–689. doi:10.1080/1828051X.2018.1557019.
- Marsman GJ, Gruppen H, Van der Poel AF, Kwakkel RP, Verstegen MW . 1997. The effect of thermal processing and enzyme treatments of soybean meal on growth performance, ileal nutrient digestibilities, and chyme characteristics in broiler chicks. Poult Sci. 76:864–872. doi:10.1093/ps/76.6.864.
- Middendorf L, Radko D, Düngelhoef K, Sieverding E, Windhaus H. 2019. Amino acid pattern in the liver and blood of fattening turkeys suffering from hepatic lipidosis. Poult Sci. 98:3950–3962. doi:10.3382/ps/pez131.
- Morales A, Buenabad L, Castillo G, Vázquez L, Espinoza S. 2017. Dietary levels of protein and free amino acids affect pancreatic proteases activities, amino acids transporters expression and serum amino acid concentrations in starter pigs. J Anim Physiol An N. 101:723–732. doi: 10.3382/ps.2013-03739 doi: 10.1111/jpn.12515
- Naveed A, Acamovic T, Bedford MR. 1998. Effect of enzyme supplementation of UK-known Lupinis albus on growth performance in broiler chickens. Br Poult Sci. 39:S36–S37. doi:10.1080/00071669888250.
- Ndazigaruye G, Kim D-H, Chang-Won K, Kyung-Rae K, Yong-Jin J. 2019. Effects of low-protein diets and exogenous protease on growth performance, carcass Traits, intestinal morphology, cecal volatile fatty acids and serum parameters in broilers. Animals (Basel). 9:226. doi:10.3390/ani9050226.
- Niban M, Mahgna A. 1993. Comparative growth and development of the digestive organs and of some enzymes in broiler and egg type chicks after hatching. Br Poult Sci. 34:523–532. doi:10.1080/00071669308417607.
- Nitsan Z, Duntington EA, Siegel PB. 1991. Organ growth and digestive enzyme levels to fifteen days of age in lines of chickens differing in body weight. Poult Sci. 70:2040–2048. doi:10.3382/ps.0702040.
- Noy Y, Sklan D. 1979. Digestion and absorption in the young chick. Poult Sci. 74:366–373. doi:10.3382/ps.0740366.
- Saleh AA. 2014. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Anim Sci Papers and Reports. 32(1):65–79.
- Saleh AA. 2016. Effect of low-protein in iso-energetic diets on performance, carcass characteristics. Digestibilities and plasma lipids of broiler chickens. Egypt Poult Sci J. 36(I):251–262. doi: 10.21608/epsj.2016.33259
- Saleh AA. 2017. Influence of Escherichia coli 6-Phytase supplementation on performance and egg quality in Hi-sex laying hens fed phosphorus deficient diets. Egypt Poult Sci. 37:1105–1117. doi: 10.21608/epsj.2017.5382
- Saleh AA, Amber K, El-Magd MA, Atta MS, Mohammed AA. 2014. Integrative effects of feeding Aspergillus awamori and fructooligosaccharide on growth performance and digestibility in broilers: Promotion muscle protein metabolism. BioMed Res Inter. 2014: 8. doi:10.1155/2014/946859. Article ID 946859.
- Saleh AA, El-Far AH, Abdel-Latif MA, Emam MA, Ghanem R. 2018. Exogenous dietary enzyme formulations improve growth performance of broiler chickens fed a low-energy diet targeting the intestinal nutrient transporter genes. PLoS ONE. 13(5):e0198085. doi:10.1371/journal pone.0198085 doi: 10.1371/journal.pone.0198085
- Saleh AA, Kirrella AA, Abdo SE, Mousa MM, Badwi NA. 2019a. Effects of dietary xylanase and arabinofuranosidase combination on the growth performance, lipid peroxidation, blood constituents, and immune response of broilers fed low-energy diets. Animals (Basel). 9:467. doi:10.3390/ani9070467.
- Saleh AA, Kirrella AA, Dawood MAO, Ebeid TA. 2019b. Effect of dietary inclusion of cumin seed oil on the performance, egg quality, immune response and ovarian development in laying hens under high ambient temperature. J Anim Physiol An N. 1–8. doi: 10.1111/jpn.13206.
- Saleh AA, Ohtsuka A, Yamamoto M, Hayashi K. 2013. Aspergillus awamori feeding modifies lipid metabolism in rats. BioMed Res Inter. 2013:7. doi:10.1155/2013/594393. Article ID 594393
- Simbaya J, Slominski BA, Guenter W, Morgan A, Campbell LD. 1996. The effects of protease and carbohydrase supplementation on the nutritive value of canola meal for poultry: in vitro and in vivo studies. Anim Feed Sci Technol. 61:219–234. doi:10.1016/0377-8401(95)00939-6.
- Sorbara JOB. 2009. Use of a protease to enhance the utilisation of soybean meal amino acids by broilers. Poultry. Sci. Assoc. 98thAnnual Meeting, Raleigh, North Carolina. Poult Sci. Abstract 88: 221, p. 68.
- Stilborn HL, Moran ET Jr, Gous RM, Harrison MD. 2010. Influence of age on carcass (feather-free) amino acid content for two broiler strain-crosses and sexes. J Applied Poult Res. 19:13–23. doi:10.3382/japr.2009-00053.
- Tactacan GB, Cho SY, Cho JH, Kim IH. 2016. Performance responses, nutrient digestibility, blood characteristics, and measures of gastrointestinal health in weanling pigs fed protease enzyme. Asian Austral J Anim. 29(7):998. doi: 10.5713/ajas.15.0886.
- Vieira SL, Angel CR, Miranda DJA, Favero A, Cruz RFA. 2013. Effects of a monocomponent protease on performance and protein utilization in 1-to 26-day-of-age Turkey poults. J Applied Poult Res. 22(4):680–688. doi:10.3382/japr.2012-00558.
- Wang X, Parsons CM. 1998. Effect of raw material source, processing systems, and processing temperatures on amino acid digestibility of meat and bone meals. Poult Sci. 77:834–841. doi:10.1093/ps/77.6.834.
- Wang D, Piao XS, Zeng ZK, Lu T, Zhang Q, Li PF. 2011. Effects of keratinase on performance, nutrient utilization, intestinal morphology, intestinal ecology and inflammatory response of weaned piglets fed diets with different levels of crude protein. Asian Austral J Anim. 24:1718–1728. doi:10.5713/ajas.2011.11132.
- Wecke C, Khan DR, Sünder A, Liebert F. 2018. Age and gender dependent amino acid concentrations in the feather, feather-free and whole empty body protein of fast growing meat-type chickens. Open J Anim Sci. 25:223–238. doi: 10.4236/ojas.2018.83017.
- Yegani M, Korver DR. 2013. Effects of corn source and exogenous enzymes on growth performance and nutrient digestibility in broiler chickens. Poult Sci. 92:1208–1220. doi: 10.3382/ps.2012-02390.